| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Case Report
Volume 11, Number 3, June 2022, pages 109-112
Non-Hepatosplenic Extramedullary Hematopoiesis Masquerading as Metastatic Malignant Melanoma
Aquino Williamsa, c, Rajiv M. Mallipudib, John A. Contia
aDepartment of Internal Medicine, Hackensack Meridian Health Mountainside Medical Center, Montclair, NJ 07042, USA
bDivision of Hospital Medicine, Yale New Haven Health Bridgeport Hospital, Bridgeport, CT 06610, USA
cCorresponding Author: Aquino Williams, Department of Internal Medicine, Hackensack Meridian Health Mountainside Medical Center, Montclair, NJ 07042, USA
Manuscript submitted February 15, 2022, accepted May 6, 2022, published online June 16, 2022
Short title: NHS-EMH as Metastatic Malignant Melanoma
doi: https://doi.org/10.14740/jh981
| Abstract | ▴Top |
Non-hepatosplenic extramedullary hematopoiesis (NHS-EMH), the formation of blood cellular elements outside the medulla of the bone marrow and outside the liver and spleen, has been noted among patients with myeloproliferative neoplasms and other serious hematological diseases. However, NHS-EMH is rarely identified among individuals without concurrent hematological disease. Since the radiologic features of NHS-EMH are nonspecific, lesions may be mistaken for metastatic disease when observed in patients with known solid tumors. We report an unusual case of a patient with a simultaneous presentation of malignant melanoma and multiple NHS-EMH lesions. The biopsy revealed trilineage hematopoiesis resembling normal bone marrow tissue, in the absence of abnormalities of peripheral blood counts or presence of driver mutations associated with myeloproliferative neoplasms. The biopsy results were critical in downstaging the patient and thus permitted avoidance of unnecessary malignant melanoma therapy. This case emphasizes the importance of surgical biopsy of suspect lesions when treatment strategies will be impacted.
Keywords: Non-hepatosplenic extramedullary hematopoiesis; Extramedullary hematopoiesis; Malignant melanoma; Myeloproliferative neoplasm
| Introduction | ▴Top |
Extramedullary hematopoiesis (EMH) is the formation of blood cellular elements outside of medulla of the bone marrow [1]. Although essential during fetal development, EMH is considered pathological after birth [2]. Mirroring embryology, the most common sites of EMH post-birth are the liver and spleen. However, rare cases of EMH outside of these organs, termed non-hepatosplenic extramedullary hematopoiesis (NHS-EMH), have been described within breast, gastrointestinal, lung, and other tissues [2, 3]. NHS-EMH has been also associated with hematological conditions including sickle cell anemia, myelofibrosis with myeloid metaplasia, polycythemia vera, and myelodysplastic syndrome [2]. To date there is limited literature on the association between malignant melanoma and NHS-EMH. A systematic review revealed that out of 42 cases of NHS-EMH, only one (2.4%) was associated with malignant melanoma [4]. EMH has also been described in a patient with choroidal melanoma [5].
Given the rarity of NHS-EMH without co-existing hematological diseases and only sparse information regarding its imaging characteristics, EMH may be misdiagnosed as metastatic disease in patients with solid tumors. We present a clinical case showcasing how serial imaging and subsequent biopsy were critical in diagnosing EMH in a patient with malignant melanoma, thereby facilitating accurate malignancy staging and avoidance of unnecessary treatment.
| Case Report | ▴Top |
Investigations
A male in his sixth decade presented for evaluation of a new mid-back skin lesion. Twelve years earlier he had been diagnosed with early-stage malignant melanoma involving the right upper bicep and was treated with excisional resection, but he did not require adjuvant therapy. A diagnostic biopsy of the new lesion confirmed superficial spreading malignant melanoma (13 mm diameter spread; depth 1.2 mm; ulceration present). Definitive resection with a wide local excision and sentinel lymph node dissection were negative for additional malignancy. Staging evaluation with positron emission tomography-computed tomography (PET-CT) scan demonstrated an infiltrative focus within the posterior segment of the right upper lobe with pathologic hypermetabolic increased fluorodeoxyglucose (FDG) uptake. There were also large paraspinal lesions at T10 and T11 (maximum FDG standardized uptake value (SUV) 2.7) and a presacral mass in the lower right pelvis (SUV 3.0) (Fig.1).
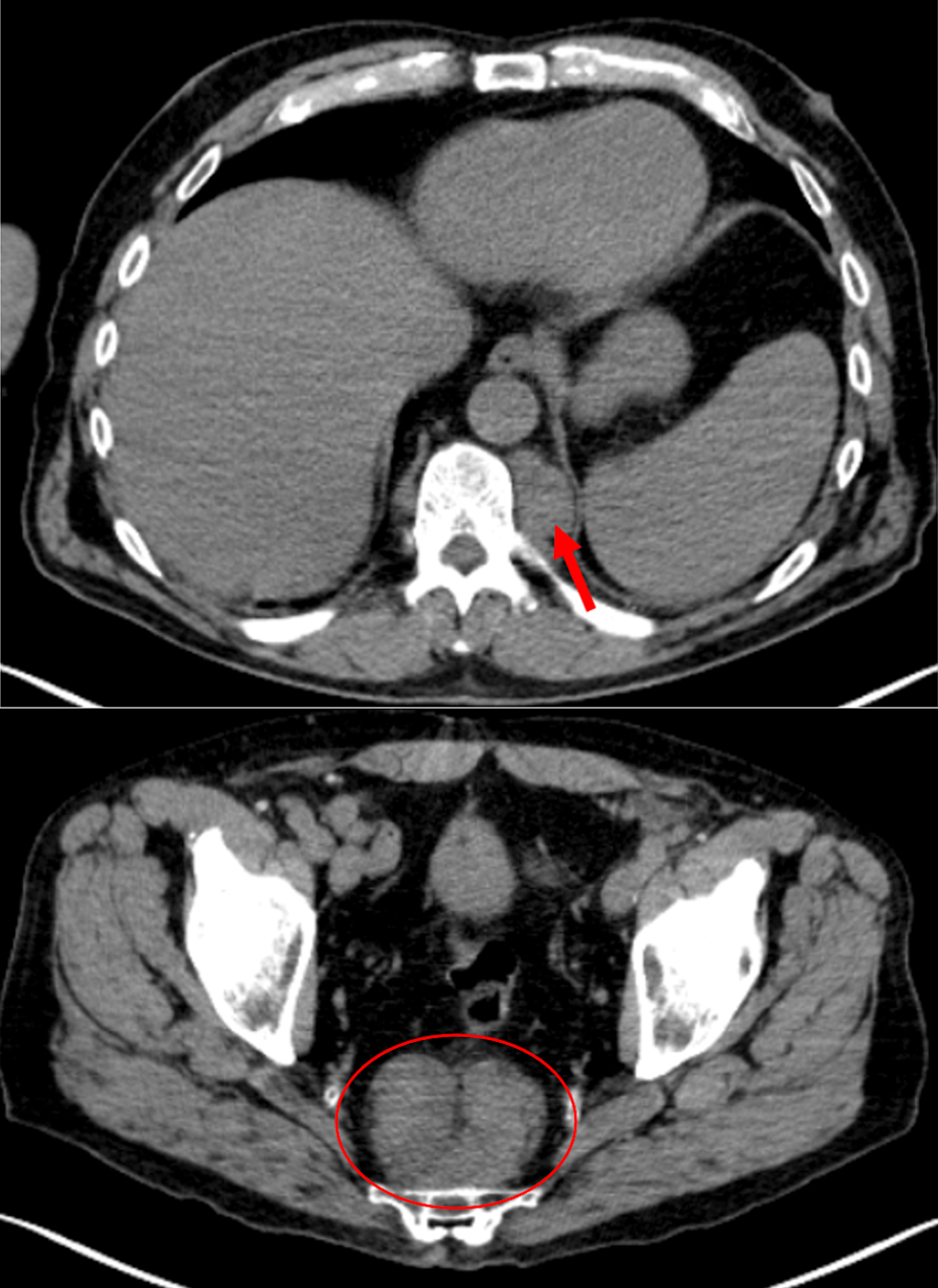 Click for large image | Figure 1. PET-CT images revealing a paraspinal mass at the level of T11 measuring 3.7 × 2.4 cm with SUV 2.7 (arrow) and the biopsy site presacral nodule measuring 7.5 × 5 cm with SUV 3.0 (circle). PET-CT: positron emission tomography-computed tomography; SUV: standardized uptake value. |
Diagnosis
Due to concerns of possible metastatic disease, a needle biopsy of the presacral mass was performed and identified trilineage hematopoietic cells supporting a diagnosis of NHS-EMH (Fig. 2a, b). The specimen was cellular with a normal myeloid to erythroid ratio. The predominant myeloid population stained for myeloperoxidase and demonstrated full maturation. Erythropoiesis was confirmed by the presence of glycophorin-A. Megakaryocytes were identified by CD61 and did not show overt dysplasia. Malignant melanoma was excluded by morphology and absence of S-100 and melan-A staining. Peripheral blood counts were normal and therefore a biopsy of the bone marrow was not performed. Genomic testing for mutations commonly associated with myeloproliferative neoplasms (JAK2, MPL, and BCR-ABL1) was negative.
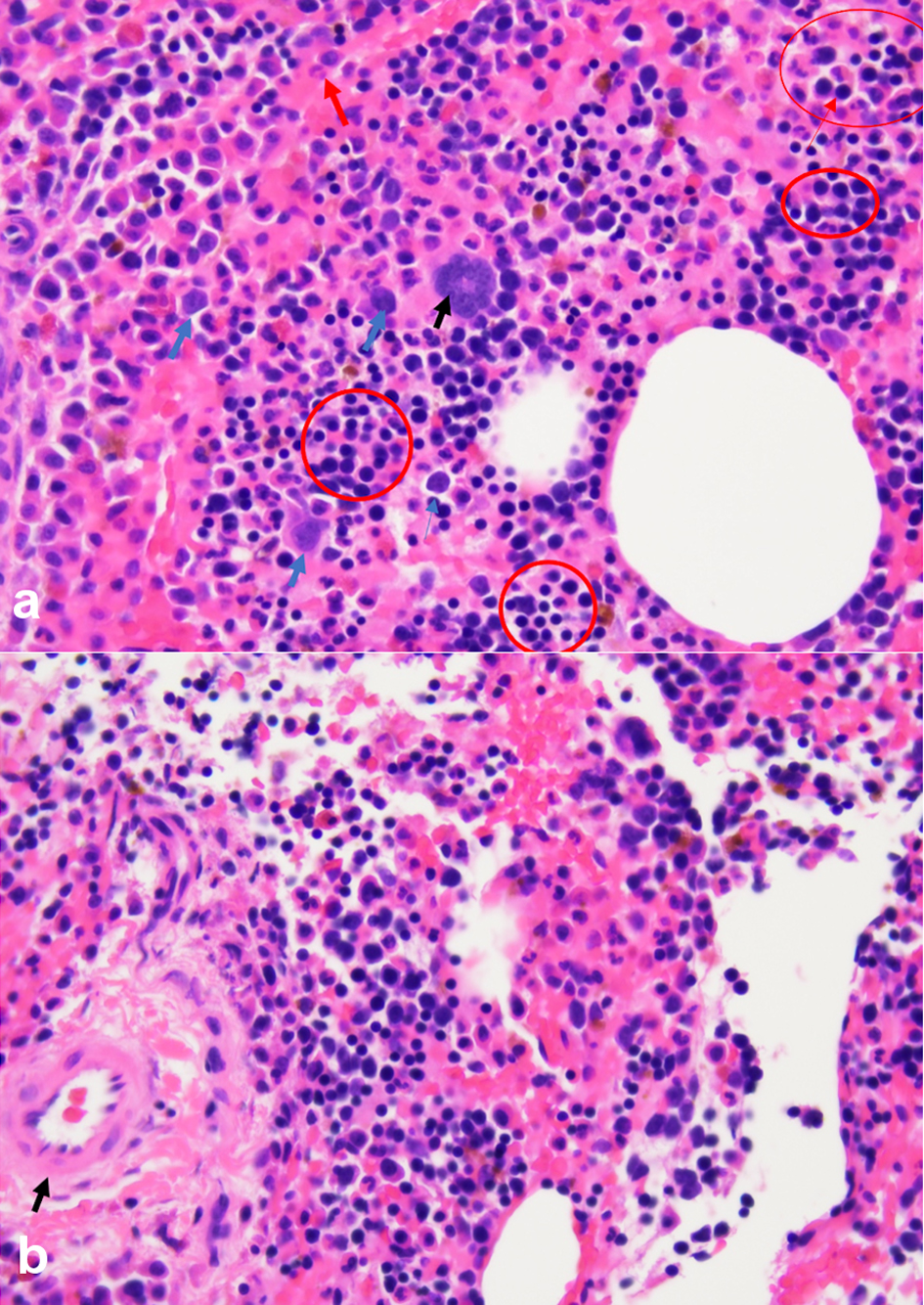 Click for large image | Figure 2. (a) Biopsy of presacral mass demonstrates NHS-EMH with trilineage hematopoietic cells including megakaryocytes (black arrows), myelocytes (blue arrows) and erythrocytes (red circles). (b) Blood vessel identified with surrounding areas trilineage hematopoiesis within the presacral mass (arrow). NHS-EMH: non-hepatosplenic extramedullary hematopoiesis. |
Treatment
As his melanoma was stage IIA (T2b, N0, M0) adjuvant therapy was not indicated.
Follow-up and outcomes
Approximately 6 months later repeat PET-CT scans revealed a slight increase in FDG uptake in the pre-sacral nodules but no other significant changes. Myeloproliferative neoplasm was deemed unlikely given the normal blood counts, absence of genomic driver mutations, and stability over long-term follow-up. The patient is currently in good health without recurrence and without significant changes in peripheral blood counts more than 2 years later.
| Discussion | ▴Top |
This case illustrates an extremely rare dual presentation of malignant melanoma and NHS-EMH and emphasizes the importance of obtaining a biopsy of suspected distant metastatic sites when treatment strategies would be impacted. Given the rarity of EMH occurring outside the liver or spleen, and without abnormalities of blood counts or known hematologic disease, our patient’s diagnosis could have easily been mistaken for metastatic malignancy and thus might have resulted in unnecessary treatment.
Though NHS-EMH radiographic features may be nonspecific, several characteristics have been highlighted in literature [3, 4]. Most NHS-EMH nodules are singular, although up to three distinct nodules have been reported [3]. Computed tomography (CT) imaging is considered superior to ultrasonography in identifying lesions. On CT, NHS-EMH lesions typically appears as well-circumscribed, inhomogeneous, hypovascular masses, often interspersed with areas of fat attenuation and without calcification or bone destruction. Heterogeneous enhancement on contrast-enhanced CT is common [3, 4, 6-9]. On magnetic resonance imagining, NHS-EMH demonstrates high-signal intensity on both T1- and T2- weighted images, similar to metastatic disease [4, 6-9]. Variability of the lipid content may lead to inconsistency in contrast enhancement whenever identifying NHS-EMH. Thus, in the absence of distinctive radiologic criteria, suspected NHS-EMH lesions require biopsy and pathological confirmation [4].
In patients with a known primary malignancy who have distant radiographic lesions, a biopsy is mandatory to exclude non-malignant causes when treatment strategies would be affected. Fine needle aspiration using CT imaging guidance permits definitive cytological diagnosis in the majority of EMH cases [10, 11]. Since EMH masses are highly vascular and have a propensity to hemorrhage, selection of an appropriate biopsy location which reduces bleeding risk is important [12]. In our patient we avoided the paraspinal lesions and noted the expected neovascularization within the presacral biopsy site (Fig. 2b). Biopsy features of NHS-EMH demonstrate trilineage hematopoiesis, resembling normal marrow hematopoiesis, but occasionally may reveal only granulopoiesis or megakaryopoiesis [2]. Irregular aggregates of immature granulocytes, erythroblast, and speckled megakaryocytes with a backdrop of small lymphocytes have been reported [2]. Nodal lesions may occur in the paracortical region of the lymph node [2]. Interestingly, simultaneous bone marrow evaluations are frequently normal in NHS-EMH [4].
The mainstay of management of NHS-EMH lesions is a conservative approach, although treatment may be required if the patient becomes symptomatic due to direct compression. In cases of secondary EMH, where a known hematologic disease exists, treatment of the underlying primary disorder may be effective. Treatment options have included surgical excision and radiotherapy for localized lesions, and hydroxyurea or chemotherapy for diffuse lesions [2-4]. One interesting observation has been the development of non-splenic EMH following splenectomy in patients with myelofibrosis with myeloid metaplasia, possibly demonstrating the ability of the malignant clone to seek new sites for proliferation [4].
A recent mouse model has suggested a possible mechanism for an association between NHS-EMH and malignant melanoma. During melanoma tumor growth, mice experienced splenic EMH and an expansion of myeloid lineages, but a reduction in erythroid and megakaryocyte lineages. The increased expansion of myeloid lineages occurred directly at the level of stem and progenitor cells and was principally driven by interleukin (IL)-3. The authors hypothesized that melanoma growth resulted in a reprogramming of the host immune system by impacting hematopoiesis directly at the level of the stem cell compartment [13].
Learning points
This novel case report of a patient with malignant melanoma who had concurrent NHS-EMH emphasizes the importance of obtaining a specimen of suspected distant metastatic disease prior to initiating oncologic treatment. The radiographic features in this patient were highly suggestive of malignancy, but the biopsy provided an unexpected alternative diagnosis sparing him from unnecessary treatment. Our patient has one of the few reported cases of NHS-EMH associated with malignant melanoma and with nearly 2 years of follow-up, both his hematologic picture is unchanged and malignant melanoma has not recurred. It is notable that recent mouse models suggest hematopoietic dysregulation in malignant melanoma and lead us to suspect that additional cases of NHS-EMH in this malignancy may be unrecognized.
Acknowledgments
The authors wish to thank Stuart Goldberg MD for critical reviews of the manuscript; Dr. Rakesh Abbi for reviewing histopathology and Dr. Prashant Parashurama for review of radiographic studies.
Financial Disclosure
There are no financial disclosures or funding sources related to this manuscript.
Conflict of Interest
The authors have no conflict of interest to disclose.
Informed Consent
Consent was obtained from the patient.
Author Contributions
Dr. Williams was the primary author. Both Dr. Conti and Dr. Mallipudi helped with the conception, editorial content, and guidance and direction of the paper. During inceptions of the paper, it was heavily edited by Dr. Mallipudi. The final draft was further edited also with guidance on how to relay the objectives of the paper by Dr. Conti, who is a hematologist oncologist and was able to provide expert insight. All authors approved the final version of the manuscript.
Data Availability
Additional details of the case (deidentified) from the corresponding author are available upon reasonable request.
| References | ▴Top |
- Sorsdahl OS, Taylor PE, Noyes WD. Extramedullary hematopoiesis, mediastinal masses, and spinal cord compression. JAMA. 1964;189:343-347.
doi pubmed - Koch CA, Li CY, Mesa RA, Tefferi A. Nonhepatosplenic extramedullary hematopoiesis: associated diseases, pathology, clinical course, and treatment. Mayo Clin Proc. 2003;78(10):1223-1233.
doi pubmed - Zhou PP, Clark E, Kapadia MR. A systematic review of presacral extramedullary haematopoiesis: a diagnosis to be considered for presacral masses. Colorectal Dis. 2016;18(11):1033-1040.
doi pubmed - Bao Y, Liu Z, Guo M, Li B, Sun X, Wang L. Extramedullary hematopoiesis secondary to malignant solid tumors: a case report and literature review. Cancer Manag Res. 2018;10:1461-1470.
doi pubmed - Gallo B, Al-Jamal RT, Thaung C, Cohen VML. Iris extramedullary hematopoiesis in choroidal melanoma. Saudi J Ophthalmol. 2020;34(2):82-84.
doi pubmed - Sohawon D, Lau KK, Lau T, Bowden DK. Extra-medullary haematopoiesis: a pictorial review of its typical and atypical locations. J Med Imaging Radiat Oncol. 2012;56(5):538-544.
doi pubmed - Yang X, Bhuiya T, Esposito M. Sclerosing extramedullary hematopoietic tumor. Ann Diagn Pathol. 2002;6(3):183-187.
doi pubmed - Badr NM, Roberts C, Shaaban AM. Extramedullary haematopoiesis in axillary lymph nodes of breast carcinoma patients receiving neoadjuvant chemotherapy: a potential diagnostic pitfall. Pathobiology. 2019;86(2-3):167-172.
doi pubmed - Shawker TH, Hill M, Hill S, Garra B. Ultrasound appearance of extramedullary hematopoiesis. J Ultrasound Med. 1987;6(6):283-290.
doi pubmed - Policarpio-Nicolas ML, Bregman SG, Ihsan M, Atkins KA. Mass-forming extramedullary hematopoiesis diagnosed by fine-needle aspiration cytology. Diagn Cytopathol. 2006;34(12):807-811.
doi pubmed - Al-Marzooq YM, Al-Bahrani AT, Chopra R, Al-Momatten MI. Fine-needle aspiration biopsy diagnosis of intrathoracic extramedullary hematopoiesis presenting as a posterior mediastinal tumor in a patient with sickle-cell disease: Case report. Diagn Cytopathol. 2004;30(2):119-121.
doi pubmed - Roberts AS, Shetty AS, Mellnick VM, Pickhardt PJ, Bhalla S, Menias CO. Extramedullary haematopoiesis: radiological imaging features. Clin Radiol. 2016;71(9):807-814.
doi pubmed - Kamran N, Li Y, Sierra M, Alghamri MS, Kadiyala P, Appelman HD, Edwards M, et al. Melanoma induced immunosuppression is mediated by hematopoietic dysregulation. Oncoimmunology. 2018;7(3):e1408750.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


