| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Letter to the Editor
Volume 11, Number 2, April 2022, pages 81-85
Transmission of Human T-Lymphotropic Virus Type I to Donor-Origin T Cells During Allogeneic Hematopoietic Stem Cell Transplantation
Makoto Hirosawaa, Daisuke Niinob, Junichi Tsukadaa, c
aDepartment of Hematology, University of Occupational and Environmental Health, 1-1 Iseigaoka, Yahatanishi-ku, Kitakyushu 807-8556, Japan
bDepartment of Pathology and Cell Biology, School of Medicine, University of Occupational and Environmental Health, 1-1 Iseigaoka, Yahatanishi-ku, Kitakyushu 807-8556, Japan
cCorresponding Author: Junichi Tsukada, Department of Hematology, University of Occupational and Environmental Health, 1-1 Iseigaoka, Yahatanishi-ku, Kitakyushu 807-8556, Japan
Manuscript submitted January 10, 2022, accepted February 15, 2022, published online March 12, 2022
Short title: Transmission of HTLV-I to Donor-Origin T Cells
doi: https://doi.org/10.14740/jh973
| To the Editor | ▴Top |
Human T-lymphotropic virus type I (HTLV-I) is a retrovirus that causes adult T-cell leukemia/lymphoma (ATLL), an aggressive CD4-positive mature T-cell malignancy with a dismal prognosis [1, 2]. In addition, the virus is associated with chronic inflammatory diseases such as HTLV-I-associated myelopathy (HAM)/tropical spastic paraparesis (TSP), arthritis, uveitis, dermatitis, and bronchioloalveolar disorders. It is estimated that HTLV-I infects 5 - 20 million individuals worldwide [3, 4]. HTLV-I is transmitted via breast feeding, sexual intercourse, needle sharing, and blood products containing cells. Organ transplantation has also been considered a rare route of HTLV-I transmission [5].
Here, we describe a case of HTLV-I transmission to T cells of an HTLV-I-negative donor through allogeneic hematopoietic stem cell transplantation (allo-HSCT). A 56-year-old man with a history of ATLL was referred to our hospital for dyspnea. Two years before the presentation, he underwent allogeneic peripheral blood stem cell transplantation (allo-PBSCT) from an HTLV-I-negative female donor with a reduced intensity conditioning (RIC) regimen consisting of fludarabine, melphalan, and total body irradiation (4 Gy). T-cell depletion was carried out using thymoglobulin. At the time of transplantation, the patient had systemic lymphadenopathy due to refractory ATLL. After transplantation, the patient achieved a complete remission. The HTLV-I-negative donor had neither atypical lymphocytes in the peripheral blood (PB) nor lymphadenopathy. The seronegativity of anti-HTLV-I antibody was confirmed by chemiluminescence enzyme immunoassay. Serum immunoglobulin levels of the donor were within normal limits.
When the patient was referred to our hospital with dyspnea, he was receiving tacrolimus therapy for chronic graft-vs-host disease (GVHD). He immediately underwent a complete blood count, which revealed 11 × 109/L white blood cells with 17% of atypical lymphocytes. The morphologic feature of the atypical lymphocytes was pleomorphic with condensed chromatin and convoluted or lobulated nucleus (Fig. 1a). Flow cytometric analysis (FCM) revealed that the atypical lymphocytes were positive for CD3 (Fig. 1b), CD25 (Fig. 1b) and CD4, and negative for CD8. Furthermore, the atypical lymphocytes, unlike his pretransplant ATLL cells, expressed CD7 (Fig. 1c). An increased HTLV-I proviral load (PVL) of 397.2 copies/1,000 PB mononuclear cells (PBMCs) was detected. Although no hypercalcemia was observed, serum lactate dehydrogenase and soluble interleukin (IL)-2 receptor levels were elevated to 290 U/L (normal: 124 - 222 U/L) and 30,573 U/mL (normal: 145 - 519 U/mL), respectively. Epstein-Barr virus (EBV) and cytomegalovirus were negative in real-time quantitative polymerase chain reaction. Computed tomography of the chest revealed a mediastinal tumor and pleural effusion (arrows: Fig. 1d). Tumor biopsy showed diffuse infiltration of medium to large, atypical cells with irregular nuclear contour (Fig. 1e). The infiltrating tumor cells showed strong immunohistochemical staining for CD3 (Fig. 1f), CD4 (Fig. 1g), CD7 (Fig. 1h), and CD25 (Fig. 1i), but they were negative for CD8 (Fig. 1j) and EBV-encoded small RNA. FCM of the infiltrating tumor cells showed the same results as those of PB.
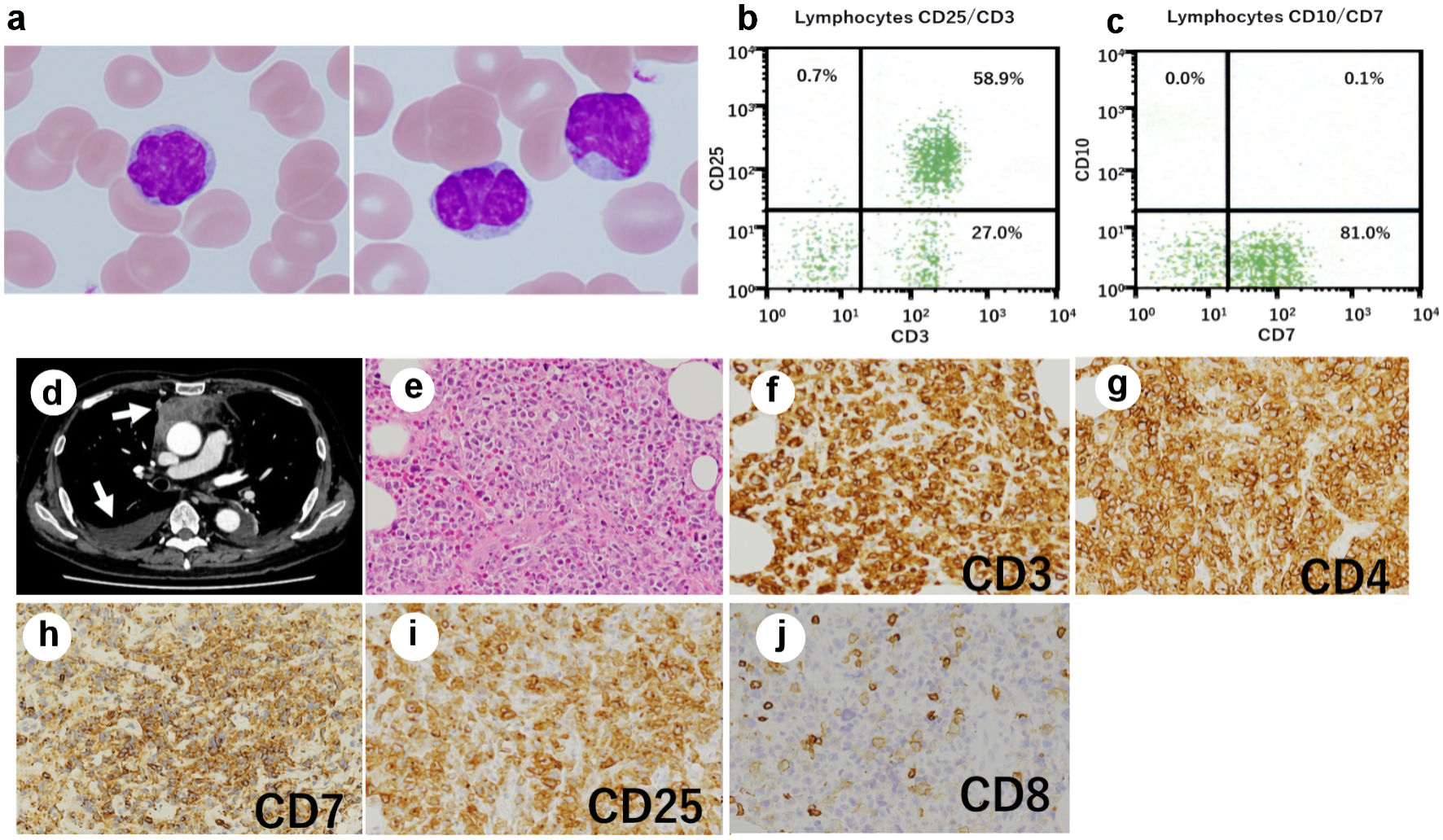 Click for large image | Figure 1. Posttransplant expansion of atypical T cells in the recipient. (a) Atypical lymphocytes with nuclear lobulation were observed in the recipient’s peripheral blood after transplantation; original magnification × 1,000, Wright-Giemsa stain. (b, c) Flow cytometry revealed T cells expressing CD3 (b), CD25 (b), and CD7 (c). (d) Computed tomography of the chest showed a mediastinal tumor and pleural effusion (arrows). (e-j) Biopsy of the mediastinal tumor was performed, showing an infiltrate of medium- to large-sized atypical lymphocytes (original magnification × 200). (e) Hematoxylin and eosin stain. The atypical cells were positive for CD3 (f), CD4 (g), CD7 (h), and CD25 (i), and negative for CD8 (j). |
Southern blot analysis (SBA) of the atypical lymphocytes using HTLV-I DNA as a probe (Fig. 2a) revealed internal bands following Pst1 digestion (lane 6; arrowheads). However, no monoclonal band pattern was observed in EcoR1 digestion (lane 5; arrow). In contrast, SBA results obtained from his pretransplant ATLL cells (Fig. 2b) revealed monoclonal integration of a defective HTLV-I provirus (lane 5; arrow). Moreover, XY-fluorescence in situ hybridization (XY-FISH) of the atypical lymphocytes was performed to determine the origin of the atypical lymphocytes. All tested cells revealed a typical XX pattern of a female donor (Fig. 2c). The G-band karyotype of the lymphocytes was also 46, XX. Bone marrow examination confirmed complete donor chimerism. Thus, our results demonstrated acquired HTLV-I infection of donor-origin T cells in the recipient.
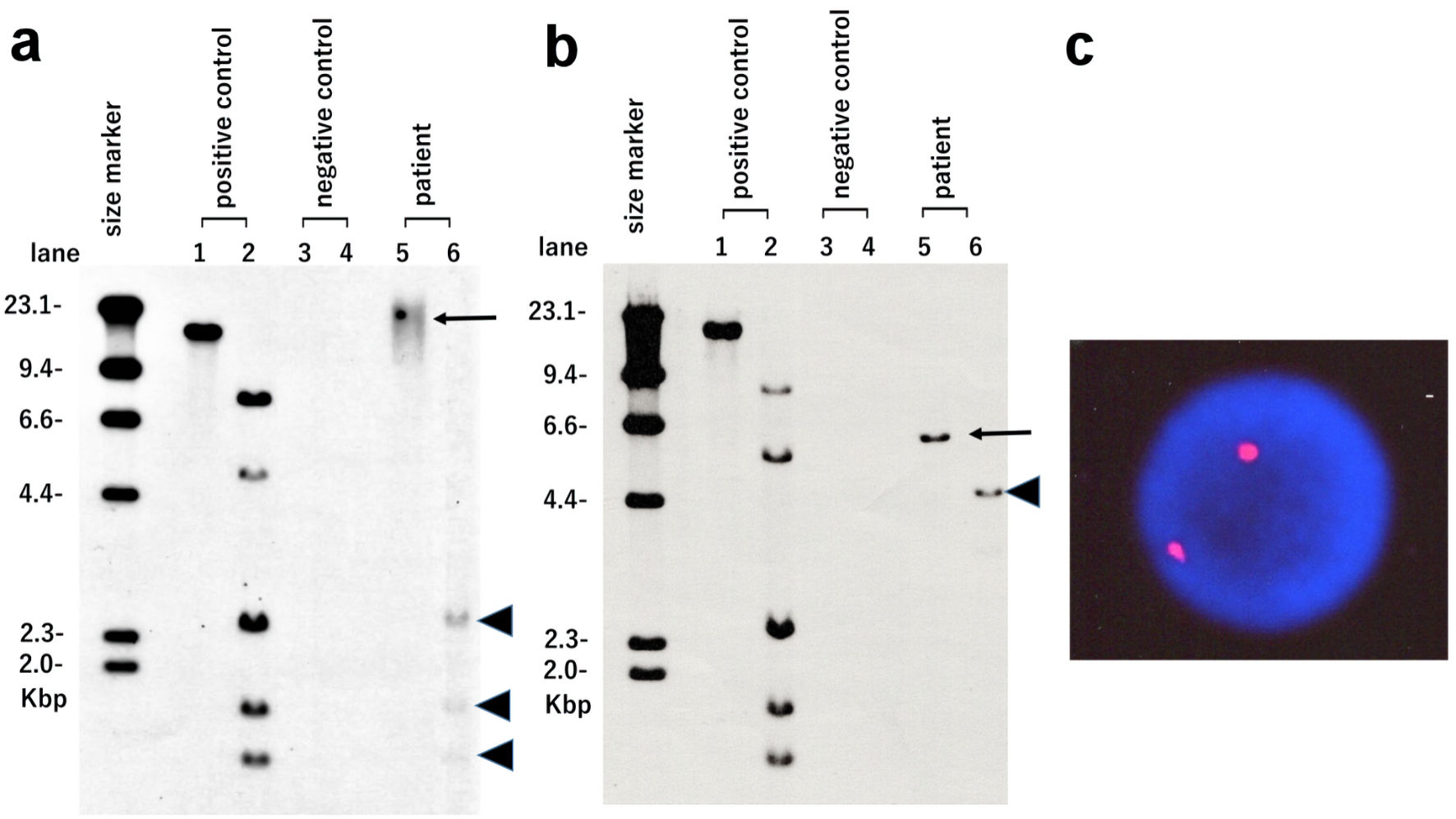 Click for large image | Figure 2. HTLV-I integration analysis using Southern blotting and XY-fluorescence in situ hybridization (XY-FISH). Atypical lymphocytes observed in the recipient after transplantation (a) and pretransplant ATLL cells of the recipient (b) were used. Lane 1: positive control in EcoR1 digestion. Lane 2: positive control in Pst1 digestion. Lane 3: negative control in EcoR1 digestion. Lane 4: negative control in Pst1 digestion. Lane 5: patient sample in EcoR1 digestion (arrows). Lane 6: patient sample in Pst1 digestion (arrowheads). (c) XY-FISH (X chromosome red and Y chromosome green) of atypical lymphocytes observed in the recipient after transplantation was performed. No XY signal was found in the nuclei of atypical lymphocytes. HTLV-I: human T-lymphotropic virus type I. |
Two months after the discontinuation of tacrolimus, white blood cell counts in PB returned to normal, and no atypical lymphocytes were observed in PB (Fig. 3a). The mediastinal tumor and pleural effusion also regressed and then disappeared (Fig. 3b, c). The PVL decreased to 31 copies/1,000 PBMCs. No exacerbation of GVHD was observed. Peripheral blood cell counts of CD4+CD3+ T cells, CD8+CD3+ T cell, CD4+CD25+CD127-/low regulatory T cells (Treg cells) and CD56+CD3- natural killer (NK) on FCM were 1,232/µL, 457/µL, 76/µL and 167/µL, respectively, showing T-cell immune reconstitution.
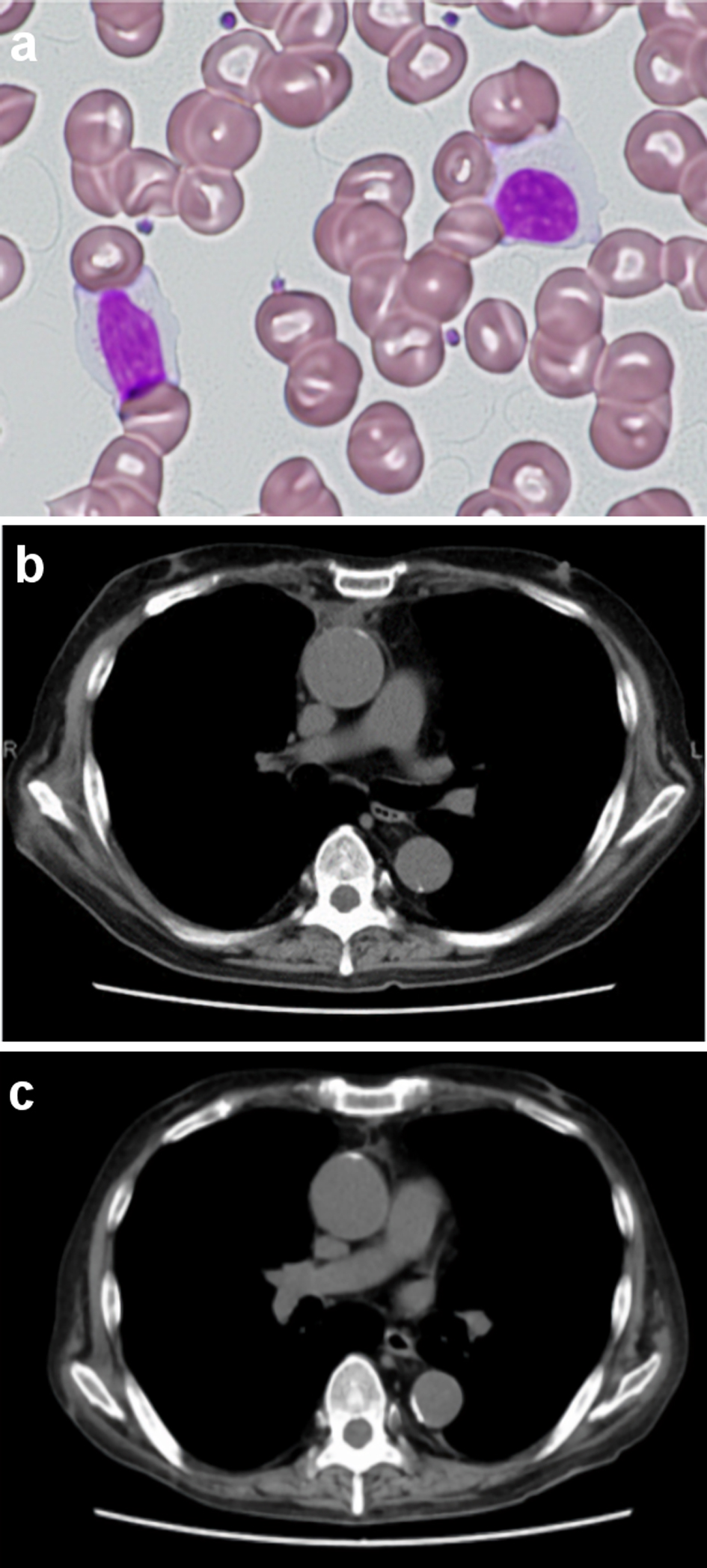 Click for large image | Figure 3. Peripheral blood lymphocytes and the mediastinal tumor after discontinuation of tacrolimus. No atypical lymphocytes with nuclear lobulation were observed in the recipient’s peripheral blood 2 months after discontinuation of tacrolimus (a) Original magnification × 400, Wright-Giemsa stain. (b, c) Computed tomography of the chest showed that the mediastinal tumor and pleural effusion regressed and then disappeared following discontinuation of tacrolimus ((b) Two months after discontinuation of tacrolimus. (c) Four months after discontinuation of tacrolimus). |
Donor-to-recipient transmission of HTLV-I through solid organ transplantation has been reported previously [5]. In a study on transplantation-acquired HTLV-I-infection of three solid organ recipients from a single HTLV-I-positive donor, Cook et al reported rapid dissemination of HTLV-I with a median peak proviral doubling time of 1.43 days. Moreover, they indicated that the median number of clones estimated from the integration sites was 1.3 × 105 [6]. In two of three solid organ recipients from a single HTLV-I-positive donor, cutaneous T-cell lymphoma occurred 2 and 3 years after transplantation [7]. In addition, early onset (within 2 years) of HAM has been observed in organ recipients from infected donors [8, 9]. Thus, when HTLV-I infection is acquired during organ transplantation, the latency appears to be short due to immunosuppression and large size of the viral inoculum.
However, limited evidence is available for HTLV-I transmission from recipients to donor-origin lymphocytes during allo-HSCT. Consistent with that observed in our patient, Ljungman et al reported posttransplant HTLV-I infection of donor-origin lymphocytes in an ATLL patient who died of encephalitis 11 months after bone marrow transplantation from an HTLV-I-negative donor [10]. Furthermore, a retrospective study of 22 long-term ATLL survivors who received allo-HSCT in Nagasaki, Japan indicated the persistent existence of donor-origin T cells newly infected with recipient-derived HTLV-I in one survivor, although they were present at very low levels [11]. In addition, our colleagues reported progressive posttransplant lymphocytosis associated with de novo HTLV-I infection of donor-origin T cells in a recipient [12]. This argument is supported by our observation that unlike ATLL cells, the atypical lymphocytes, which proliferated after transplantation, expressed CD7. Downregulation of CD7 expression in HTLV-I-infected T cells represents disease progression during multistep leukemogenesis [13, 14]. Moreover, our patient showed a high PVL of 397.2 copies/1,000 PBMCs, which predisposed him to HTLV-I-associated diseases [15, 16].
T cells are infected with HTLV-I via direct cell-cell contact between infected cells and target cells via the virologic synapse. In addition to T cells, epithelial cells, dendritic cells, B-cells, monocytes/macrophages, and hematopoietic progenitors/stem cells can be infected with HTLV-I and are believed to be an important part of the virus reservoir. Since our patient presented with multiple lymph node (LN) lesions at the time of transplantation, it is possible that a certain number of HTLV-I-infected cells remained and that some of the viral transmission occurred in the LNs, where lymphocytes concentrate.
Our SBA results using the posttransplant atypical cells exhibited a provirus integration pattern distinct from that of the pretransplant ATLL cells. Multiple subclones of HTLV-I-infected T cells with distinct genetic aberrations have been demonstrated in infected individuals during the clinical course and/or among samples obtained from different sites [17]. A study of high-resolution oligo-array comparative genomic hybridization analysis on paired samples of PB and LNs from acute ATLL patients demonstrated that LNs contain multiple subclones of varying sizes and that one of the subclones in the LNs then appears in the PB, indicating clonal exchange during disease progression [18].
Conversely, the development of donor-derived ATLL has been observed after allo-HSCT from asymptomatic HTLV-I carrier donors [19, 20]. Unlike our case, Tamaki et al reported that a patient with donor-derived ATLL after allo-HSCT from an asymptomatic HTLV-I carrier died of ATLL progression despite administration of chemotherapeutic agents as well as discontinuation of immunosuppressive therapy [19]. Thus, de novo infection of donor-origin T cells with HTLV-I during allo-HSCT may have clinical features distinct from those of donor-derived ATLL after transplantation using HTLV-I-positive donors.
Moreover, in our case, the mediastinal lesion responded well to the withdrawal of tacrolimus, suggesting the possibility that profound suppression of HTLV-I-specific immune responses attributing to long-term use of tacrolimus accelerates the expansion of infected T cells. The de novo HTLV-I infection during allo-HSCT might have been underdiagnosed or misdiagnosed as a relapse. A large prospective study is necessary to evaluate the de novo HLTV-I infection of donor-derived lymphocytes.
Acknowledgments
None to declare.
Financial Disclosure
This study received no funding from public, private, or not-for-profit sectors.
Conflict of Interest
The authors declare that they have no competing interests to disclose.
Informed Consent
Written informed consent was obtained from the patient.
Author Contributions
MH and DN analyzed data and wrote the paper. JT designed the research, analyzed data, and wrote the paper. All authors read and approved the manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Ishitsuka K, Tamura K. Human T-cell leukaemia virus type I and adult T-cell leukaemia-lymphoma. Lancet Oncol. 2014;15(11):e517-526.
doi - Katsuya H, Ishitsuka K, Utsunomiya A, Hanada S, Eto T, Moriuchi Y, Saburi Y, et al. Treatment and survival among 1594 patients with ATL. Blood. 2015;126(24):2570-2577.
doi pubmed - de The G, Bomford R. An HTLV-I vaccine: why, how, for whom? AIDS Res Hum Retroviruses. 1993;9(5):381-386.
doi pubmed - Gessain A, Cassar O. Epidemiological aspects and world distribution of HTLV-1 infection. Front Microbiol. 2012;3:388.
doi pubmed - Martin-Davila P, Fortun J, Lopez-Velez R, Norman F, Montes de Oca M, Zamarron P, Gonzalez MI, et al. Transmission of tropical and geographically restricted infections during solid-organ transplantation. Clin Microbiol Rev. 2008;21(1):60-96.
doi pubmed - Cook LB, Melamed A, Demontis MA, Laydon DJ, Fox JM, Tosswill JH, de Freitas D, et al. Rapid dissemination of human T-lymphotropic virus type 1 during primary infection in transplant recipients. Retrovirology. 2016;13:3.
doi pubmed - Glowacka I, Korn K, Potthoff SA, Lehmann U, Kreipe HH, Ivens K, Barg-Hock H, et al. Delayed seroconversion and rapid onset of lymphoproliferative disease after transmission of human T-cell lymphotropic virus type 1 from a multiorgan donor. Clin Infect Dis. 2013;57(10):1417-1424.
doi pubmed - Toro C, Rodes B, Poveda E, Soriano V. Rapid development of subacute myelopathy in three organ transplant recipients after transmission of human T-cell lymphotropic virus type I from a single donor. Transplantation. 2003;75(1):102-104.
doi pubmed - Zarranz Imirizaldu JJ, Gomez Esteban JC, Rouco Axpe I, Perez Concha T, Velasco Juanes F, Allue Susaeta I, Corral Carranceja JM. Post-transplantation HTLV-1 myelopathy in three recipients from a single donor. J Neurol Neurosurg Psychiatry. 2003;74(8):1080-1084.
doi pubmed - Ljungman P, Lawler M, Asjo B, Bogdanovic G, Karlsson K, Malm C, McCann SR, et al. Infection of donor lymphocytes with human T lymphotrophic virus type 1 (HTLV-I) following allogeneic bone marrow transplantation for HTLV-I positive adult T-cell leukaemia. Br J Haematol. 1994;88(2):403-405.
doi pubmed - Yamasaki R, Miyazaki Y, Moriuchi Y, Tsutsumi C, Fukushima T, Yoshida S, Taguchi J, et al. Small number of HTLV-1-positive cells frequently remains during complete remission after allogeneic hematopoietic stem cell transplantation that are heterogeneous in origin among cases with adult T-cell leukemia/lymphoma. Leukemia. 2007;21(6):1212-1217.
doi pubmed - Kitamura N, Nakanishi T, Yoshida Y, Higashi T, Tsukada J. HTLV-I-associated posttransplant lymphoproliferative disorder following virus transmission from recipient to donor cells. Blood. 2017;130(1):84-86.
doi pubmed - Kobayashi S, Tian Y, Ohno N, Yuji K, Ishigaki T, Isobe M, Tsuda M, et al. The CD3 versus CD7 plot in multicolor flow cytometry reflects progression of disease stage in patients infected with HTLV-I. PLoS One. 2013;8(1):e53728.
doi pubmed - Kobayashi S, Nakano K, Watanabe E, Ishigaki T, Ohno N, Yuji K, Oyaizu N, et al. CADM1 expression and stepwise downregulation of CD7 are closely associated with clonal expansion of HTLV-I-infected cells in adult T-cell leukemia/lymphoma. Clin Cancer Res. 2014;20(11):2851-2861.
doi pubmed - Nagai M, Usuku K, Matsumoto W, Kodama D, Takenouchi N, Moritoyo T, Hashiguchi S, et al. Analysis of HTLV-I proviral load in 202 HAM/TSP patients and 243 asymptomatic HTLV-I carriers: high proviral load strongly predisposes to HAM/TSP. J Neurovirol. 1998;4(6):586-593.
doi pubmed - Hodson A, Laydon DJ, Bain BJ, Fields PA, Taylor GP. Pre-morbid human T-lymphotropic virus type I proviral load, rather than percentage of abnormal lymphocytes, is associated with an increased risk of aggressive adult T-cell leukemia/lymphoma. Haematologica. 2013;98(3):385-388.
doi pubmed - Gillet NA, Malani N, Melamed A, Gormley N, Carter R, Bentley D, Berry C, et al. The host genomic environment of the provirus determines the abundance of HTLV-1-infected T-cell clones. Blood. 2011;117(11):3113-3122.
doi pubmed - Umino A, Nakagawa M, Utsunomiya A, Tsukasaki K, Taira N, Katayama N, Seto M. Clonal evolution of adult T-cell leukemia/lymphoma takes place in the lymph nodes. Blood. 2011;117(20):5473-5478.
doi pubmed - Tamaki H, Matsuoka M. Donor-derived T-cell leukemia after bone marrow transplantation. N Engl J Med. 2006;354(16):1758-1759.
doi pubmed - Nakamizo A, Akagi Y, Amano T, Suzuki SO, Otsuka R, Abe Y, Yoshimoto K, et al. Donor-derived adult T-cell leukaemia. Lancet. 2011;377(9771):1124.
doi
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


