| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Original Article
Volume 11, Number 2, April 2022, pages 45-54
Site-Specific Survival of Extra Nodal Diffuse Large B-Cell Lymphoma and Comparison With Gastrointestinal Diffuse Large B-Cell Lymphoma
Varsha Guptaa, e, Vinit Singhb, Ravneet Bajwaa, Trishala Meghalc, Shuvendu Send, David Greenberga, Madhurima Annea, Michael J. Levitta
aDepartment of Hematology and Oncology, Jersey Shore University Medical Center, Neptune, NJ, USA
bDepartment of Internal Medicine, Monmouth Medical Center, Long Branch, NJ, USA
cDepartment of Hematology and Oncology, Monmouth Medical Center, Long Branch, NJ, USA
dDepartment of Internal Medicine, Jersey Shore University Medical Center, Neptune, NJ, USA
eCorresponding Author: Varsha Gupta, Department of Hematology and Oncology, Jersey Shore University Medical Center, Neptune, NJ, USA
Manuscript submitted February 17, 2022, accepted April 11, 2022, published online April 22, 2022
Short title: Site-Specific Survival of EN-DLBCL
doi: https://doi.org/10.14740/jh984
| Abstract | ▴Top |
Background: Diffuse large B-cell lymphoma (DLBCL) constitutes 30% of all non-Hodgkin’s lymphomas. It can present as a nodal disease or as an extra nodal disease. Based on the site of origin, extra nodal DLBCL (EN-DLBCL) may have a distinct clinical outcome. Apart from the site of origin, factors including demographics, stage, and presence of any other primary malignancy also affect the outcome. The purpose of our study was to characterize prognostically distinct groups based on the site of presentation of EN-DLBCL.
Methods: We used 18 registries in Surveillance, Epidemiology, and End Results database to identify the patients with EN-DLBCL for 2000 - 2015 with last follow-up till December 31, 2018. A total of 30,290 EN-DLBCL patients were selected and categorized based on 13 broad sites grouping. Demographic variables were summarized. We did overall survival analysis with univariate and multivariate Cox-proportional hazard modeling. Short-term survival trend was calculated as well.
Results: The percentage of EN-DLBCL of all DLBCLs is 34.48%. EN-DLBCL was comparatively seen more in males (54.94%) and non-Hispanic whites (71.52%). In terms of clinical characteristics, patients with EN-DLBCL were mostly diagnosed at age ≥ 60 years (66.11%), early stage (69.33%), and presentation as first primary cancer (81.89%). A higher risk of mortality was seen in non-Hispanic black (hazard ratio (HR) 1.36), with late age of onset (HR 2.69), late stage at presentation (HR 1.42), and with history of other malignancy (HR 1.29). Compared to the intestinal tract, the risk of overall mortality was higher in individuals with involvement of nervous system (HR 1.85), pancreas and hepatobiliary system (HR 1.22), and respiratory system (HR 1.18) and the best outcomes were seen in heart and mediastinal site (HR 0.58) of DLBCL.
Conclusion: Based upon our population-based study, we conclude that primary site of presentation of EN-DLBCL is an important prognostic factor with significant difference in survival based on histological and epidemiological characteristics.
Keywords: Diffuse large B-cell lymphoma; Non-Hodgkin’s lymphoma; Extra nodal diffuse large B-cell lymphoma; Epidemiology; SEER
| Introduction | ▴Top |
Diffuse large B-cell lymphoma (DLBCL) is one of the most common lymphoid neoplasms and represents 25-35% of all non-Hodgkin’s lymphomas (NHLs) [1, 2]. Most of the DLBCLs originate in lymph nodes, also known as the nodal DLBCL, but about 30-40% may present initially in extra nodal sites which is also known as extra nodal diffuse large B-cell lymphoma (EN-DLBCL) [3]. The most common extra nodal site of origin is the gastrointestinal tract specifically in stomach and ileocecal region [4]. In practicality any other organ can be involved with DLBCL, e.g., head/neck, nervous system, genitourinary (especially testis), breast, heart/mediastinum, bone marrow, spleen, musculoskeletal, skin/soft tissue, pancreaticobiliary, respiratory tract, endocrine glands, among others. Known prognostic factors of nodal DLBCL include age, race, stage, performance status, immunodeficiency, B-symptoms, central nervous system (CNS) recurrence, bone marrow involvement, molecular subtypes (i.e., germinal center B cell (GCB) and activated B cell (ABC) subtype), and genetics (relevant oncogene translocation, e.g., MYC gene, TP53 loss) [5]. It is expected that EN-DLBCL also has similar prognostic factors. Extra nodal involvement of DLBCL is considered a poor prognostic factor as per revised international prognostic index (IPI) [6, 7].
Despite common morphological characteristics, these tumors behave very differently than nodal DLBCL and need to be managed as a distinct entity. With ongoing research and newer modalities of diagnosis, pathological description, risk stratification and management, there has been a significant improvement in the outcomes of nodal DLBCL. Although similar outcome trends are seen in cases of EN-DLBCL as well, descriptive studies and universal guidelines are still lacking. The purpose of our study was to use the large population-based data to identify prognostically important determinant of survival and qualify the risk of mortality based on the site of presentation of EN-DLBCL compared to gastrointestinal tract, the most common site of EN-DLBCL.
| Materials and Methods | ▴Top |
This is an IRB exempted retrospective study executed by analysis of data from the cancer patients in the Surveillance, Epidemiology, and End Results (SEER) database. Ethical review and approval were waived for this study, due to the de-identified information of the patients included in the public SEER database. The population in SEER 18 represents 27.8% of US population based on 2010 US census and includes cancer cases from 18 cancer registries across the United States which includes Alaska Native Tumor Registry, Connecticut, Detroit, Georgia Center for Cancer Statistics (Atlanta, Greater Georgia, and Rural Georgia), Greater Bay Area Cancer Registry (San Francisco-Oakland and San Jose-Monterey), Greater California, Hawaii, Iowa, Kentucky, Los Angeles, Louisiana, New Mexico, New Jersey, Seattle-Puget Sound, and Utah. This database contains data from 2000 to 2018 of all the cancer patients in the registries. The SEER database is a standard for population study in the United States with a case ascertainment rate of 98%.
Study population
Individual patient level data were extracted from the SEER 18 database by using SEER*Stat software (version 8.0.5; Surveillance Research Program of the National Cancer Institute). The database was quarried for years 2000 - 2015 for the patients who were diagnosed with DLBCL and were selected based on the diagnostic codes from WHO-ICD-O codes, which included subtypes DLBCL, not otherwise specified (NOS)), intravascular large B-cell lymphoma, primary effusion lymphoma, and mediastinal large B-cell lymphoma. Information regarding the patients diagnosed with EN-DLBCL till December 31, 2015 was collected. Patients with age ≥ 18 were included in the study. The data were retrieved for all patients diagnosed with DLBCL, irrespective of the site. EN-DLBCL was categorized based on the site recode ICD-O-3/WHO 2008 variable in the SEER database. Details of the selection algorithm are given in Supplementary Material 1 (www.thejh.org). There were 267 sites included based on the site code mentioned in the SEER database. These 267 sites were categorized into 10 broad categories based on the organ involved. Sites included in each broad group are summarized in the Supplementary Material 2 (www.thejh.org). Patients with unknown staging data and survival status were excluded. Patients who were diagnosed after the autopsy were also excluded from the study.
Study variables
The SEER registries record data on patient characteristics including gender, age at diagnosis, ethnicity, stage on diagnosis, primary tumor site, vital status, cause of death, the month, and year of last follow-ups. Associations between the demographic, clinical, and pathologic characteristics of patients and survival were assessed. In our analysis of association between age and survival, we analyzed age as a categorical variable, with the following age intervals: ages ≤ 60 years and > 60 years. Age greater than 60 was taken as cutoff as considered as an adverse prognostic factor as per the IPI. Pathological information included stage at diagnosis and type of NHL. We also categorized stages into two broad grouping early stages which included stage 1 and stage 2 diseases, whereas late disease consisted of stage 3 and stage 4 diseases.
Statistical analysis
Descriptive statistics were used to describe patient baseline characteristics. Categorical variables were compared by using Chi-square test. Overall survival (OS) was calculated as time from diagnosis to death from any cause. Survival curves were plotted by using Kaplan-Meier curves and survival analyses were performed by using a log-rank test. Life tables were constructed to analyze the 1-year and 3-year OS as percentage with 95% confidence interval (95% CI). Patients were censored at the date of last follow-up in SEER, death or on December 31, 2018, whichever came first.
To identify the determinant of outcome, all the covariates were analyzed in a univariate model and those with P-value < 0.20 were fitted into the multivariate model to analyze the effect of each covariate independent of the others. Multivariate Cox proportional hazard model was used for the survival analyses. The strength of association between each predictor and survival was expressed as a hazard ratio (HR) along with a 95% CI. All tests with two-sided P-value of 0.05 were considered statistically significant.
| Results | ▴Top |
In the SEER database, for study duration from 2000 to 2015, 93,638 patients with age ≥ 18 were diagnosed with DLBCL, out of that 61,354 were nodal DLBCL and 32,284 were EN-DLBCL. Proportion of EN-DLBCL was 34.48% (95% CI 34.17 - 34.78). Of the patients, 1,956 were not included in the final analysis because of unavailability of stage and 38 were not included because of unavailability of survival months. A total of 30,290 patients were included in survival analysis (Fig. 1).
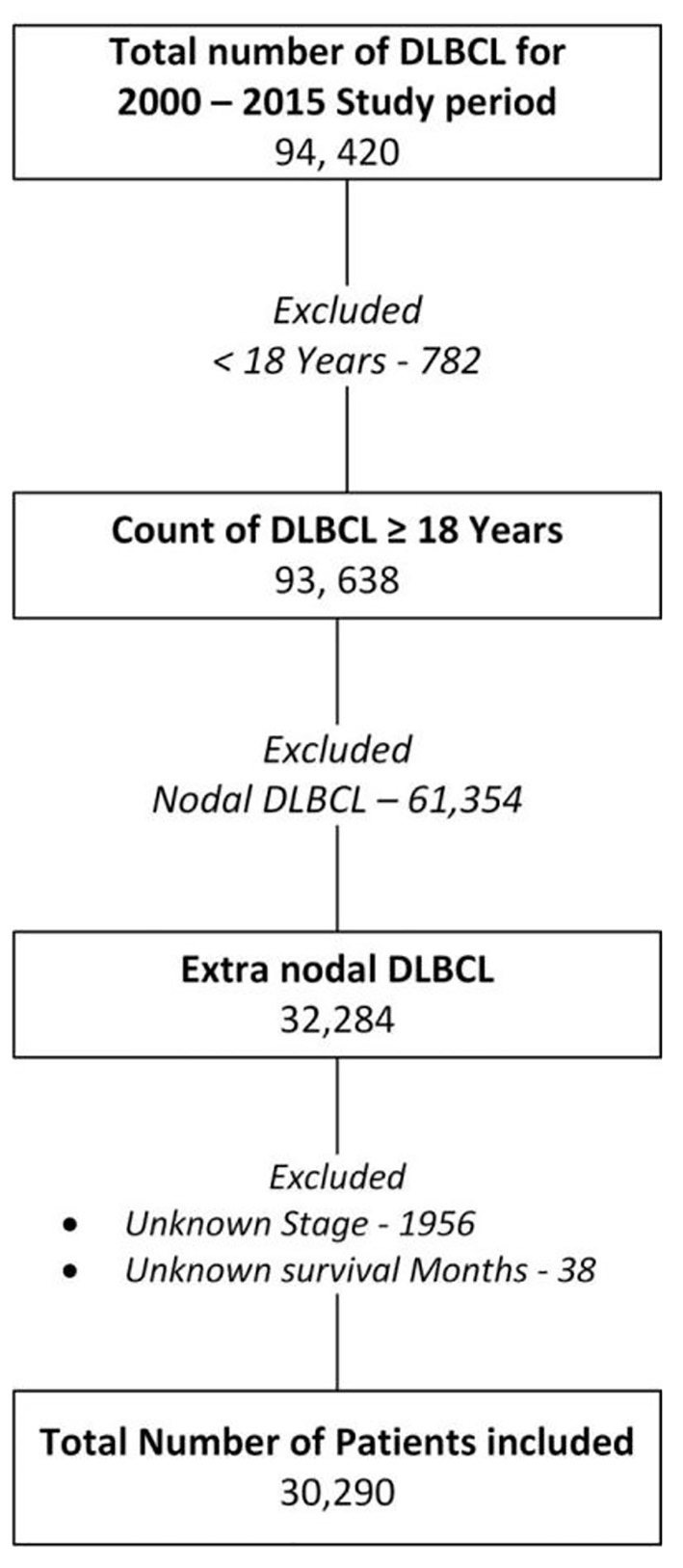 Click for large image | Figure 1. Flowchart showing the patient selection and exclusion for the study. Patients diagnosed between 2000 and 2015 and listed in SEER 18 registries database were included in the study. Date cutoff for last follow-up was December 31, 2018. DLBCL: diffuse large B-cell lymphoma. |
Patient characteristics
We have summarized the patient demography in Table 1. The median age of the study population was 69 years (95% CI 35 - 88). Majority of the patients aged ≥ 60 years (66.11%) at the time of diagnosis, except patients with heart and mediastinal DLBCL who were diagnosed at an earlier age with proportion of DLBCL diagnosed at or after 60 years were only 20.87%. Majority of the patients were male (54.94%). However, in subgroup analysis, males were noted to have comparatively lesser involvement of the endocrine organs (36.46%) and expectedly in the breast tissue (3.71%). EN-DLBCL was more common in non-Hispanic white (NHW) population representing 71.52%, followed by Hispanic (13%), African American (8.6%) and Asian (8%). Among NHW, incidence was disproportionately more in endocrine organs (82.04%), skin and soft tissues (79.52%), respiratory tract (77.27%) and musculoskeletal (76.66%) sites. Majority of cancer was diagnosed early in the course i.e., stage 1 and stage 2 diseases, as seen in 69.33% patients. Late-stage diagnosis was seen in case of involvement of bone marrow (94.53%). Most of the patients did not have any prior history of cancer diagnosis at the time of diagnosis of EN-DLBCL (81.89%). As expected, gastrointestinal tract (28.31%) was the most common site, followed by nervous system (13.78%), and head and neck site (11.79%). Description of site-specific demography and comparison with gastrointestinal tract is given in the Supplementary Material 3 (www.thejh.org).
 Click to view | Table 1. Demographic and Clinical characteristic of the Study Population |
Survival analysis
On univariate analysis, all the variables included in the study were significant and hence, included for multivariate analysis (Tables 2 and 3). Kaplan-Meier curves were also plotted for all the variables. On multivariate analysis, late-onset cancer (age ≥ 60 years) had poor prognosis with HR of 2.69 (95% CI 2.59 - 2.79) when compared to patients diagnosed before the age of 60 years (Fig. 2a). Males were found to have small but significantly worse OS when compared to females (Fig. 2b). In case of ethnicity, African American patients had worse outcome with HR of 1.27 (95% CI 1.19 - 1.34) when compared to the NHW patients. Hispanic and Asian patients did not have significant difference when compared to NHW patients (Fig. 2c). Patients with advanced-stage disease had worse outcome with HR of 1.42 (95% CI 1.37 - 1.46) compared to the early-stage diseases (Fig. 2d). Similarly, patients with previous history of cancer had worse outcome with HR of 1.29 (95% CI 1.24 - 1.33) when compared to patient with EN-DLBCL as first malignancy (Fig. 2e). For site-specific survival study, gastrointestinal tract was taken as the reference, as it is the most common site of involvement of EN-DLBCL. On comparison to gastrointestinal tract, nervous system with HR of 2.15 (95% CI 2.04 - 2.26), pancreas and hepatobiliary sites with HR of 1.29 (95% CI 1.17 - 1.42), and respiratory tract with HR of 1.26 (95% CI 1.14 - 1.38) had significantly worse outcome. Site-specific overall median survival was reported for all, except heart and mediastinal sites, as median survival was not reached. Of all the sites, heart and mediastinum site had best survival outcome, whereas the worst median survival was seen for nervous system 13 months (95% CI 11 - 14). Other site-specific survival details are listed in Table 3. Statistical significance was not met in case of EN-DLBCL involving bone marrow with HR of 0.87 (95% CI 0.56 - 1.37) and “others” site with HR of 1.11 (95% 0.84 - 1.29). Heart and mediastinal sites had the best outcome with HR of 0.59 (95% CI 0.50 - 0.69). In addition, sites involving musculoskeletal, endocrine organs, head and neck, breast, skin and soft tissues and genitourinary tract were seen to have significantly better outcome when compared to the gastrointestinal tract. Kaplan-Meier curves are plotted for site-specific OS in Figure 3.
 Click to view | Table 2. Univariate and Multivariate analysis for Overall Survival with Cox-Proportional Hazard Model |
 Click to view | Table 3. Site-Specific Univariate and Multivariate analysis for Overall Survival With Cox-Proportional Hazard Model |
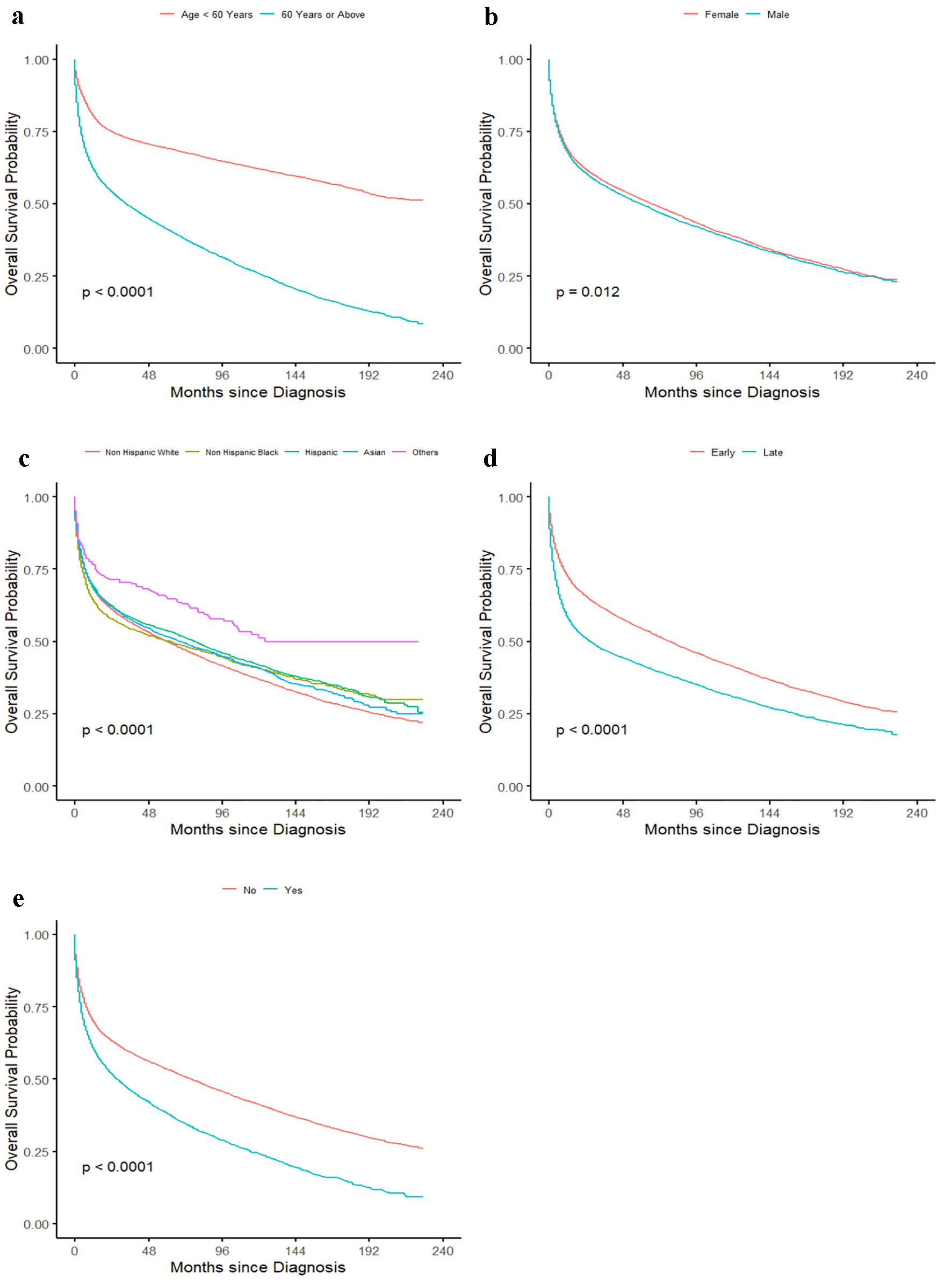 Click for large image | Figure 2. Kaplan-Meier curve showing effect of (a) age of onset categorized as age < 60 years and age ≥ 60 years, (b) gender, (c) ethnicity categorized as non-Hispanic white, non-Hispanic black, Hispanic, Asian, and others, (d) stage categorized as early stage which includes stage 1 and stage 2 diseases, and late stage which includes stage 3 and stage 4 diseases, and (e) past history of cancer. |
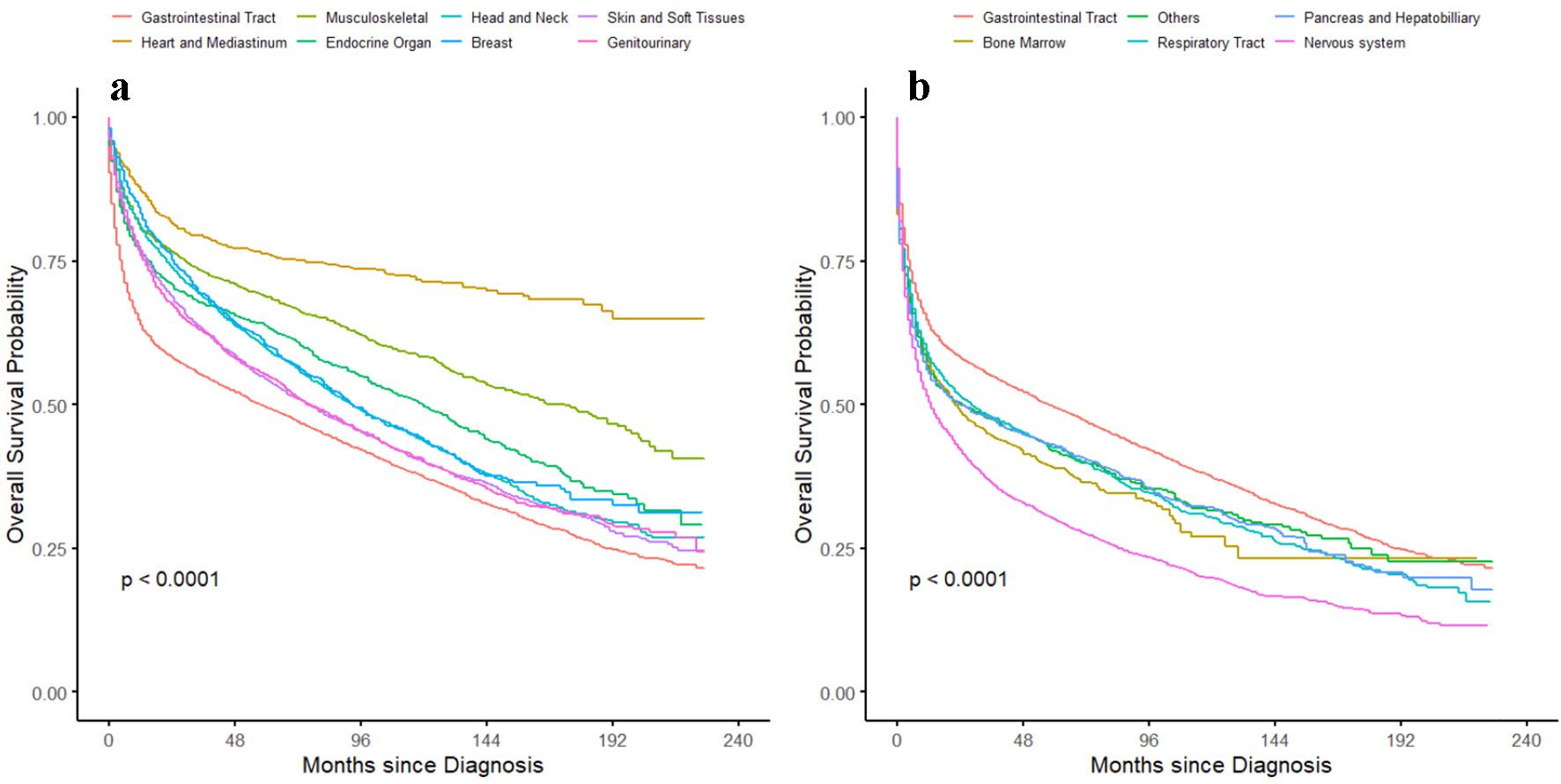 Click for large image | Figure 3. Kaplan-Meier curve showing overall survival probability based on the site of the EN-DLBCL. (a) Overall survival probability for the site with better survival than the gastrointestinal tract. (b) Overall survival probability for the site with worse survival than the gastrointestinal tract. EN-DLBCL: extra nodal diffuse large B-cell lymphoma. |
Changing trend in survival
We calculated the 1-year and 3-year probability of OS for EN-DLBCL. The 1-year OS probability steadily increased from 66.70% (95% CI 66.70-69.10%) in the year 2000 to 70.60% (95% CI 68.70-72.60%) in the year 2015. Similar but better trends were seen in the 3-year OS probability which increased from 53.71% (95% CI 51.28-56.26%) in the year 2000 to 60.60% (95% CI 58.60-62.70%) in the year 2015.
| Discussion | ▴Top |
To the best of our knowledge, this is the largest SEER database based observational study focusing on epidemiology, prognosis, and outcomes of patients with EN-DLBCL. We confirmed that the prognosis of EN-DLBCL is determined and influenced by the site of involvement, age of presentation, and stage of presentation, among various other factors. In accordance with previous reports, EN-DLBCL accounted for 34.35% of all DLBCLs [8, 9]. The most common sites as expected were gastrointestinal tract, head and neck, and nervous system [4, 8]. Most of the patients presented with early-stage EN-DLBCL, which could be attributed to the fact that the patients may present earlier with localizing symptoms [8]. Patients with bone marrow involvement of DLBCL were classified as late stage because of the current Ann Arbor staging of DLBCL, which reflects the need of a separate staging system for EN-DLBCL. Most of the patients in our study presented at an older age. In agreement with the previous studies, gender distribution showed male predominance. Highest incidence was seen in the white population, which is again like prior reported studies [10].
Our study showed that the African American population had worse outcome when compared to NHW patients. In congruence to our study, comparable results have been reported in prior studies. A retrospective cohort analysis done in southern United States showed that a greater percentage of African American population had earlier age of diagnosis of DLBCL when compared to white population [11]. They also had worse lactate dehydrogenase (LDH), B-symptoms and Eastern Cooperative Oncology Group (ECOG) status, when compared to white population. The difference in outcome can be attributed to the biological factors related to the tumor, healthcare services utilization, and the socioeconomic differences among different ethnic groups [12-14].
Although only limited geographical variation is observed in the distribution and incidence of NHL within the United States, across the globe, there has been significant difference seen in incidence, etiology, and site of NHL [15]. Over the last decade, incidence of DLBCL remains high but is decreasing in the western world whereas it is increasing in East Asia and Middle East [16]. In terms of etiology, EN-DLBCL associated with infectious etiology like hepatitis C virus (HCV), Epstein-Barr virus (EBV) and human immunodeficiency virus (HIV) is comparatively more in East Asian and South American population [17-19]. Although the SEER database lacks this information, it should always be considered while discussing the epidemiological and clinical perspective.
Our knowledge and classification of these lymphoid neoplasms will continue to evolve as the understanding of these diseases progresses further. The current classification system used is the World Health Organization (WHO) Classification of Tumors of Hematopoietic and Lymphoid Tissues, which was updated in 2017. It incorporates new clinical, pathological, genetic, and molecular information that occurred since the previous 2008 publication [20]. WHO has identified certain extra nodal sites as a separate entity for, e.g., primary DLBCL of the, primary cutaneous diffuse large B-cell lymphoma-leg type, primary mediastinal (thymic) large B-cell lymphoma, primary effusion lymphoma and intravascular large B-cell lymphoma. Prior entity known as “B-cell lymphoma, unclassifiable, with features intermediate between DLBCL and Burkitt lymphoma” in 2008 has been replaced by “high-grade B-cell lymphoma, NOS and “high-grade B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements.”
Numerous literatures can be found regarding EN-DLBCL and their notable difference in terms of prognosis and preferred treatment approaches. Overall, the prognosis of both nodal DLBCL and EN-DLBCL has improved in the rituxan era, post 2006. However, in a recent study by Bobillo et al, stage I EN-DLBCLs were seen to have poorer prognosis than those of stage I nodal DLBCLs. They concluded that consolidative radiation therapy to patients with end of immunochemotherapy positive positron emission tomography (PET) scan, improved OS in patients with extra nodal involvement [21, 22]. This treatment approach is quite different from nodal DLBCL, where many times radiation therapy is not needed. There were indeed many limitations in this retrospective study, including their inclusion criteria, non-uniformity of chemotherapy used, and small number of patients enrolled in the study especially in the nodal DLBCL group [22].
We found that nervous system, respiratory system, pancreas, and hepatobiliary involvement were associated with poorer prognosis, when compared to the gastrointestinal tract. Similarly, involvement of the heart, mediastinum, and musculoskeletal system had better prognosis, when compared to the gastrointestinal tract. Castillo et al conducted a similar study with smaller patient population [23]. They also concluded that specific sites of DLBCL were associated with either a better or a worse prognosis. In their study, worse outcome was seen in gastrointestinal tract, respiratory system, liver, and pancreas. These findings are similar to the results of our study. There are many factors that may impact the prognosis of EN-DLBCL. Certain factors are unique and characteristic to that site of involvement of DLBCL, as described below [9, 24].
Previous literature has reported that CNS lymphomas and testicular lymphomas generally have high Ki-67 expression [8]. However, correlation of Ki-67 expression and prognosis has remained controversial. Ki-67 expression has been used as a proliferation marker and so is an indicator of the nature of the disease. He et al conducted a meta-analysis to study the relation of Ki-67 expression to the outcome for patients with various subtypes of lymphoma. They investigated its impact especially after introduction of rituximab. Although the study supported the prognostic significance of Ki-67 expression in DLBCL, the study was associated with many limitations [25].
Yin et al, in their study, described site-specific risk patterns of developing secondary malignant neoplasm in patients with EN-DLBCL [26]. Highest risk was seen among patients with lung and liver/pancreas DLBCL. This finding explains, to a certain extent, a poorer prognosis of EN-DLBCL involving pancreas/hepatobiliary and respiratory system.
One of the other factors leading to poorer prognosis is CNS relapse. Testicular, breast and uterine DLBCLs are known to have a high prevalence of non-GCB phenotype and the MYD88/CD79B mutated genotype, resembling primary CNS lymphoma [27]. This could be a reason of its increased propensity of CNS recurrence. Bone marrow involvement is also known to cause CNS relapse and may explain poorer outcome [28]. DLBCLs involving the kidneys, adrenal glands, and ovaries are other sites associated with an increased risk of spread to the CNS [21, 29, 30]. Ollila et al recommended individualized consideration of CNS prophylaxis based on the CNS-IPI score for these patients [27].
The increased risk could be attributed to the treatment-related factors, including the use of radiation and surgery. Previously surgery was recommended for certain sites of involvement like the intestinal, gastric, breast, bone, or thyroid area. However, recently there are studies published describing the detrimental effects of surgery in these patients, especially considering development of surgical complications, unnecessary delay in chemotherapy and worse healing [31]. Consolidative radiation therapy has been recommended for EN-DLBCL of testicle, breast, mediastinum, and bone, per European Society for Medical Oncology (ESMO) guidelines [3]. Considering the above discrepancies in management approach and no available standard universal guidelines, poorer prognosis seen with EN-DLBCL can be explained.
Some extra nodal sites have a better prognosis than the others because of unique clinical symptoms at presentation and therefore the possibility of diagnosis at an earlier stage. Primary bone DLBCL (PB-DLBCL) presents with characteristic and localizing symptoms of pain, bone fractures, localized swelling or periprosthetic joint infection. This may result in earlier diagnosis and subsequent earlier treatment [32, 33]. Similarly, DLBCL involving the breast presents many times as a palpable lump and may be detected earlier on screening mammograms [34]. Primary mediastinal B-cell lymphoma (PMBCL) which now constitutes a separate WHO entity, usually affects the adolescents and young adults with unique symptoms of chest pain, superior vena cava syndrome and shortness of breath. PMBCL has shown excellent outcomes after the use of dose-adjusted EPOCH-R chemotherapy and the fact that younger individuals tolerate the chemotherapy regimens better [35].
Some studies have indicated that certain immunophenotypes are associated with worse outcomes, e.g., studies have been published with findings that indicate adverse prognostic impact of immunoblastic morphology [36]. Immunoblastic variant is also associated with MYC translocations. De Groen et al studied a large cohort of patients with PB-DLBCL and provided a comprehensive evaluation of immunohistochemistry (IHC), gene-expression profiling (GEP) and targeted deep sequencing. PB-DLBCL is characterized by a centrocyte-like GCB phenotype with a specific GEP pattern. GCB-associated mutation profile is associated generally with a favorable survival [32]. Further studies are needed to describe these factors for other extra nodal sites and thus may help to explain the differences in their outcomes.
The diversity in clinical presentation, morphology, genetic alterations, and response to treatment proves that EN-DLBCL is a heterogenous group of NHLs and must be individually studied [2]. Based on the unique behavior, although 2017 WHO classification identified DLBCL of CNS, cutaneous and mediastinal region as a separate entity, there is a need of further classification and research [20]. Considering the heterogeneity of these lymphomas, clinical practice guidelines have been published by ESMO for management of certain extra nodal sites of DLBCL [3]. They described the diagnostic workup and treatment strategies of primary testicular lymphoma, primary CNS lymphoma, primary mediastinal lymphoma, primary breast lymphoma and primary bone lymphoma. We believe that a comprehensive and unique set of guidelines is needed for other sites of EN-DLBCL as well.
There are certain limitations to our study. SEER database provides limited data regarding the type of treatment these patients received. Various treatment modalities of DLBCL including chemoimmunotherapy, CNS prophylaxis, surgery and radiation therapy can result in different outcomes for these patients, which is unknown to us. Pathology including mutations of these patients was also not accounted, which is known to have a significant impact on the OS. We could not evaluate the relationship between genetic predisposition and prognosis of the patients. Ki-67 expression was not known. A well-established prognostic tool, IPI score was not evaluated for these patients. Apart from the database-related limitations, the definition of EN-DLBCL has always been controversial [37]. The cases involving both nodal and extra nodal sites are difficult to categorize. The WHO classification of DLBCL has also been ever evolving, based on advancements in research. Although primary mediastinal (thymic) B-cell lymphoma continues to be categorized as a subtype of DLBCL in many literatures [35, 38], it is certainly controversial after understanding its unique molecular alterations and immunophenotypic presentation. We have included PMBCL as a subtype of DLBCL in our study based upon the morphology of the disease and so the findings should be interpreted accordingly. There is also vast discrepancy regarding the true definition of primary bone marrow lymphoma, which needs to be addressed. Only cases with primarily bone marrow involvement without any other nodal or extra nodal involvement should be included, as per prior studies [39-41]. However, the criteria are not clearly defined when it comes to stage IV disease.
In conclusion, our study confirms that there is a significant difference in the natural history of EN-DLBCL, particularly regarding outcomes. EN-DLBCL thus should be considered as distinct entities, based on each specific site rather than a homogenous group. There is a strong need for site-specific staging system, prognostic criteria, and therapeutic approach in the management of EN-DLBCL. The varied histopathological profiles of EN-DLBCL also need to be further explored.
| Supplementary Material | ▴Top |
Suppl 1. Details of the Selection Algorithm.
Suppl 2. Sites Included in Each Broad Group.
Suppl 3. Site-Specific Comparison of Demographic and Clinical Characteristics.
Acknowledgments
None to declare.
Financial Disclosure
There was no specific financial disclosure or funding source for this study.
Conflict of Interest
The authors declare that they have no conflict of interest.
Informed Consent
De-identified patient data were collected from SEER database under data user agreement with NCI. Ethical review and approval were waived for this study, due to the de-identified information of the patients included in the public SEER database.
Author Contributions
VG and VS designed and performed the study. VS, VG, and RB assisted and supported in data collection, editing and subsequent analysis with statistics. VG, VS, RB, and SS drafted the manuscript and did critical editing. TM, MA, DG, and MJL carefully supervised this manuscript preparation and writing.
Data Availability
All data were extracted from and available on the SEER Program (https://seer.cancer.gov/, accessed on November 11, 2021).
| References | ▴Top |
- Sehn LH, Salles G. Diffuse large B-cell lymphoma. N Engl J Med. 2021;384(9):842-858.
doi pubmed - Moller MB, Pedersen NT, Christensen BE. Diffuse large B-cell lymphoma: clinical implications of extranodal versus nodal presentation—a population-based study of 1575 cases. Br J Haematol. 2004;124(2):151-159.
doi pubmed - Vitolo U, Seymour JF, Martelli M, Illerhaus G, Illidge T, Zucca E, Campo E, et al. Extranodal diffuse large B-cell lymphoma (DLBCL) and primary mediastinal B-cell lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27(suppl 5):v91-v102.
doi pubmed - Psyrri A, Papageorgiou S, Economopoulos T. Primary extranodal lymphomas of stomach: clinical presentation, diagnostic pitfalls and management. Ann Oncol. 2008;19(12):1992-1999.
doi pubmed - Zhou Z, Sehn LH, Rademaker AW, Gordon LI, Lacasce AS, Crosby-Thompson A, Vanderplas A, et al. An enhanced International Prognostic Index (NCCN-IPI) for patients with diffuse large B-cell lymphoma treated in the rituximab era. Blood. 2014;123(6):837-842.
doi pubmed - El-Galaly TC, Villa D, Alzahrani M, Hansen JW, Sehn LH, Wilson D, de Nully Brown P, et al. Outcome prediction by extranodal involvement, IPI, R-IPI, and NCCN-IPI in the PET/CT and rituximab era: A Danish-Canadian study of 443 patients with diffuse-large B-cell lymphoma. Am J Hematol. 2015;90(11):1041-1046.
doi pubmed - Hui D, Proctor B, Donaldson J, Shenkier T, Hoskins P, Klasa R, Savage K, et al. Prognostic implications of extranodal involvement in patients with diffuse large B-cell lymphoma treated with rituximab and cyclophosphamide, doxorubicin, vincristine, and prednisone. Leuk Lymphoma. 2010;51(9):1658-1667.
doi pubmed - Shi Y, Han Y, Yang J, Liu P, He X, Zhang C, Zhou S, et al. Clinical features and outcomes of diffuse large B-cell lymphoma based on nodal or extranodal primary sites of origin: Analysis of 1,085 WHO classified cases in a single institution in China. Chin J Cancer Res. 2019;31(1):152-161.
doi pubmed - Lopez-Guillermo A, Colomo L, Jimenez M, Bosch F, Villamor N, Arenillas L, Muntanola A, et al. Diffuse large B-cell lymphoma: clinical and biological characterization and outcome according to the nodal or extranodal primary origin. J Clin Oncol. 2005;23(12):2797-2804.
doi pubmed - Wu XC, Andrews P, Chen VW, Groves FD. Incidence of extranodal non-Hodgkin lymphomas among whites, blacks, and Asians/Pacific Islanders in the United States: anatomic site and histology differences. Cancer Epidemiol. 2009;33(5):337-346.
doi pubmed - Flowers CR, Shenoy PJ, Borate U, Bumpers K, Douglas-Holland T, King N, Brawley OW, et al. Examining racial differences in diffuse large B-cell lymphoma presentation and survival. Leuk Lymphoma. 2013;54(2):268-276.
doi pubmed - Flowers CR, et al. Racial disparities in cell of origin among DLBCL patients. Blood. 2012;120:2686-2686.
doi - Flowers CR, Fedewa SA, Chen AY, Nastoupil LJ, Lipscomb J, Brawley OW, Ward EM. Disparities in the early adoption of chemoimmunotherapy for diffuse large B-cell lymphoma in the United States. Cancer Epidemiol Biomarkers Prev. 2012;21(9):1520-1530.
doi pubmed - Phillips AA, Smith DA. Health disparities and the global landscape of lymphoma care today. Am Soc Clin Oncol Educ Book. 2017;37:526-534.
doi pubmed - Al-Hamadani M, Habermann TM, Cerhan JR, Macon WR, Maurer MJ, Go RS. Non-Hodgkin lymphoma subtype distribution, geodemographic patterns, and survival in the US: A longitudinal analysis of the National Cancer Data Base from 1998 to 2011. Am J Hematol. 2015;90(9):790-795.
doi pubmed - Cai W, Zeng Q, Zhang X, Ruan W. Trends analysis of non-Hodgkin lymphoma at the national, regional, and global level, 1990-2019: results from the global burden of disease study 2019. Frontiers in Medicine. 2021;8:1609.
doi pubmed - Huh J. Epidemiologic overview of malignant lymphoma. Korean J Hematol. 2012;47(2):92-104.
doi pubmed - Hofscheier A, Ponciano A, Bonzheim I, Adam P, Lome-Maldonado C, Vela T, Cortes E, et al. Geographic variation in the prevalence of Epstein-Barr virus-positive diffuse large B-cell lymphoma of the elderly: a comparative analysis of a Mexican and a German population. Mod Pathol. 2011;24(8):1046-1054.
doi pubmed - Lu CH, Lee KF, Chen CC, Chen YY, Huang CE, Tsai PS, Tsou HY, et al. Clinical characteristics and treatment outcome in a Taiwanese population of patients with Epstein-Barr virus-positive diffuse large B-cell lymphoma. Jpn J Clin Oncol. 2014;44(12):1164-1171.
doi pubmed - WHO classification of tumours of haematopoietic and lymphoid tissues. 2017.
- Bobillo S, Joffe E, Lavery JA, Sermer D, Ghione P, Noy A, Caron PC, et al. Clinical characteristics and outcomes of extranodal stage I diffuse large B-cell lymphoma in the rituximab era. Blood. 2021;137(1):39-48.
doi pubmed - Lossos IS. Stage I DLBCL: extranodal may mean extra radiation. Blood. 2021;137(1):3-5.
doi pubmed - Castillo JJ, Winer ES, Olszewski AJ. Sites of extranodal involvement are prognostic in patients with diffuse large B-cell lymphoma in the rituximab era: an analysis of the Surveillance, Epidemiology and End Results database. Am J Hematol. 2014;89(3):310-314.
doi pubmed - Yacoub A, Aljitawi OS, Cui W. Primary bone marrow DLBCL, unraveling the unique biology of this very rare presentation. Blood. 2013;122:5065-5065.
doi - He X, Chen Z, Fu T, Jin X, Yu T, Liang Y, Zhao X, et al. Ki-67 is a valuable prognostic predictor of lymphoma but its utility varies in lymphoma subtypes: evidence from a systematic meta-analysis. BMC Cancer. 2014;14:153.
doi pubmed - Yin X, Xu A, Huang Z, Fan F, Wang Y, Chen L, Cui G, et al. The relationship among primary anatomic subsite and risk and distribution of second malignant neoplasms in patients with stage I/II diffuse large B-cell lymphoma: An analysis of the surveillance, epidemiology, and end results database. Transl Oncol. 2021;14(7):101106.
doi pubmed - Ollila TA, Olszewski AJ. Extranodal diffuse large B cell lymphoma: molecular features, prognosis, and risk of central nervous system recurrence. Curr Treat Options Oncol. 2018;19(8):38.
doi pubmed - Tsao C, et al. Extranodal diffuse large B cell lymphoma in the rituximab era and the risk of central nervous system (CNS) relapse. A single center experience from 2008-2012. Blood. 2013;122:4330-4330.
doi - Takahashi H, Tomita N, Yokoyama M, Tsunoda S, Yano T, Murayama K, Hashimoto C, et al. Prognostic impact of extranodal involvement in diffuse large B-cell lymphoma in the rituximab era. Cancer. 2012;118(17):4166-4172.
doi pubmed - Boussios S, Zerdes I, Vassou A, Bareta E, Seraj E, Papoudou-Bai A, Pavlidis N, et al. Extranodal diffuse large B-cell lymphomas: A retrospective case series and review of the literature. Hematol Rep. 2018;10(1):7070.
doi pubmed - Shen H, Wei Z, Zhou D, Zhang Y, Han X, Wang W, Zhang L, et al. Primary extra-nodal diffuse large B-cell lymphoma: A prognostic analysis of 141 patients. Oncol Lett. 2018;16(2):1602-1614.
doi pubmed - de Groen RAL, van Eijk R, Bohringer S, van Wezel T, Raghoo R, Ruano D, Jansen PM, et al. Frequent mutated B2M, EZH2, IRF8, and TNFRSF14 in primary bone diffuse large B-cell lymphoma reflect a GCB phenotype. Blood Adv. 2021;5(19):3760-3775.
doi pubmed - Jain A, Alam K, Maheshwari V, Khan R, Nobin H, Narula V. Primary bone lymphomas-Clinical cases and review of literature. J Bone Oncol. 2013;2(3):132-136.
doi pubmed - Hosein PJ, Maragulia JC, Salzberg MP, Press OW, Habermann TM, Vose JM, Bast M, et al. A multicentre study of primary breast diffuse large B-cell lymphoma in the rituximab era. Br J Haematol. 2014;165(3):358-363.
doi pubmed - Dunleavy K, Wilson WH. Primary mediastinal B-cell lymphoma and mediastinal gray zone lymphoma: do they require a unique therapeutic approach? Blood. 2015;125(1):33-39.
doi pubmed - Heyning FH, Hogendoorn PC, Kramer MH, Hermans J, Kluin-Nelemans JC, Noordijk EM, Kluin PM. Primary non-Hodgkin's lymphoma of bone: a clinicopathological investigation of 60 cases. Leukemia. 1999;13(12):2094-2098.
doi pubmed - Krol AD, le Cessie S, Snijder S, Kluin-Nelemans JC, Kluin PM, Noordijk EM. Primary extranodal non-Hodgkin's lymphoma (NHL): the impact of alternative definitions tested in the Comprehensive Cancer Centre West population-based NHL registry. Ann Oncol. 2003;14(1):131-139.
doi pubmed - Zhou H, Xu-Monette ZY, Xiao L, Strati P, Hagemeister FB, He Y, Chen H, et al. Prognostic factors, therapeutic approaches, and distinct immunobiologic features in patients with primary mediastinal large B-cell lymphoma on long-term follow-up. Blood Cancer J. 2020;10(5):49.
doi pubmed - Wang HY, et al. Veterans general hospital. Cancer Medicine. 2018;7:3713-3721.
doi pubmed - Chang H, Hung YS, Lin TL, Wang PN, Kuo MC, Tang TC, Wu JH, et al. Primary bone marrow diffuse large B cell lymphoma: a case series and review. Ann Hematol. 2011;90(7):791-796.
doi pubmed - Martinez A, Ponzoni M, Agostinelli C, Hebeda KM, Matutes E, Peccatori J, Campidelli C, et al. Primary bone marrow lymphoma: an uncommon extranodal presentation of aggressive non-hodgkin lymphomas. Am J Surg Pathol. 2012;36(2):296-304.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


