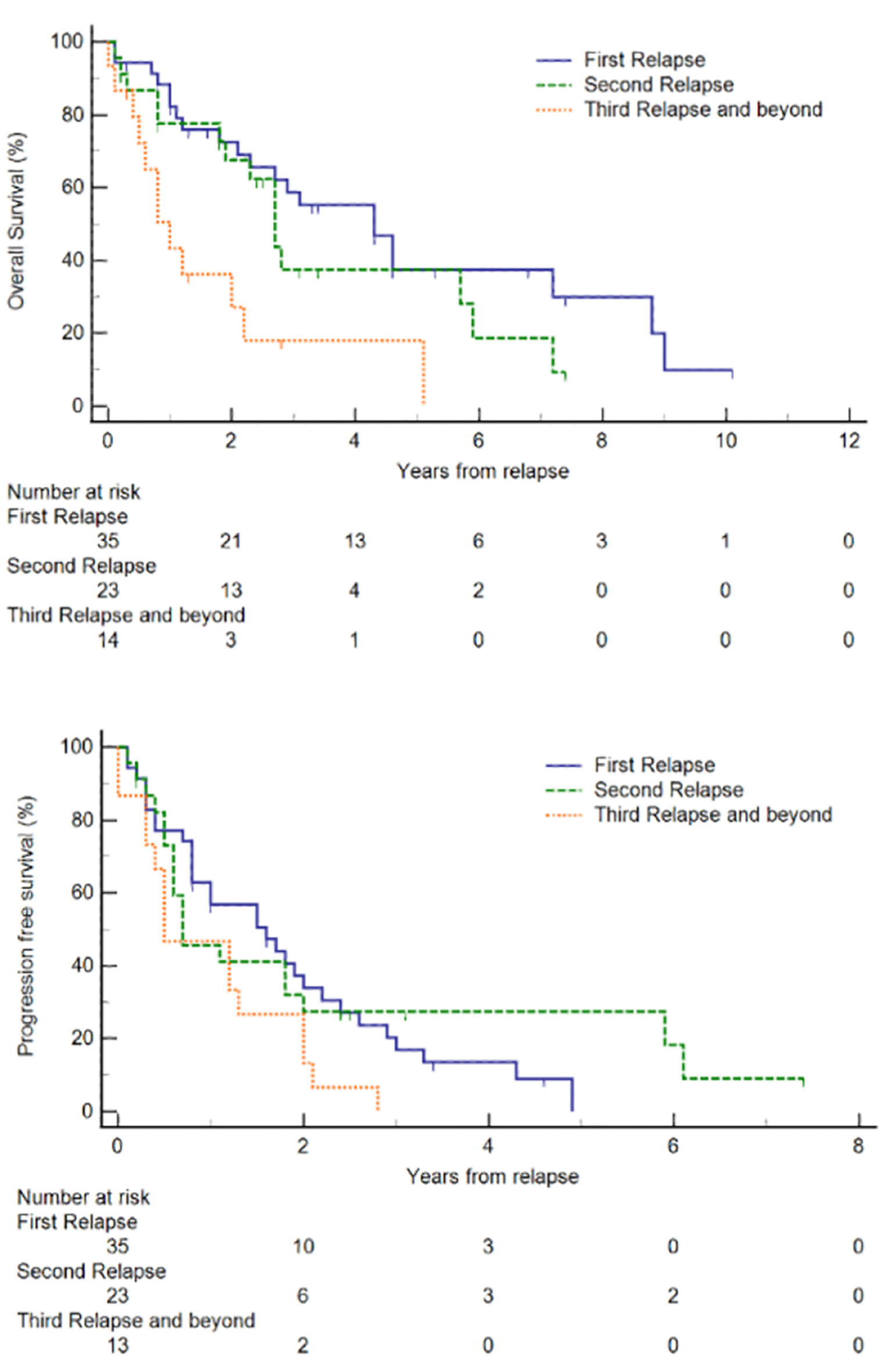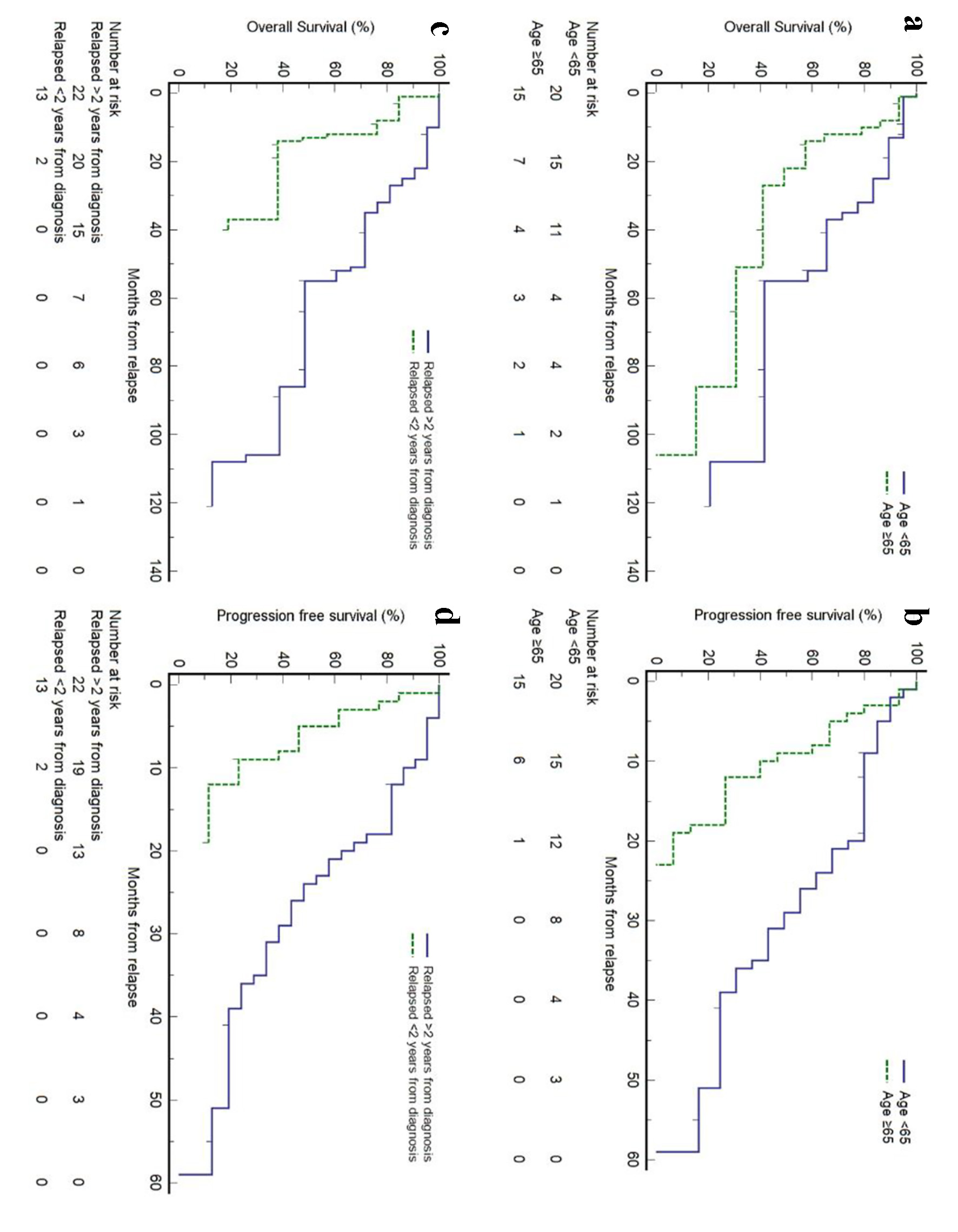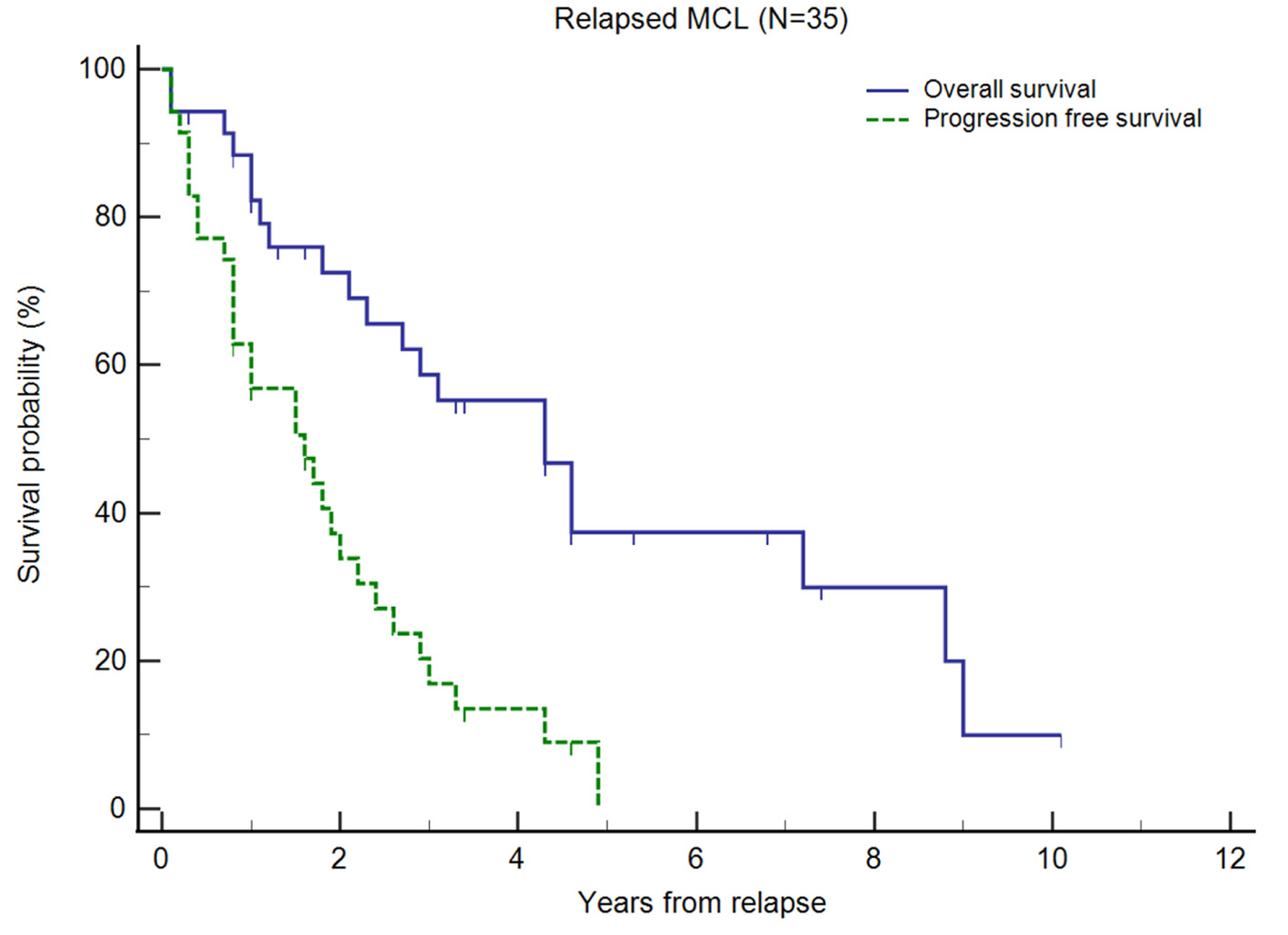
Figure 1. Kaplan-Meier plots of overall survival (OS) and progression-free survival (PFS) of relapsed/refractory (R/R) mantle cell lymphoma (MCL) from first relapse. Small vertical lines on the graph represent censored observations when patients are lost to follow-up.