| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Original Article
Volume 10, Number 6, December 2021, pages 246-254
Blood Graft and Outcome After Autologous Stem Cell Transplantation in Patients With Primary Central Nervous System Lymphoma
Anu Partanena, j, Outi Kuittinenb, c, d, Antti Turunena, Jaakko Valtolaa, Marja Pyoralaa, Hanne Kuitunend, Kaija Vasalae, Taru Kuittinena, Pentti Mantymaaf, Jukka Pelkonenf, g, Esa Jantunena, b, h, Ville Varmavuoi
aDepartment of Medicine, Kuopio University Hospital, Kuopio, Finland
bInstitute of Clinical Medicine, University of Eastern Finland, Kuopio, Finland
cDepartment of Oncology, Kuopio University Hospital, Kuopio, Finland
dDepartment of Oncology, Oulu University Hospital, Oulu, Finland
eDepartment of Oncology, Central Hospital of Central Finland, Jyvaskyla, Finland
fEastern Finland Laboratory Centre, Kuopio, Finland
gDepartment of Clinical Microbiology, University of Eastern Finland, Kuopio, Finland
hDepartment of Medicine, North Karelia Hospital District, Joensuu, Finland
iDepartment of Medicine, Kymenlaakso Central Hospital, Kotka, Finland
jCorresponding Author: Anu Partanen, Department of Medicine, Kuopio University Hospital, PO Box 100, 70029 Kuopio, Finland
Manuscript submitted October 21, 2021, accepted November 30, 2021, published online December 13, 2021
Short title: Autograft in Central Nervous System Lymphoma
doi: https://doi.org/10.14740/jh939
| Abstract | ▴Top |
Background: Autologous stem cell transplantation (auto-SCT) is a treatment option for patients with primary central nervous system lymphoma (PCNSL).
Methods: In this prospective multicenter study, the effects of blood graft cellular content on hematologic recovery and outcome were analyzed in 17 PCNSL patients receiving auto-SCT upfront.
Results: The infused viable CD34+ cell count > 1.7 × 106/kg correlated with more rapid platelet engraftment (10 vs. 31 days, P = 0.027) and with early neutrophil recovery (day + 15) (5.4 vs. 1.6 × 109/L, P = 0.047). A higher number of total collected CD34+ cells > 3.3 × 106/kg infused predicted worse 5-year progression-free survival (PFS) (33% vs. 100%, P = 0.028). In addition, CD3+CD8+ T cells > 78 × 106/kg in the infused graft impacted negatively on the 5-year PFS (0% vs. 88%, P = 0.016).
Conclusion: The cellular composition of infused graft seems to impact on the hematologic recovery and PFS post-transplant. Further studies are needed to verify the optimal autograft cellular content in PCNSL.
Keywords: CD34+ cell mobilization; Primary central nervous lymphoma; Autologous stem cell transplantation; Autograft cellular composition; Outcome
| Introduction | ▴Top |
Primary central nervous system lymphoma (PCNSL) accounts only about 1-2% of all non-Hodgkin lymphomas. Diffuse large B-cell lymphoma (DLBCL) is the most common histopathological entity of this challenging extranodal disease entity with increasing incidence [1-3]. Autologous stem cell transplantation (auto-SCT) is a widely used treatment option in transplant-eligible patients with PCNSL [4-8], although the superiority of auto-SCT after methotrexate-based induction therapy has not been validated due to a paucity of randomized studies [4, 5, 9-11]. A randomized PRECIS study [8] concluded that upfront auto-SCT is an effective treatment option in PCNSL in line with a previous phase II study [12] and a more recent retrospective analysis [13]. In addition, a prospective German study suggested that auto-SCT as the first-line consolidation is a feasible treatment also for selected elderly patients [14].
Until now, the optimal thresholds of autograft cellular composition to be infused for lymphoma patients have not been established even if the most important parameter of graft quality has suggested to be the graft CD34+ cell content [15-17]. In the most recent prospective study of DLBCL patients including also some PCNSL patients [18], a cut-off point > 2.65 × 106/kg of CD34+ cells infused was associated with not only more rapid hematologic recovery but in line with previous retrospective observations [19, 20] also with better overall survival (OS). Higher CD34+CD133+CD38- cell content of the graft has been shown to hasten hematologic recovery after auto-SCT [21] and in DLBCL patients also to correlate with better 5-year OS [18]. In previous studies, a higher amount of natural killer (NK) cells in the grafts has been linked with earlier lymphocyte recovery and also with better outcome [22-24]. In addition, the most recent prospective study showed a positive correlation between the number of infused CD3+ cells and outcome in DLBCL patients mostly with systemic disease [18].
At present, the more detailed data on the significance of CD34+ cell mobilization and blood grafts cellular composition on outcome in PCNSL patients are lacking. The aim of this prospective study was to analyze the impact of mobilization characteristics and cellular composition of blood grafts on hematologic recovery and outcome in PCNSL patients with an upfront auto-SCT as a part of a prospective multicenter graft and outcome in autologous stem cell transplantation (GOA) study.
| Materials and Methods | ▴Top |
Patients
The present study population consisted of 17 patients with PCNSL who received an upfront auto-SCT between May 2012 and December 2018 at the University Hospitals of Kuopio and Oulu in Finland. As an induction treatment (median four cycles), nine patients (53%) received therapy according to Bonn-protocol [25] consisting of systemic and intrathecal methotrexate- and cytarabine-based chemotherapy added to blood-brain barrier disruption (BBBD) treatment [26], four patients (23%) MATRix treatment (methotrexate, cytarabine, thiotepa and rituximab) combined to BBBD therapy, two patients (12%) MATRix-induction alone and two patients (12%) Bonn-protocol therapy alone. The patients participated from the time point of apheresis in the prospective, non-interventional multicenter GOA study. The main aims in the GOA study were to evaluate the impact of different mobilization methods on the cellular composition of blood grafts collected and possible correlations of graft cellular composition with hematologic and immune recovery as well as outcome after auto-SCT. The main patient and mobilization characteristics of the patients are shown in Table 1.
 Click to view | Table 1. Patient and Mobilization Characteristics in Patients With PCNS Lymphoma |
Mobilization and collection of blood grafts
All the patients received chemotherapy combined with granulocyte colony-stimulating factor (G-CSF) in order to mobilize CD34+ cells (Table 1). The mobilizing chemotherapy was chosen according to the institutional standards of care. The majority of patients received BBBD treatment as a mobilization therapy [27]. BBBD treatment composed of intra-arterial mannitol infusion followed by methotrexate and carboplatin with intravenous rituximab, etoposide and cyclophosphamide. Blood CD34+ cell counts were measured with a flow cytometry using an International Society of Hemotherapy and Graft Engineering (ISHAGE) protocol [28]. Aphereses were initiated when blood CD34+ cell counts rose over 10 × 106/L, and the minimal collection target of 2.0 × 106/kg CD34+ cells to proceed to auto-SCT was used. In hard-to-mobilize patients, pre-emptive plerixafor (PLER) was given when blood CD34+ cell counts were still less than 10 × 106/L and simultaneously rising white blood cell counts were over 5 × 109/L [29]. PLER use was also thought of, if the yield of the first apheresis was unsatisfactory (< 1 × 106/kg CD34+ cells) or if blood CD34+ cell counts were decreasing before the adequate apheresis yield was achieved.
The aphereses were performed with COBE Spectra (Terumo BCT) AutoPBSC apheresis machine for one patient and since April 2013 with the Spectra Optia leukapheresis system (Spectra Optia, software 7.2., Terumo BCT, Lakewood, USA) at KUH. The Spectra Optia apheresis system was used throughout the study at Oulu University Hospital. The blood volume processed was 2 - 3 times of the estimated total blood volume of each patient. The number of CD34+ cells of each apheresis product was measured by flow cytometry at the stem cell laboratory of each hospital using an ISHAGE protocol with a single platform method [28]. In order to protect the cells from stress and death during cryopreservation, dimethylsulfoxide (DMSO) was added to apheresis products at a final concentration of 10%. After freezing the final products were maintained in the vapor phase of a liquid nitrogen freezer.
Graft analysis
After each collection, two additional 0.5 mL specimens were taken from the apheresis product to facilitate the graft cellular composition analyses in the future. DMSO was added to the samples as a final concentration of 10% and they were cryopreserved in a freezer with a controlled-rate freezing program likewise the graft bags.
An experienced flow cytometrist (AR) later analyzed the thawed cryopreserved graft specimens at the Department of Clinical Microbiology, University of Eastern Finland by flow cytometry (FACSCanto, Becton Dickinson, San Jose, CA) using an ISHAGE protocol [28]. In order to determinate both CD34+ cells and subclasses, CD34, CD38, CD45 and CD133 antibodies were used. All antibodies were delivered by Becton Dickinson except for CD133 (Miltenyi Biotec GmbH). Viable CD34+ cells were assorted by using 7-aminoactinomycin (7-AAD) staining. The absolute counts of B, T and NK cells as well as the CD3+CD4+ and CD3+CD8+ subpopulations were specified by using both CD3/CD8/CD45/CD4 and CD3/CD16 + CD56/CD45/CD19 reagents (BD Multitest, Becton Dickinson) with tubes (BD Trucount, Becton Dickinson).
High-dose therapy (HDT) and post-transplant course
The response to the induction treatment before HDT was evaluated with magnetic resonance imaging (MRI). All patients received the combination of carmustine (400 mg/m2 on day -6) and thiotepa (5 mg/kg b.i.d. on days -5 and -4) as an HDT. After the graft infusion, four patients (23%) received filgrastim, three patients (18%) received pegfilgrastim and 10 patients received (59%) lipegfilgrastim, respectively.
The definition of engraftment was composed of the days from the graft infusion (day 0) until absolute neutrophil count was > 0.5 × 109/L and platelet (PLT) count was > 20 × 109/L without PLT transfusions for previous 3 days, respectively. To assess the hematopoietic recovery complete blood counts were obtained at day +15 and at 1, 3, 6 and 12 months after the graft infusion. Infections and need for intensive care unit (ICU) admission during the early post-transplant period were also recorded.
Statistical analysis
All calculations and analyses were performed with the statistical program package SPSS (IBM SPSS Statistics Version 26, Chicago, USA). Descriptive statistics for continuous variables were described using medians with ranges and categorical variables were presented with frequencies and percentages. In order to determine optimal cut-off points for apheresis parameters and graft cellular components correlating with hematologic recovery, PFS and OS, receiver operating characteristic (ROC) curves were used and Youden’s index was applied. Spearman’s rank was performed to define associations between variables. In survival analyses, log rank test and Kaplan-Meier’s method were used. Two-tailed P values < 0.05 were considered statistically significant.
Ethics
The GOA study protocol was approved by the Research Ethics Committee of the North Savo Hospital District (13/2012) until 12/2016 as well as an amendment of GOA study including non-Hodgkin lymphoma (NHL) patients transplanted in the Kuopio University Hospital (KUH) catchment area in 2017 - 2018, but without analysis of the infused grafts. The study was conducted according to the Declaration of Helsinki.
| Results | ▴Top |
Mobilization efficiency and collection of CD34+ cells
All patients achieved the minimum collection target ≥ 2 × 106/kg CD34+ cells. Altogether seven patients (41%) received pre-emptive PLER to augment CD34+ cell mobilization. The median graft CD34+ cell yield was 3.7 × 106/kg and for majority of the patients, only one apheresis session was needed to reach the adequate graft to proceed to auto-SCT (Table 2).
 Click to view | Table 2. Mobilization and Collection Parameters for CD34+ Cells in Patients With PCNS Lymphoma |
Graft cellular composition
The detailed data on cellular composition of the infused grafts were available in 10 patients included in the study between 2012 and 2016. The median cryopreservation time before the auto-SCT was 41 days (23 - 91 days). The median loss of CD34+ cells was 48% (range 0-73%) during cryopreservation. The detailed cellular composition of cryopreserved grafts is presented in Table 3.
 Click to view | Table 3. Cellular Composition of Infused Grafts in Patients With PCNS Lymphoma |
Hematologic recovery after auto-SCT
A median hospitalization time during auto-SCT was 23 days (range 17 - 80 days). The incidence of febrile neutropenia was 94% (16 patients) and of those only one patient (6%) had positive blood culture. One patient (6%) had a need for ICU admission due to pneumonia.
The neutrophil engraftment took place in a median of 10 days (8 - 18 days) and the median time to reach a PLT count > 20 × 109/L without transfusions was 13 days (8 - 98 days). A higher CD34+ cell yield at the first apheresis > 3.1 × 106/kg and total collected CD34+ cell yield > 3.3 × 106/kg correlated with faster platelet engraftment (9 vs. 15 days, P = 0.006 and 9 vs. 23 days, P = 0.009).
Several correlations were observed with the cellular composition of infused graft and the post-transplant hematologic recovery (Table 4). A higher number of infused viable CD34+ cells > 1.7 × 106/kg was linked to more rapid platelet engraftment (10 vs. 31 days, P = 0.027) and neutrophil recovery at day +15 (5.4 vs. 1.6 × 109/L, P = 0.047). A higher amount of cytotoxic CD3+CD8+ cells infused (> 78 × 106/kg) was correlated with slower neutrophil engraftment (9 vs. 12 days, P = 0.005). CD3+ cell count > 181 × 106/kg in the infused graft was associated with the lower number of neutrophils both at day +15 (1.2 vs. 4.4 × 109/L, P = 0.028), and also at 1 month (0.9 vs. 2.2 × 109/L, P = 0.029) after the graft infusion. In addition, a higher number of infused CD3+ cells was linked with slower platelet engraftment (22 vs. 11 days, P = 0.027) and early platelet recovery (day +15) after the graft infusion (24 vs. 111 × 109/L, P = 0.022). Also, the infused CD3+CD8+ cell count > 78 × 106/kg was also linked with the slower early post-transplant (day +15) platelet (30 vs. 84 × 109/L, P = 0.004) and leukocyte (2.8 vs. 7.8 × 109/L, P = 0.029) recovery. In addition, the graft CD3+CD4+ cell count > 94 × 106/kg was associated with lower hemoglobin levels at 1 month (103 vs. 108 × 109/L, P = 0.029) and higher number of lymphocytes at 12 months (2.0 vs. 1.7 × 109/L, P = 0.035) after auto-SCT.
 Click to view | Table 4. Correlation Between Graft Cellular Composition and Hematologic Recovery After Auto-SCT |
Post-transplant outcome
By October 31, 2020, the median follow-up time from auto-SCT was 46 months (range 3 - 95 months). A disease recurrence or progression has been observed in four patients (24%). The median time to relapse was 253 days (range 64 - 1,925 days). Altogether two patients (12%) have died due to disease progression during the follow-up and one of those within 100 days after auto-SCT. In Kaplan-Mayer analysis, 5-year PFS for total patient population was 69% (Fig. 1) and OS was as high as 88% (Fig. 2), respectively.
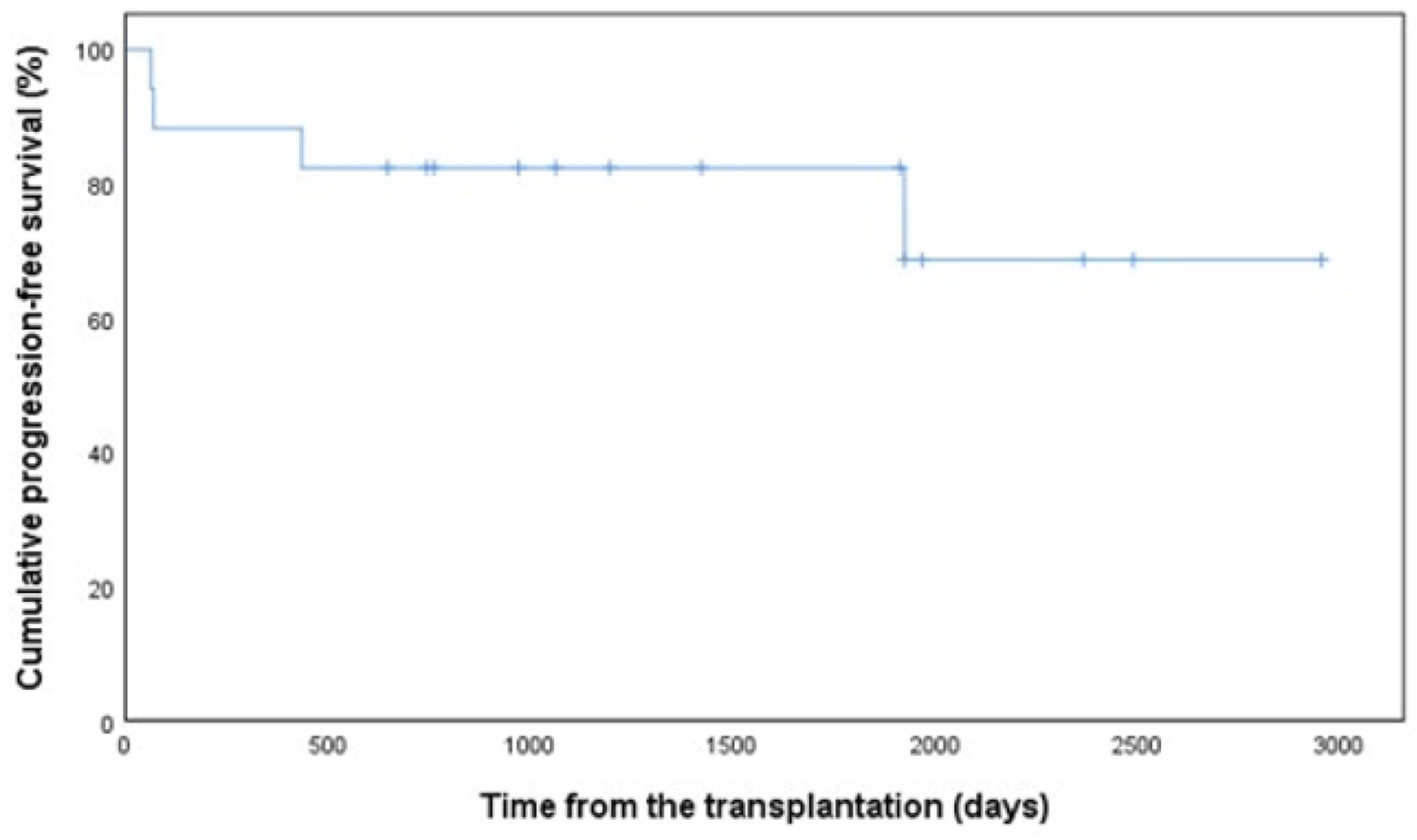 Click for large image | Figure 1. Progression-free survival in 17 patients with PCNSL. PCNSL: primary central nervous system lymphoma. |
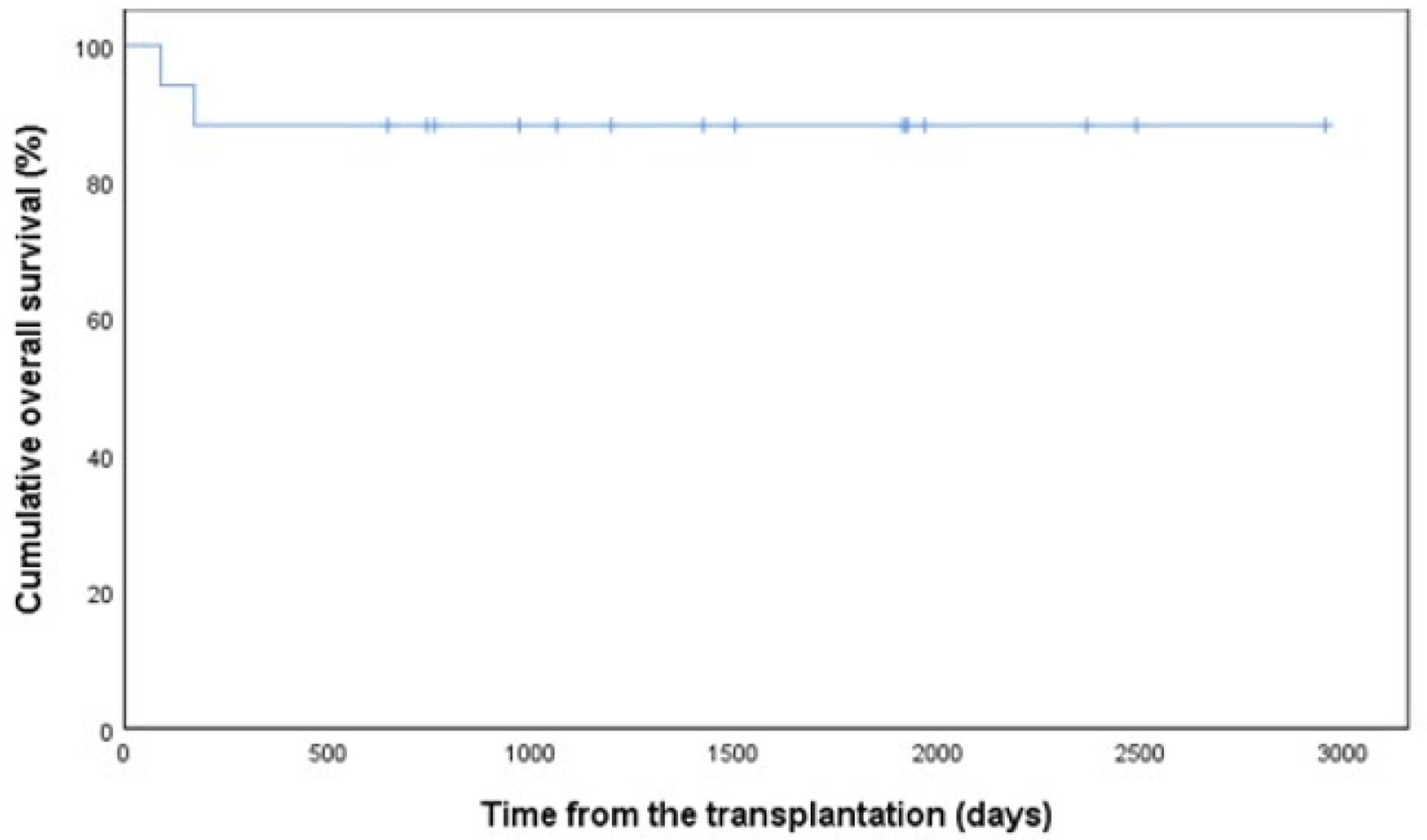 Click for large image | Figure 2. Overall survival in 17 patients with PCNSL. PCNSL: primary central nervous system lymphoma. |
The first-line therapy before auto-SCT had no meaningful effect on survival (P = 0.130). Disease status complete remission (CR) before auto-SCT was not significantly associated with PFS (P = 0.651) or OS (P = 0.244) either. The better mobilizing capacity of CD34+ cells neither had significant impact on 5-year OS. A higher number of total graft CD34+ yield collected with a cut-off point > 3.3 × 106/kg (AUC 0.444, sensitivity 1.000, specificity 0.444, P = 0.028) was associated with worse 5-year PFS (33% vs. 100%), but the number of viable CD34+ cells after thawing with a cut-off point > 1.7 × 106/kg did not correlate with PFS (P = 0.688) or OS (P = 0.414). Regarding the impact on detailed graft cellular content on outcome, also higher amount of CD3+CD4+ T cells with a cut-off point > 94 × 106/kg (AUC 0.778, sensitivity 1.000, specificity 0.778, P = 0.069) showed a trend for negative impact on 5-year PFS (30% vs. 100%). In addition, the patients with infused CD3+CD8+ T cells with a cut-off point > 78 × 106/kg (AUC 0.889, sensitivity 1.000, specificity 0.889, P = 0.016) had poorer PFS (0% vs. 88%) at 5 years.
| Discussion | ▴Top |
This prospective multicenter study aimed to analyze the correlations of CD34+ cell mobilization parameters and graft cellular content with hematologic recovery and outcome in PCNSL patients after an upfront auto-SCT. Higher yield of CD34+ cells of the first apheresis and total yield of CD34+ cells harvested correlated with faster platelet engraftment. Instead, higher number of CD3+ lymphocytes infused was associated with slower platelet recovery and higher amount of graft CD3+CD8+ cells with slower neutrophil engraftment. Surprisingly, higher number of total CD34+ cells in the graft predicted worse 5-year PFS. Also, higher number of CD3+CD8+ T cells infused affected negatively on the 5-year PFS. However, these associations should be handled with reservation due to small number of patients.
A good response to treatment before auto-SCT has been suggested to predict a better long-term outcome in a retrospective analysis by the Mayo Clinic [11] and also, in a previous intent-to-treat analysis in the subgroup of the patients with PCNSL [30]. The optimal treatment strategy in order to produce deep remission rates in PCNSL has not been validated. A recent randomized IELSG32 study highlighted MATRix induction therapy as a representative of effective high-dose methotrexate-based treatment [31]. Omuro et al [12] concluded in a prospective study that R-MPV (rituximab, methotrexate, procarbazine, vincristine) before auto-SCT was effective and safe without detectable neurotoxicity. The MATRix combination was used in our study for the majority of the patients before BBBD treatment, which in turn has been proven to result in high remission rates before auto-SCT in a recent retrospective study [32] and also in a successful strategy for disease control during very long follow-up [32]. In the present study, a great majority (82%) of patients achieved CR before auto-SCT, but in contrast to previous report regarding DLBCL patients [18], we did not find any correlations with pretransplant disease status and survival possibly due to small study population.
The variable mobilizing agents were perhaps less effective in the present patient population resulting in an excess need for PLER use compared to the patients with systemic DLBCL in a previous study [18]. Mobilizing therapies used as well as previous treatment history have an impact on the cellular content of collected grafts [33, 34]. Interestingly, the lower mobilizing capacity regarding the cut-off point of the total collected CD34+ cell yield < 3.3 × 106/kg seemed to predict better 5-year PFS in the present analysis. Regarding other graft cellular components, higher number of CD3+CD4+ T-helper cells and cytotoxic CD3+CD8+ cells were associated with inferior PFS. Otherwise, we found a median of 48% loss of viable CD34+ cells during the cryopreservation indicating possibly a need for higher goals for collected CD34+ cells. The reasons for these interesting novel observations are unknown.
The long-term outcome of the PCNSL patients has been historically inferior compared to patients with systemic DLBCL. A plausible explanation for divergent outcomes may be the pharmacological differences in therapies used in addition to the different biology of these subtypes. In a recent phase II study, a 2-year OS of up to 81% was observed after auto-SCT consolidation in PCNSL patients [12] in line with a retrospective study with a 71% 2-year OS in patients with either upfront or later auto-SCT [13]. We found a promising 5-year OS of 88% in patients receiving upfront auto-SCT. Of note, 65% of our patients had received BBBD therapy before auto-SCT.
There are obvious limitations in this study. The mobilization chemotherapy varied during the course of this non-interventional study. In addition, small number of enrolled patients hampered more sophisticated statistical analyses. The obvious strengths of this prospective multicenter study are comparable graft collection practices and centrally analyzed graft cellular composition by a single experienced cytometrist.
To conclude, according to this prospective study, the higher mobilizing capacity with a threshold of 3.3 × 106/kg CD34+ cells in the collected graft influenced negatively PFS. Graft cellular composition and especially the number of cytotoxic T cells had an impact on both the hematologic recovery and PFS. Excellent long-term OS rates were observed after an upfront auto-SCT. A larger study is required to verify the optimal autograft cellular content in patients with PCNSL.
Acknowledgments
Antti Ropponen is acknowledged for graft analyses by flow cytometry, Tuomas Selander M.Sc. for the statistical support and David Laaksonen M.D., Ph.D. for linguistic revision.
Financial Disclosure
AP is grateful for the scholarships granted by the Finnish Society of Hematology and the Cancer Foundation of North Savo. A research grant from Sanofi Genzyme for the GOA study is also acknowledged.
Conflict of Interest
AP reports honoraria from Behring and Abbvie and has participated in Scientific Advisory Board meetings organized by Abbvie, Novartis and Takeda. JV reports honoraria from Amgen, Janssen-Cilag and Sanofi. VV reports consultancy fees from Abbvie, Amgen, Celgene, Janssen-Cilag, Roche and Sanofi. EJ reports honoraria from Amgen and Sanofi and has participated in Scientific Advisory Board meetings organized by Amgen, Takeda and TEVA. The other authors declare no conflict of interest.
Informed Consent
Informed consent was obtained from all patients.
Author Contributions
AP, EJ and VV designed the study, obtained patient data, analyzed the data, interpreted the results and wrote the manuscript. AP, OK, AT, JV, MP, HK, KV, TK, PM, JP, EJ and VV participated in the revision and approval of the final version of this manuscript.
Data Availability
Any inquiries regarding supporting data availability of this study should be directed to the corresponding author.
| References | ▴Top |
- Han CH, Batchelor TT. Diagnosis and management of primary central nervous system lymphoma. Cancer. 2017;123(22):4314-4324.
doi pubmed - Enblad G, Martinsson G, Baecklund E, Hesselager G, Sundstrom C, Amini RM, Hagberg H. Population-based experience on primary central nervous system lymphoma 2000-2012: the incidence is increasing. Acta Oncol. 2017;56(4):599-607.
doi pubmed - Shiels MS, Pfeiffer RM, Besson C, Clarke CA, Morton LM, Nogueira L, Pawlish K, et al. Trends in primary central nervous system lymphoma incidence and survival in the U.S. Br J Haematol. 2016;174(3):417-424.
doi pubmed - Soussain C, Choquet S, Fourme E, Delgadillo D, Bouabdallah K, Ghesquieres H, Damaj G, et al. Intensive chemotherapy with thiotepa, busulfan and cyclophosphamide and hematopoietic stem cell rescue in relapsed or refractory primary central nervous system lymphoma and intraocular lymphoma: a retrospective study of 79 cases. Haematologica. 2012;97(11):1751-1756.
doi pubmed - Welch MR, Sauter CS, Matasar MJ, Faivre G, Weaver SA, Moskowitz CH, Omuro AM. Autologous stem cell transplant in recurrent or refractory primary or secondary central nervous system lymphoma using thiotepa, busulfan and cyclophosphamide. Leuk Lymphoma. 2015;56(2):361-367.
doi pubmed - Illerhaus G, Kasenda B, Ihorst G, Egerer G, Lamprecht M, Keller U, Wolf HH, et al. High-dose chemotherapy with autologous haemopoietic stem cell transplantation for newly diagnosed primary CNS lymphoma: a prospective, single-arm, phase 2 trial. Lancet Haematol. 2016;3(8):e388-397.
doi - Ferreri AJ, Illerhaus G. The role of autologous stem cell transplantation in primary central nervous system lymphoma. Blood. 2016;127(13):1642-1649.
doi pubmed - Houillier C, Taillandier L, Dureau S, Lamy T, Laadhari M, Chinot O, Molucon-Chabrot C, et al. Radiotherapy or autologous stem-cell transplantation for primary CNS lymphoma in patients 60 years of age and younger: results of the intergroup ANOCEF-GOELAMS randomized phase II PRECIS study. J Clin Oncol. 2019;37(10):823-833.
doi pubmed - Kiefer T, Hirt C, Spath C, Schuler F, Al-Ali HK, Wolf HH, Herbst R, et al. Long-term follow-up of high-dose chemotherapy with autologous stem-cell transplantation and response-adapted whole-brain radiotherapy for newly diagnosed primary CNS lymphoma: results of the multicenter Ostdeutsche Studiengruppe Hamatologie und Onkologie OSHO-53 phase II study. Ann Oncol. 2012;23(7):1809-1812.
doi pubmed - Ferreri AJ, Cwynarski K, Pulczynski E, Ponzoni M, Deckert M, Politi LS, Torri V, et al. Chemoimmunotherapy with methotrexate, cytarabine, thiotepa, and rituximab (MATRix regimen) in patients with primary CNS lymphoma: results of the first randomisation of the International Extranodal Lymphoma Study Group-32 (IELSG32) phase 2 trial. Lancet Haematol. 2016;3(5):e217-227.
doi - Khurana A, Micallef IN, LaPlant BR, Patrick O'Neill B, Habermann TM, Ansell SM, Inwards DJ, et al. Outcomes of autologous stem cell transplant consolidation in primary central nervous system lymphoma: A Mayo clinic experience. Biol Blood Marrow Transplant. 2020;26(12):2217-2222.
doi pubmed - Omuro A, Correa DD, DeAngelis LM, Moskowitz CH, Matasar MJ, Kaley TJ, Gavrilovic IT, et al. R-MPV followed by high-dose chemotherapy with TBC and autologous stem-cell transplant for newly diagnosed primary CNS lymphoma. Blood. 2015;125(9):1403-1410.
doi pubmed - Dholaria BR, Kumar A, Azzuqua AG, Nishihori T, Kharfan-Dabaja MA, Tun HW, Ayala E. Autologous stem cell transplantation in central nervous system lymphoma: A multicenter retrospective series and a review of the literature. Clin Lymphoma Myeloma Leuk. 2019;19(6):e273-e280.
doi pubmed - Schorb E, Finke J, Ihorst G, Kasenda B, Fricker H, Illerhaus G. Age-adjusted high-dose chemotherapy and autologous stem cell transplant in elderly and fit primary CNS lymphoma patients. BMC Cancer. 2019;19(1):287.
doi pubmed - Weaver CH, Hazelton B, Birch R, Palmer P, Allen C, Schwartzberg L, West W. An analysis of engraftment kinetics as a function of the CD34 content of peripheral blood progenitor cell collections in 692 patients after the administration of myeloablative chemotherapy. Blood. 1995;86(10):3961-3969.
doi pubmed - Allan DS, Keeney M, Howson-Jan K, Popma J, Weir K, Bhatia M, Sutherland DR, et al. Number of viable CD34(+) cells reinfused predicts engraftment in autologous hematopoietic stem cell transplantation. Bone Marrow Transplant. 2002;29(12):967-972.
doi pubmed - Stiff PJ, Micallef I, Nademanee AP, Stadtmauer EA, Maziarz RT, Bolwell BJ, Bridger G, et al. Transplanted CD34(+) cell dose is associated with long-term platelet count recovery following autologous peripheral blood stem cell transplant in patients with non-Hodgkin lymphoma or multiple myeloma. Biol Blood Marrow Transplant. 2011;17(8):1146-1153.
doi pubmed - Partanen A, Turunen A, Valtola J, Pyorala M, Vasala K, Kuittinen O, Kuitunen H, et al. Mobilization characteristics, blood graft composition, and outcome in diffuse large B-cell lymphoma after autologous stem cell transplantation: Results from the prospective multicenter GOA study. Transfusion. 2021;61(2):516-525.
doi pubmed - Blystad AK, Delabie J, Kvaloy S, Holte H, Valerhaugen H, Ikonomou I, Kvalheim G. Infused CD34 cell dose, but not tumour cell content of peripheral blood progenitor cell grafts, predicts clinical outcome in patients with diffuse large B-cell lymphoma and follicular lymphoma grade 3 treated with high-dose therapy. Br J Haematol. 2004;125(5):605-612.
doi pubmed - Bolwell BJ, Pohlman B, Rybicki L, Sobecks R, Dean R, Curtis J, Andresen S, et al. Patients mobilizing large numbers of CD34+ cells ('super mobilizers') have improved survival in autologous stem cell transplantation for lymphoid malignancies. Bone Marrow Transplant. 2007;40(5):437-441.
doi pubmed - Henon P, Sovalat H, Bourderont D, Ojeda-Uribe M, Arkam Y, Wunder E, Raidot JP, et al. Role of the CD34+ 38- cells in posttransplant hematopoietic recovery. Stem Cells. 1998;16(Suppl 1):113-122.
doi pubmed - Porrata LF, Gertz MA, Inwards DJ, Litzow MR, Lacy MQ, Tefferi A, Gastineau DA, et al. Early lymphocyte recovery predicts superior survival after autologous hematopoietic stem cell transplantation in multiple myeloma or non-Hodgkin lymphoma. Blood. 2001;98(3):579-585.
doi pubmed - Valtola J, Varmavuo V, Ropponen A, Selander T, Kuittinen O, Kuitunen H, Keskinen L, et al. Early immune recovery after autologous transplantation in non-Hodgkin lymphoma patients: predictive factors and clinical significance. Leuk Lymphoma. 2016;57(9):2025-2032.
doi pubmed - Porrata LF. Autograft immune effector cells and survival in autologous peripheral blood hematopoietic stem cell transplantation. J Clin Apher. 2018;33(3):324-330.
doi pubmed - Pels H, Schmidt-Wolf IG, Glasmacher A, Schulz H, Engert A, Diehl V, Zellner A, et al. Primary central nervous system lymphoma: results of a pilot and phase II study of systemic and intraventricular chemotherapy with deferred radiotherapy. J Clin Oncol. 2003;21(24):4489-4495.
doi pubmed - Kuitunen H, Tokola S, Siniluoto T, Isokangas M, Sonkajarvi E, Alahuhta S, Turpeenniemi-Hujanen T, et al. Promising treatment results with blood brain barrier disruption (BBBD) based immunochemotherapy combined with autologous stem cell transplantation (ASCT) in patients with primary central nervous system lymphoma (PCNSL). J Neurooncol. 2017;131(2):293-300.
doi pubmed - Doolittle ND, Muldoon LL, Culp AY, Neuwelt EA. Delivery of chemotherapeutics across the blood-brain barrier: challenges and advances. Adv Pharmacol. 2014;71:203-243.
doi pubmed - Gratama JW, Kraan J, Keeney M, Sutherland DR, Granger V, Barnett D. Validation of the single-platform ISHAGE method for CD34(+) hematopoietic stem and progenitor cell enumeration in an international multicenter study. Cytotherapy. 2003;5(1):55-65.
doi pubmed - Jantunen E, Varmavuo V, Juutilainen A, Kuittinen T, Mahlamaki E, Mantymaa P, Nousiainen T. Kinetics of blood CD34(+) cells after chemotherapy plus G-CSF in poor mobilizers: implications for pre-emptive plerixafor use. Ann Hematol. 2012;91(7):1073-1079.
doi pubmed - Abrey LE, Moskowitz CH, Mason WP, Crump M, Stewart D, Forsyth P, Paleologos N, et al. Intensive methotrexate and cytarabine followed by high-dose chemotherapy with autologous stem-cell rescue in patients with newly diagnosed primary CNS lymphoma: an intent-to-treat analysis. J Clin Oncol. 2003;21(22):4151-4156.
doi pubmed - Ferreri AJ, Reni M, Foppoli M, Martelli M, Pangalis GA, Frezzato M, Cabras MG, et al. High-dose cytarabine plus high-dose methotrexate versus high-dose methotrexate alone in patients with primary CNS lymphoma: a randomised phase 2 trial. Lancet. 2009;374(9700):1512-1520.
doi - Angelov L, Doolittle ND, Kraemer DF, Siegal T, Barnett GH, Peereboom DM, Stevens G, et al. Blood-brain barrier disruption and intra-arterial methotrexate-based therapy for newly diagnosed primary CNS lymphoma: a multi-institutional experience. J Clin Oncol. 2009;27(21):3503-3509.
doi pubmed - Saraceni F, Shem-Tov N, Olivieri A, Nagler A. Mobilized peripheral blood grafts include more than hematopoietic stem cells: the immunological perspective. Bone Marrow Transplant. 2015;50(7):886-891.
doi pubmed - Jantunen E, Fruehauf S. Importance of blood graft characteristics in auto-SCT: implications for optimizing mobilization regimens. Bone Marrow Transplant. 2011;46(5):627-635.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


