| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Original Article
Volume 10, Number 4, August 2021, pages 187-195
Treatment Outcomes and Survival Patterns of Asian Patients With Relapsed/Refractory Mantle Cell Lymphoma
Jing Yuan Tana, f, Tian Yu Qiua, f, Jianbang Chiangb, c, Ya Hwee Tanb, c, Valerie Shiwen Yangb, c, d, Esther Wei Yin Changb, c, Eileen Poonb, c, Nagavalli Somasundaramb, c, e, Mohamad Faridb, c, e, Miriam Taob, c, e, Soon Thye Limb, c, e, g, Jason Yongsheng Chanb, c, e, g
aSinghealth Internal Medicine Residency, Singapore General Hospital, Singapore
bDivision of Medical Oncology, National Cancer Centre Singapore, Singapore
cSingHealth Duke-NUS Blood Cancer Centre, Singapore
dInstitute of Molecular and Cell Biology, Singapore
eOncology Academic Clinical Program, Duke-NUS Medical School, Singapore
fThese authors contributed equally to this article.
gCorresponding Author: Jason Yongsheng Chan, Division of Medical Oncology, National Cancer Centre Singapore, 11 Hospital Drive, 169610, Singapore; Soon Thye Lim, Division of Medical Oncology, National Cancer Centre Singapore, 11 Hospital Drive, 169610, Singapore
Manuscript submitted July 8, 2021, accepted August 10, 2021, published online August 30, 2021
Short title: Outcomes of R/R MCL
doi: https://doi.org/10.14740/jh890
| Abstract | ▴Top |
Background: Mantle cell lymphoma (MCL) is widely considered an incurable malignancy even with current therapies and relapsed/refractory (R/R) disease to primary treatment remains common. With improved treatment guidelines and the advent of novel agents, patients are increasingly being treated with more lines of regimens. However, outcomes after each line of treatment remain poorly characterized, especially in the Asian population. In this paper, we described the survival outcomes in a group of R/R MCL patients.
Methods: We retrospectively studied 35 patients with R/R MCL between 1998 and 2020 at the National Cancer Centre Singapore. Patients were followed longitudinally throughout their disease course. Overall survival (OS) and progression-free survival (PFS) were determined by the Kaplan-Meier method.
Results: The median OS and PFS from diagnosis were 105 and 40 months, respectively. After first relapse, the median OS and PFS were 52 and 19 months, post-second relapse 32 and 8 months, and post-third relapse 12 and 6 months, respectively. Patients older than 65 years at first relapse had shorter survival (median OS: 22 vs. 55 months, P = 0.0417; median PFS: 9 vs. 29 months, P = 0.001). Early treatment failure after first line therapy was also associated with worse survival outcomes (median OS: 13 vs. 55 months, P < 0.001; median PFS: 9 vs. 26 months, P < 0.001).
Conclusion: With each relapse, survival outcomes for patients with MCL are worse. Novel treatment and contemporary outcomes of R/R MCL are encouraging and support the need for continued research in this area.
Keywords: Mantle cell lymphoma; Novel therapies; Chemotherapy; Bruton’s tyrosine kinase
| Introduction | ▴Top |
Mantle cell lymphoma (MCL) is an uncommon subtype of B-cell non-Hodgkin lymphoma (NHL) [1]. While it has a heterogeneous clinical course, it is generally considered to be an aggressive disease [2]. It is widely thought to be an incurable malignancy, and most patients will have refractory/recurrent (R/R) disease and require multiple lines of therapy [3]. Clinical practice varies greatly between managing physicians and multiple chemotherapy regimens have been tried in R/R MCL [4, 5]. Currently, chemoimmunotherapy with or without autologous stem cell transplant is considered first line standard of care treatment [6]. There is however no universal consensus on subsequent lines of treatment of R/R MCL [7]. The development of lenalidomide, temsirolimus and bortezomib provided significant albeit modest and temporary responses in the relapse setting [8-10]. Most recently, the advent of Bruton’s tyrosine kinase (BTK) inhibitors represented a breakthrough in the treatment of B-cell NHL and has proven beneficial in clinical trials on MCL [11, 12]. A recent consensus paper by the Asian Lymphoma Study Group has also recommended the use of BTK inhibitors for R/R MCL [13]. However, treatment outcomes with each subsequent line of therapy, especially involving the use of novel-therapies, remain poorly characterized.
In this study, we investigated the treatment patterns and examined the survival patterns in Asian patients with R/R MCL.
| Materials and Methods | ▴Top |
Study cohort
This was a retrospective study involving patients who had R/R MCL and seen at the National Cancer Centre Singapore between April 1998 and September 2020. Patients’ clinical data were extracted from the Singapore Lymphoma Study database. At the time of analysis, 37 patients with R/R MCL were included in our study. This was from an initial cohort of 66 patients with a histologically confirmed diagnosis of MCL, of which 56 had received first-line chemoimmunotherapy with or without autologous stem cell transplant and subsequently. Of the 37 patients with R/R MCL, we excluded two patients due to incomplete clinical data. Thirty-five patients were included in the final analysis. The median follow-up was 79 months for the final cohort of R/R MCL patients (Supplementary Material 1, www.thejh.org). Relevant demographical, clinico-pathological and treatment information at diagnosis and at each relapse were collected and analyzed. Demographical information included sex, age, ethnicity and smoking history. Response to treatment was determined by the treating clinician’s assessment which was guided by both clinical and radiological evaluation. Outcome measures included 1) percentage of patients with an objective response, which included complete remission (CR), partial remission (PR), stable disease (SD), progressive disease (PD) and death; 2) survival outcomes including progression-free survival (PFS) and overall survival (OS). Early relapse was defined as relapse or progression of disease within 2 years from initiating first-line treatment. Late relapse was defined as relapse or progression of disease after 2 years from initiating first-line treatment. We also described in detail the clinical progress and outcomes of patients who received BTK inhibitors (ibrutinib and acalabrutinib) as part of their treatment regimen for R/R MCL.
This research study was carried out as part of the Singapore Lymphoma Study with approval from the SingHealth Centralised Institutional Review Board (CIRB 2018/3084). This study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration.
Statistical analysis
The primary outcomes of this study were OS and PFS. PFS was calculated from date of diagnosis/date of relapse until the date of disease progression/further relapse or death from any cause. Disease progression/relapse was defined as a presentation with new symptoms or signs of the disease that was confirmed radiographically or pathologically. OS was defined as the time from date of diagnosis/date of relapse until death from any cause or was censored at the date of last follow-up for survivors. Kaplan-Meier survival curves were plotted to estimate survival for each individual clinico-pathological parameter. The log-rank test was then used to determine hazard ratios (HRs), the corresponding 95% confidence intervals (95% CIs) of mortality and the P-values. Comparisons of the frequencies of categorical variables were performed using Pearson’s Chi-squared test or Fisher’s exact test, as appropriate. All statistical evaluations were made assuming a two-sided test with significance level of 0.05 unless otherwise stated. All tests were performed using MedCalc statistical Software for Windows version 19.0.4 (MedCalc Software, Ostend, Belgium).
| Results | ▴Top |
Patient characteristics
Thirty-five patients with R/R MCL were included in our final analysis. The median age of our cohort was 58 years old with the majority being males (74.3%). The majority (80%) had low to intermediate simplified Mantle Cell Lymphoma International Prognostic Index (sMIPI) scores at initial diagnosis. More than half (68.6%) had Ann Arbor stage 4 at initial diagnosis. The rest of the patient characteristics are shown in Table 1.
 Click to view | Table 1. Clinical Features of R/R MCL Cohort at Diagnosis |
Treatments received
Of the 35 patients who relapsed or had refractory disease, 18 (51.4%) received cytarabine-based chemotherapy regimens and 17 (48.6%) received non-cytarabine-based chemotherapy regimens as first-line treatment. The majority (13/18) of the cytarabine-based regimens was R-hyperCVAD (rituximab, cyclophosphamide, vincristine, doxorubicin and dexamethasone, alternating with high dose methotrexate and cytarabine) and the majority (9/17) of the non-cytarabine-based regimen was R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine and prednisolone). Thirty-three (94.3%) patients received rituximab as part of their first-line treatment and 11 (31.4%) patients also received maintenance rituximab. Five patients (14.3%) received autologous hematopoietic stem cell transplant (HSCT) as part of their initial treatment regimen. Other first-line treatment regimens are summarized in Table 1. Twenty-nine (82.8%) patients received second-line systemic treatment (Supplementary Material 2, www.thejh.org), of which two had allogenic HSCT. Ten (34.4%) patients received novel therapies with the majority being bortezomib-based regimens (6/10). In the remaining patients who received conventional chemotherapy, bendamustine-based regimens were the most common (9/19).
Treatment patterns and survival outcomes
For our R/R MCL cohort (n = 35), after first-line treatment, the majority (74.3%) achieved CR before relapse. The median OS and PFS from diagnosis were 105 and 40 months, respectively. In comparison, the OS for patients without documented relapse (median not reached) and patients who did not receive first-line treatment (median, 9.4 months) are presented in Supplementary Material 3 (www.thejh.org). The median duration from diagnosis to first relapse was 40.1 months. The median OS and PFS from first relapse were 52 months (95% CI 25 - 86) and 19 months (95% CI 9 - 26), respectively (Fig. 1). Out of these 35 patients, 58.6% were alive and 16.9% were progression-free at 3 years after second-line treatment, and 37.4% were alive and 0% were progression-free at 5 years after second-line treatment. Treatment outcomes declined with successive lines of treatment. The proportion of patients who achieved CR or PR after subsequent lines of treatment decreased and in patients who received fourth-line treatment and beyond, CR or PR was not achieved (Table 2). With each subsequent line of treatment, there was a decrease in OS (Fig. 2). The median OS following second, third and fourth-line treatment beyond were 52 months (95% CI 25 - 86 months), 32 months (95% CI 22 - 71 months), and 12 months (95% CI 5 - 26 months), respectively (P = 0.0017). We also observed a decrease in PFS with each subsequent line of treatment, though this was not statistically significant. The median PFS following second, third and fourth-line treatment beyond were 19 months (95% CI 9 - 26 months), 8 months (95% CI 6 - 24 months), and 6 months (95% CI 3 - 15 months), respectively (P = 0.0967).
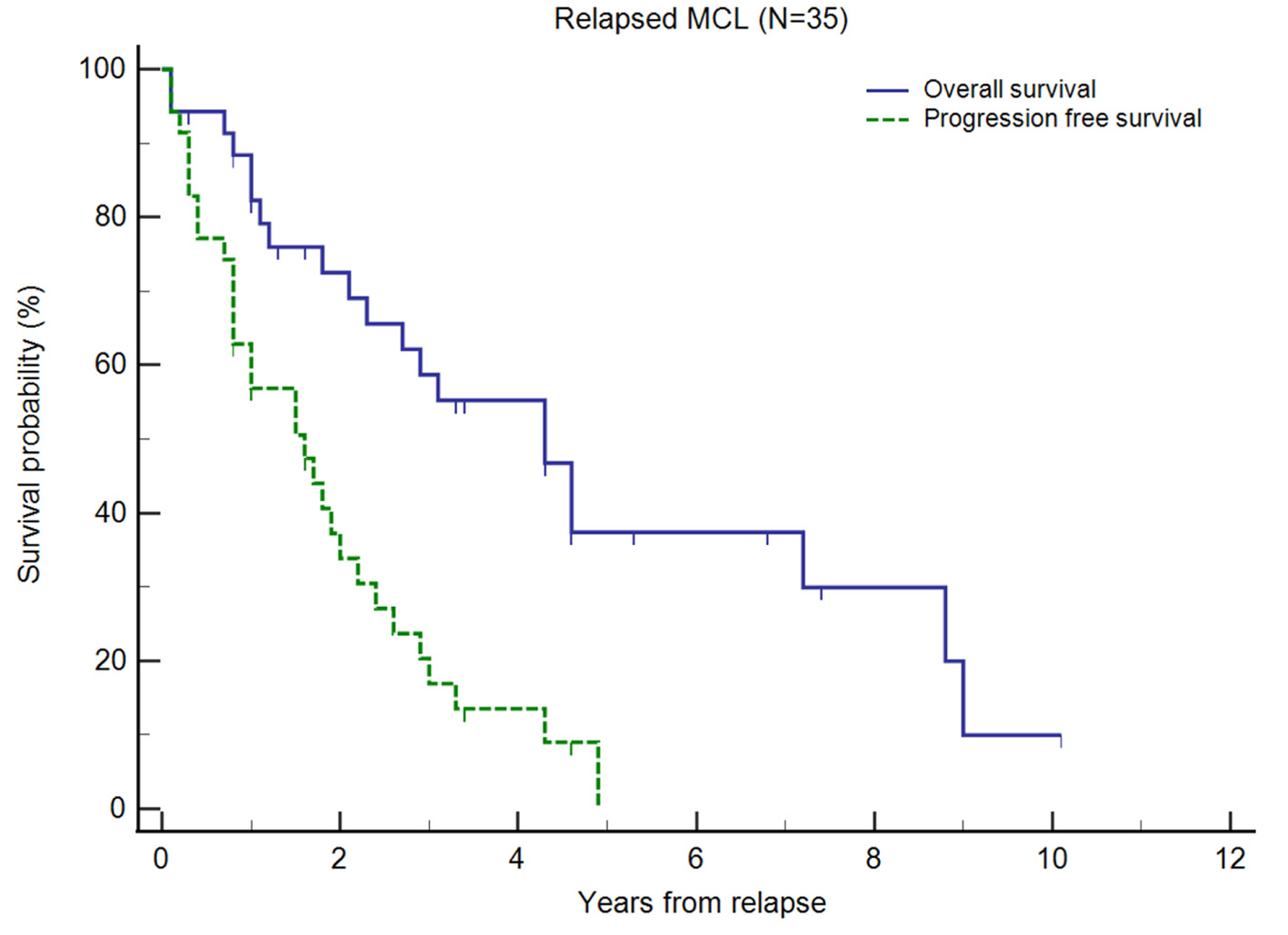 Click for large image | Figure 1. Kaplan-Meier plots of overall survival (OS) and progression-free survival (PFS) of relapsed/refractory (R/R) mantle cell lymphoma (MCL) from first relapse. Small vertical lines on the graph represent censored observations when patients are lost to follow-up. |
 Click to view | Table 2. Clinical Features of Study Cohort at Each Time of Relapse |
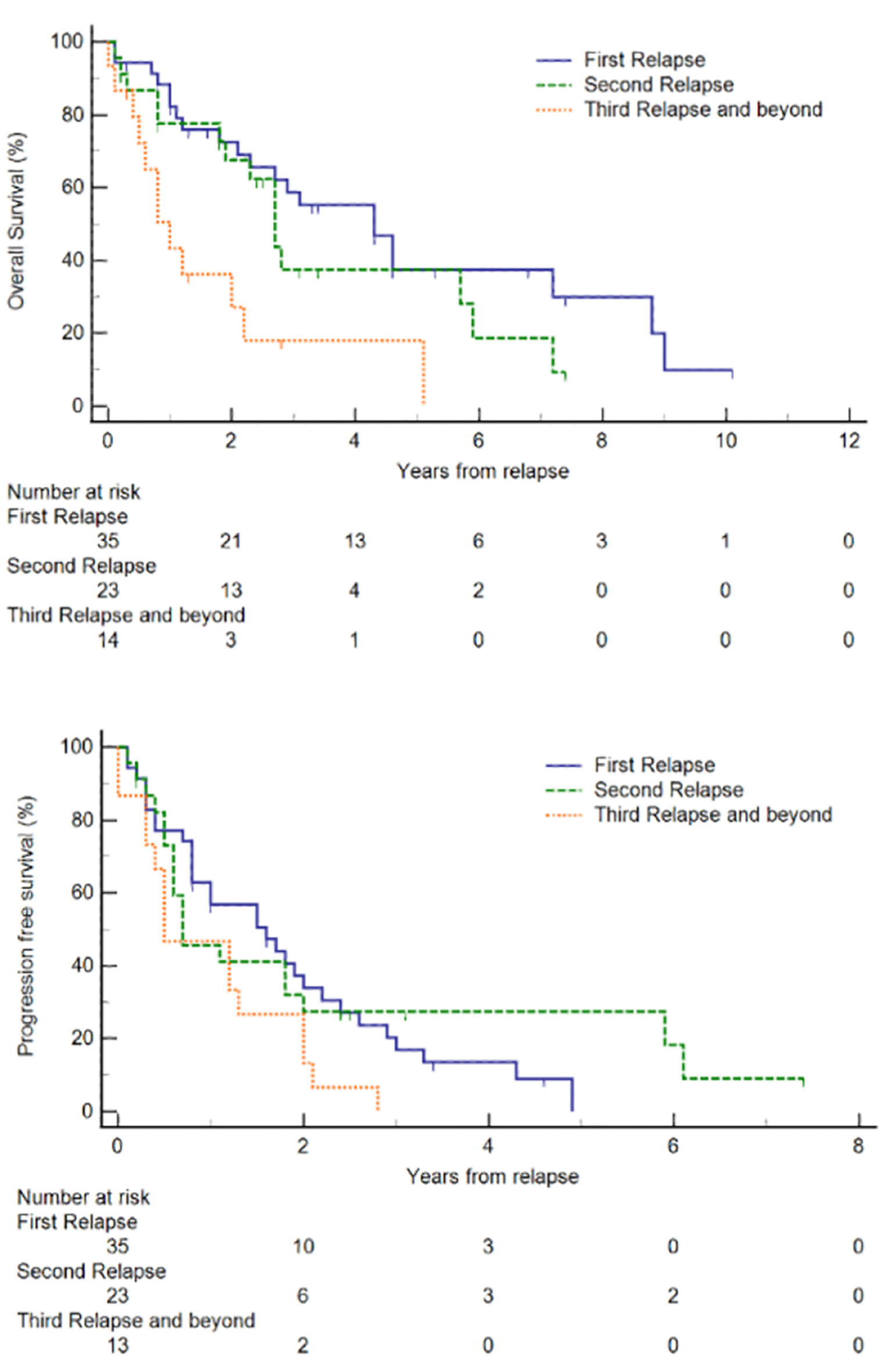 Click for large image | Figure 2. Kaplan-Meier plots of overall survival (OS) and progression-free survival (PFS) of relapsed/refractory (R/R) mantle cell lymphoma (MCL) at each relapse. |
Survival analyses and prognostic factors
In our relapsed cohort, we identified two significant prognostic factors. Firstly, patients older than 65 years at first relapse had shorter survival compared with those younger than 65 years (median OS 22 months, 95% CI 10 - 86 months vs. median OS 55 months, 95% CI 35 - 108 months, P = 0.0417; median PFS 9 months, 95% CI 3 - 12 months vs. median PFS 29 months, 95% CI 20 - 39 months, P = 0.001). Secondly, patients with early relapse, defined as relapse within 2 years from initial diagnosis, also had shorter survival compared with those who relapsed after 2 years (median OS 13 months, 95% CI 13 - 55 months vs. median OS 55 months, 95% CI 35 - 108 months, P < 0.001; median PFS 9 months, 95% CI 2 - 12 months vs. median PFS 26 months, 95% CI 18 - 36 months, P < 0.001) (Fig. 3). We also observed that in relapsed MCL patients, the use of novel therapies (lenalidomide, bortezomib, and BTK inhibitors) in any of the subsequent lines of treatment had a longer OS than patients who did not (median OS 55 months, 95% CI 27 - 106 months vs. median OS 35 months, 95% CI 10 - 37 months), though the difference was not statistically significant. Other prognostic factors analyzed included gender, sMIPI score at diagnosis, the use of novel therapy (lenalidomide, everolimus, borteozomib, and BTK inhibitor), the use of BTK inhibitors and the use of HSCT at initial treatment. These factors were found to be not significant in our study (Supplementary Material 4, www.thejh.org). In addition, we divided our R/R MCL cohort into two groups, analyzing the OS outcomes based on their dates of diagnosis (2009 onwards versus prior to 2009). The median OS was 136 months (2009 onwards; n = 17) compared to 98 months (prior to 2009; n = 18), but this was not statistically significant (HR 0.63, 95% CI 0.23 - 1.73, P = 0.373) (Supplementary Material 5, www.thejh.org).
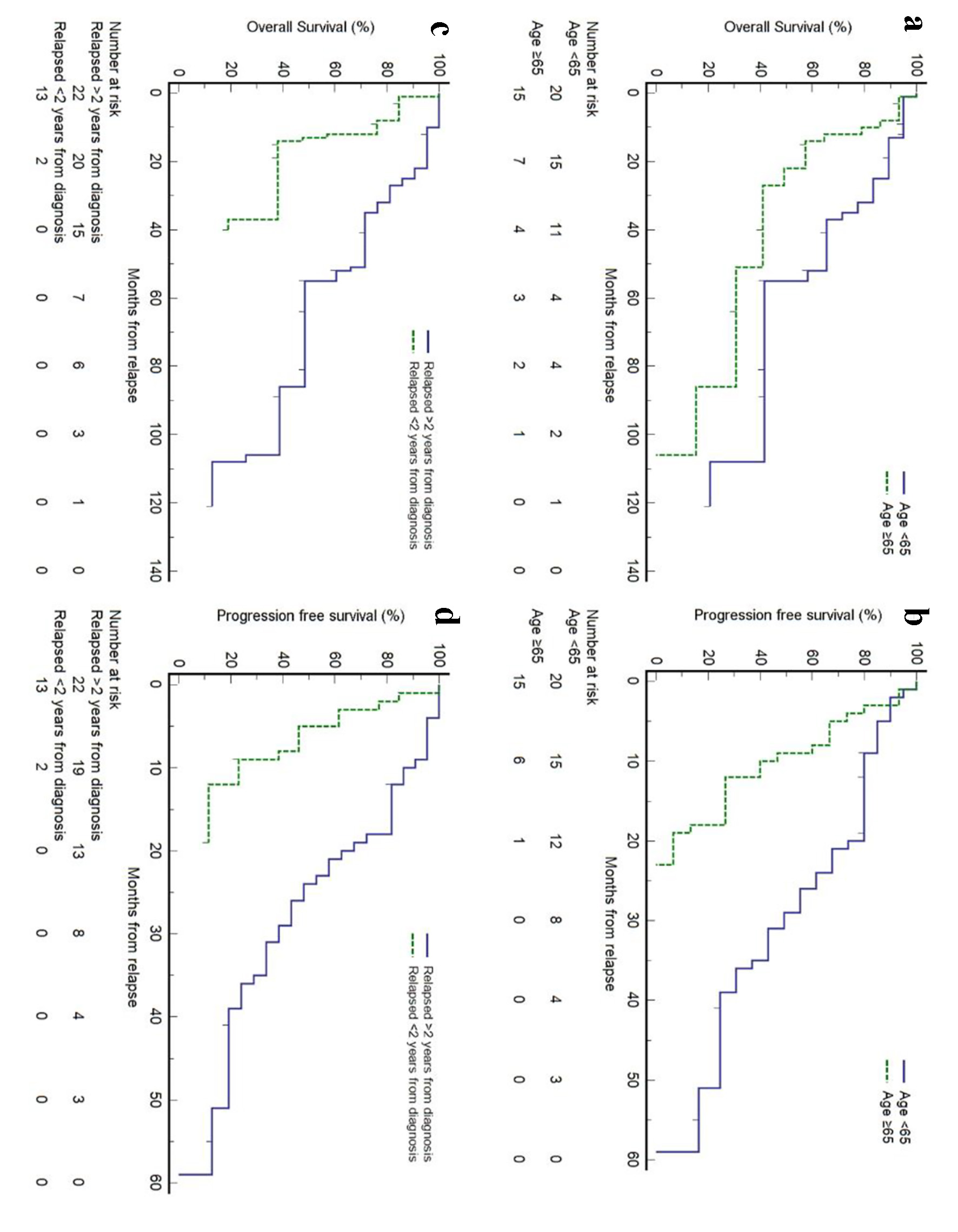 Click for large image | Figure 3. Prognostic factors for survival outcomes in patients with relapsed/refractory (R/R) mantle cell lymphoma (MCL) at first relapse. |
| Discussion | ▴Top |
MCL remains incurable with current modes of therapies and while survival of MCL patients is improving, most patients are expected to relapse over time [14]. With the constant development of new drugs to improve treatment outcomes, it is important to describe the course of the disease over time and after each relapse, so as to facilitate optimal care of the patients. Our study represents the first in Asia to study the treatment outcomes of patients with R/R MCL. The longitudinal patient treatment data collected over 20 years allowed for the observation of the use of novel therapy on treatment outcomes in addition to conventional chemoimmunotherapy. In this study, we observed a progressive shortening of both OS and PFS after each relapse. The median OS after first, second and third relapse and beyond were 52, 32 and 12 months, respectively. Median PFS also decreased from 19, 8 and 6 months, respectively. Kumar et al reported a similar observation close to our cohort with a decline in median OS and PFS after second, third and fourth line of treatment (41.4, 25.2 and 14.4 months, respectively; 14, 6.5 and 5 months, respectively) [15]. On top of acquired resistance to therapy, the older age at each relapse may further preclude patients from undergoing intensive treatment regimens with inherent risks of greater side effects [16]. In keeping with this, we also observed that older age (above 65 years) at first relapse was associated with poorer survival outcomes. Treating the elderly with relapsed MCL remains complex because of various comorbidities and frailties and the consequent increased risk of toxicity from therapies [17]. Novel therapies, including BTK inhibitors, are a reasonable therapeutic consideration in elderly patients with MCL [18].
In our study, we observed that patients with early relapse (within 2 years from diagnosis) had poorer survival outcomes. Both median OS and PFS were decreased by more than 50% in patients with early relapse. While there is no consensus definition to early relapse, previous studies have reported similar observations. In a study by Visco and colleagues, MCL patients treated with intensive frontline regimens who had progression within 2 years of diagnosis had increased mortality, while those who developed progression after 2 years had longer OS [19]. Taken together, this suggests that early relapse within 2 years from diagnosis may be a surrogate outcome measure for poor OS.
We also observed that central nervous system (CNS) relapse was very rare in our cohort. CNS relapse has significant fatal consequences with a poor median survival and continues to have very poor prognosis with current treatment options [20, 21]. Only one (2.9%) patient had CNS involvement at relapse and subsequently we did not observe CNS involvement in further relapses. CNS relapse is thought to be a rare event in MCL; reported rates are relatively low at 4-8% [20, 22]. In our cohort we observed an even lower rate. A high Ki-67 (≥ 30%) has been shown to be strongly associated CNS relapse along with factors such as blastoid histology, presence of B symptoms, increased serum lactate dehydrogenase (LDH), poor Eastern Cooperative Oncology Group (ECOG) performance status (≥ 2) and a high MIPI score [22-24]. Overall in our cohort, the majority of our patients had a good ECOG performance status, a low to intermediate MIPI score and < 50% had a high Ki-67. This could explain our observation of a very low rate of CNS involvement at relapse.
In the original cohort of 56 patients who received chemotherapy, six patients received autologous HSCT, of which five relapsed. In our cohort, there was a low proportion of patients who received HSCT as first-line treatment. Possible reasons for this include an older age at diagnosis and most of the younger patients receiving (R)-HyperCVAD. In a consensus statement by the Asian Lymphoma Study Group, HSCT was recommended in young fit patients with advanced disease, its use otherwise remains limited with lack of robust evidence [13]. The median survival of the five patients who relapsed after autologous HSCT was 52 months (95% CI 25 - 52) compared to 51 months (95% CI 22 - 86) in those who did not receive HSCT. The difference was not statistically significant. We also observed that amongst these five patients, only one was an early relapse (within 2 years of diagnosis). Relapse after HSCT has been noted to be associated with poor prognosis. In the study by Dietrich et al, which looked at outcomes of patients who relapsed after HSCT, the median OS of the whole study group was 19 months [25]. This differs substantially from the median OS of our five patients and could be due to various reasons. Aside from our very small numbers, one-third of Dietrich study patients had an early relapse (within 12 months). Early relapse after HSCT has been associated with extremely poor prognosis [26, 27] and on the contrary, a longer duration to relapse after HSCT may be associated with better prognosis.
The limitations of our study include a small sample size and thus caution is warranted in interpretation of our results. In particular, treatment strategies varied considerably and the majority of our patients did not receive maintenance rituximab as part of first-line treatment. This is likely due to patient recruitment over a long period where treatment strategies evolved and improved over the years. Given our small sample size, we were also unable to effectively study various prognostic factors and the treatment outcomes of novel therapy, in particular, the use of BTK inhibitors in our cohort of R/R MCL patients.
Conclusions
Our study represents the first to report treatment outcomes and survival patterns in an Asian population with R/R MCL. Patients more than 65 years old and patients who relapse early after first-line treatment represent a high-risk group and further studies characterizing the use of novel therapies in these patients are urgently required to improve their survival outcomes.
| Supplementary Material | ▴Top |
Suppl 1. Patient inclusion and treatment overview.
Suppl 2. Systemic Therapy at Each Relapse
Suppl 3. Overall survival of patients with (n = 35) or without (n = 19) documented relapse after first-line treatment. Median overall survival was 105 months for the relapsed group, while that was not reached for patients without documented relapse (hazard ratio 1.13, 95% confidence interval 0.38 - 3.35, P = 0.823). Survival of patients who did not receive first-line chemotherapy (n = 10) is also shown here for comparison (median, 9.4 months).
Suppl 4. Factors Associated With Overall Survival of Relapsed/Refractory Mantle Cell Lymphoma Patients
Suppl 5. Overall survival of relapsed/refractory mantle cell lymphoma patients according to year of diagnosis. Median overall survival was 136 months (2009 onwards; n = 17) compared to 98 months (prior to 2009; n = 18) (hazard ratio 0.63, 95% confidence interval 0.23 - 1.73, P = 0.373).
Acknowledgments
The authors would like to thank all patients for their participation in this study.
Financial Disclosure
This work was supported by the Tanoto Foundation Professorship in Medical Oncology, New Century Foundation Limited, Ling Foundation, Singapore Ministry of Health’s National Medical Research Council Research Training Fellowship (NMRC/Fellowship/0054/2017), SHF-Foundation Research Grant (SHF/FG653P/2017), as well as the SingHealth Duke-NUS Academic Medical Centre and Oncology ACP Nurturing Clinician Scientist Scheme (08-FY2017/P1/14-A28).
Conflict of Interest
The authors declare that they have no competing interests.
Informed Consent
Written informed consents were obtained from all subjects.
Author Contributions
JYT and TYQ analyzed the data and drafted the manuscript; JYT, TYQ, SYO, JBC, YHT, VSY, EWYC, EP, NS, MF, MT, STL, and JYC obtained patient data; JYT, TYQ and JYC designed the study, interpreted the results, and revised the manuscript; and all authors read and approved the final version of the manuscript.
Data Availability
The datasets created and analyzed during this study are available from the corresponding authors upon reasonable request.
| References | ▴Top |
- Maddocks K. Update on mantle cell lymphoma. Blood. 2018;132(16):1647-1656.
doi pubmed - Cohen JB, Zain JM, Kahl BS. Current approaches to mantle cell lymphoma: diagnosis, prognosis, and therapies. Am Soc Clin Oncol Educ Book. 2017;37:512-525.
doi pubmed - Goy A, Kahl B. Mantle cell lymphoma: the promise of new treatment options. Crit Rev Oncol Hematol. 2011;80(1):69-86.
doi pubmed - Dreyling M, Aurer I, Cortelazzo S, Hermine O, Hess G, Jerkeman M, Le Gouill S, et al. Treatment for patients with relapsed/refractory mantle cell lymphoma: European-based recommendations. Leuk Lymphoma. 2018;59(8):1814-1828.
doi pubmed - Parrott M, Rule S, Kelleher M, Wilson J. A systematic review of treatments of relapsed/refractory mantle cell lymphoma. Clin Lymphoma Myeloma Leuk. 2018;18(1):13-25 e16.
doi pubmed - Jain P, Wang M. Mantle cell lymphoma: 2019 update on the diagnosis, pathogenesis, prognostication, and management. Am J Hematol. 2019;94(6):710-725.
doi pubmed - Hanel W, Epperla N. Emerging therapies in mantle cell lymphoma. J Hematol Oncol. 2020;13(1):79.
doi pubmed - Desai M, Newberry K, Ou Z, Wang M, Zhang L. Lenalidomide in relapsed or refractory mantle cell lymphoma: overview and perspective. Ther Adv Hematol. 2014;5(3):91-101.
doi pubmed - Hambley B, Caimi PF, William BM. Bortezomib for the treatment of mantle cell lymphoma: an update. Ther Adv Hematol. 2016;7(4):196-208.
doi pubmed - Zinzani PL, Vose JM, Czuczman MS, Reeder CB, Haioun C, Polikoff J, Tilly H, et al. Long-term follow-up of lenalidomide in relapsed/refractory mantle cell lymphoma: subset analysis of the NHL-003 study. Ann Oncol. 2013;24(11):2892-2897.
doi pubmed - Wang ML, Rule S, Martin P, Goy A, Auer R, Kahl BS, Jurczak W, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2013;369(6):507-516.
doi pubmed - Owen C, Berinstein NL, Christofides A, Sehn LH. Review of Bruton tyrosine kinase inhibitors for the treatment of relapsed or refractory mantle cell lymphoma. Curr Oncol. 2019;26(2):e233-e240.
doi pubmed - Yoon DH, Cao J, Chen TY, Izutsu K, Kim SJ, Kwong YL, Lin TY, et al. Treatment of mantle cell lymphoma in Asia: a consensus paper from the Asian Lymphoma Study Group. J Hematol Oncol. 2020;13(1):21.
doi pubmed - Schieber M, Gordon LI, Karmali R. Current overview and treatment of mantle cell lymphoma. F1000Res. 2018;7(F1000 Faculty Rev):136.
doi pubmed - Kumar A, Sha F, Toure A, Dogan A, Ni A, Batlevi CL, Palomba MLM, et al. Patterns of survival in patients with recurrent mantle cell lymphoma in the modern era: progressive shortening in response duration and survival after each relapse. Blood Cancer J. 2019;9(6):50.
doi pubmed - Kluin-Nelemans HC, Hoster E, Hermine O, Walewski J, Trneny M, Geisler CH, Stilgenbauer S, et al. Treatment of older patients with mantle-cell lymphoma. N Engl J Med. 2012;367(6):520-531.
doi pubmed - Soubeyran P, Gressin R. Treatment of the elderly patient with mantle cell lymphoma. Hematology Am Soc Hematol Educ Program. 2016;2016(1):425-431.
doi pubmed - Romancik JT, Cohen JB. Management of older adults with mantle cell lymphoma. Drugs Aging. 2020;37(7):469-481.
doi pubmed - Visco C, Tisi MC, Evangelista A, Di Rocco A, Zoellner AK, Zilioli VR, Hohaus S, et al. Time to progression of mantle cell lymphoma after high-dose cytarabine-based regimens defines patients risk for death. Br J Haematol. 2019;185(5):940-944.
doi pubmed - Conconi A, Franceschetti S, Lobetti-Bodoni C, Stathis A, Margiotta-Casaluci G, Ramponi A, Mazzucchelli L, et al. Risk factors of central nervous system relapse in mantle cell lymphoma. Leuk Lymphoma. 2013;54(9):1908-1914.
doi pubmed - Campo E, Rule S. Mantle cell lymphoma: evolving management strategies. Blood. 2015;125(1):48-55.
doi pubmed - Cheah CY, George A, Gine E, Chiappella A, Kluin-Nelemans HC, Jurczak W, Krawczyk K, et al. Central nervous system involvement in mantle cell lymphoma: clinical features, prognostic factors and outcomes from the European Mantle Cell Lymphoma Network. Ann Oncol. 2013;24(8):2119-2123.
doi pubmed - Ferrer A, Bosch F, Villamor N, Rozman M, Graus F, Gutierrez G, Mercadal S, et al. Central nervous system involvement in mantle cell lymphoma. Ann Oncol. 2008;19(1):135-141.
doi pubmed - Chihara D, Asano N, Ohmachi K, Nishikori M, Okamoto M, Sawa M, Sakai R, et al. Ki-67 is a strong predictor of central nervous system relapse in patients with mantle cell lymphoma (MCL). Ann Oncol. 2015;26(5):966-973.
doi pubmed - Dietrich S, Boumendil A, Finel H, Avivi I, Volin L, Cornelissen J, Jarosinska RJ, et al. Outcome and prognostic factors in patients with mantle-cell lymphoma relapsing after autologous stem-cell transplantation: a retrospective study of the European Group for Blood and Marrow Transplantation (EBMT). Ann Oncol. 2014;25(5):1053-1058.
doi pubmed - Wu A, Graf SA, Burwick N, Grim JE, Dong ZM, Richard RE, Chauncey TR. Mantle cell lymphoma relapsed after autologous stem cell transplantation: a single-center experience. Blood Res. 2020;55(1):57-61.
doi pubmed - Cassaday RD, Guthrie KA, Budde EL, Thompson L, Till BG, Press OW, Chauncey TR, et al. Specific features identify patients with relapsed or refractory mantle cell lymphoma benefitting from autologous hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2013;19(9):1403-1406.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


