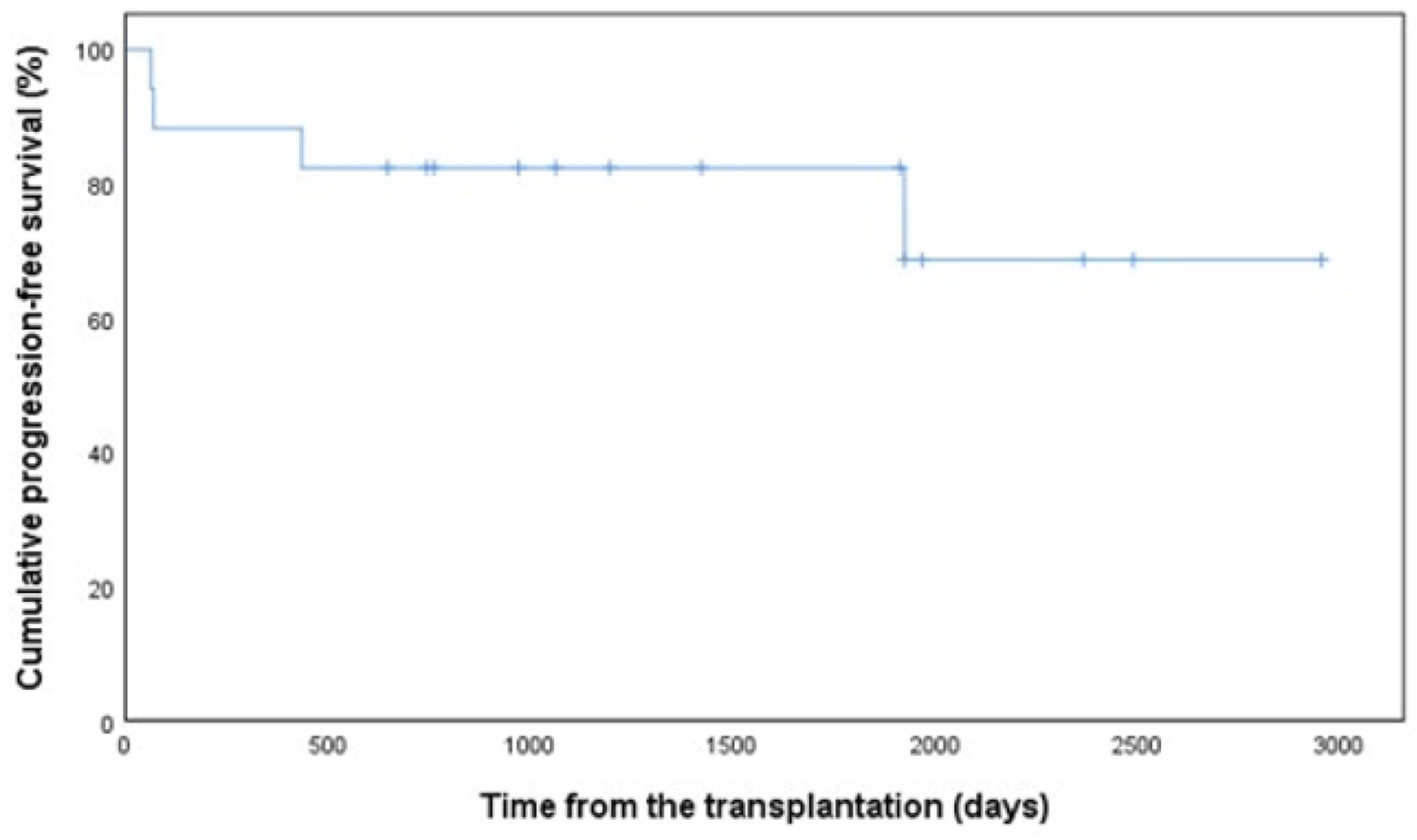
Figure 1. Progression-free survival in 17 patients with PCNSL. PCNSL: primary central nervous system lymphoma.
| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Original Article
Volume 10, Number 6, December 2021, pages 246-254
Blood Graft and Outcome After Autologous Stem Cell Transplantation in Patients With Primary Central Nervous System Lymphoma
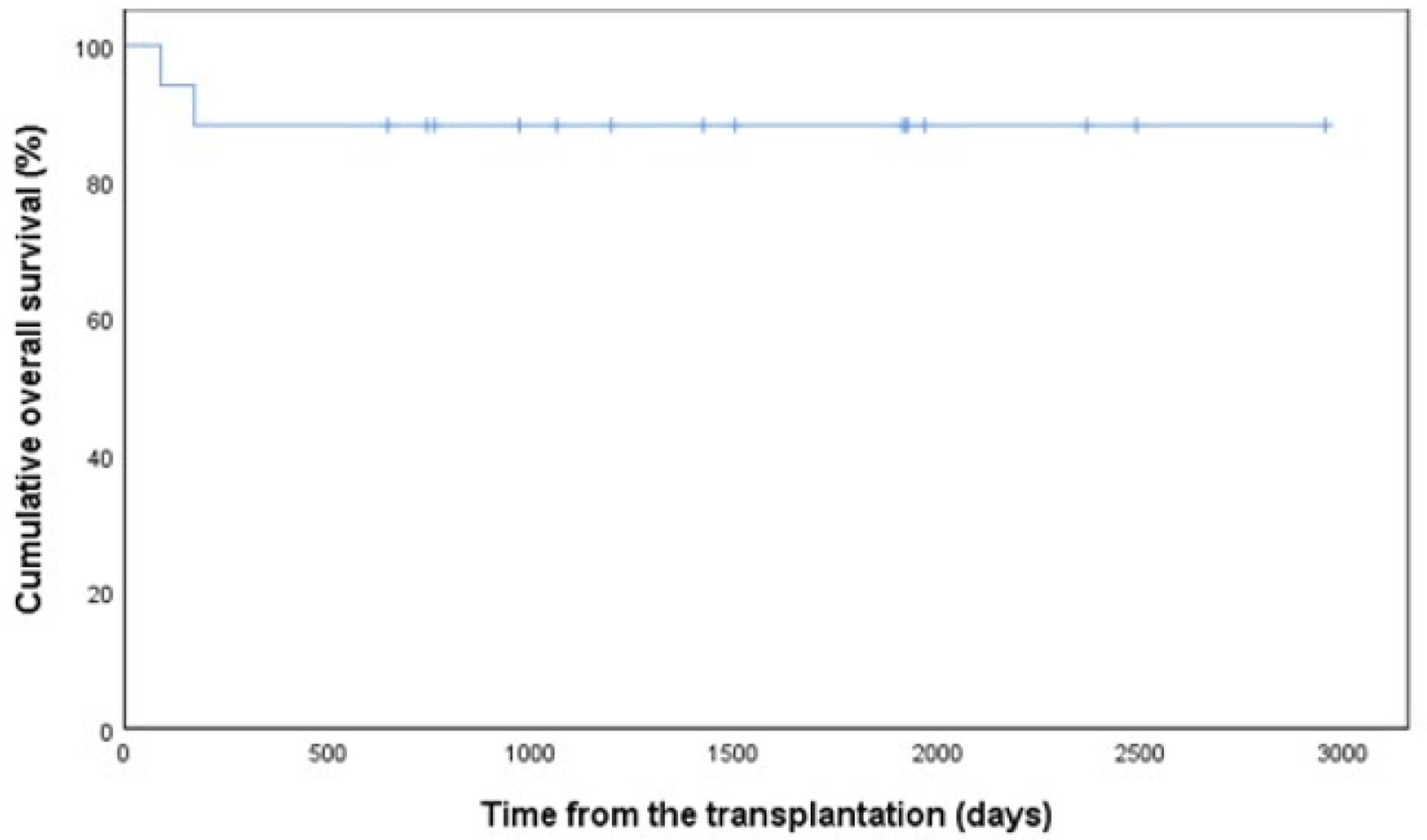
Figures

Tables
| Variables | n = 17 |
|---|---|
| aMissing data in two patients. bMissing data in three patients. BBBD: blood and brain barrier disruption including therapy with intra-arterial mannitol infusion followed by methotrexate and carboplatin and intravenous rituximab, etoposide and cyclophosphamide; Bonn protocol: methotrexate and cytarabine-based chemotherapy; CR: complete remission; CSF: cerebrospinal fluid; DHAP: dexamethasone-cytarabine-cisplatin; FIL: filgrastim; G-CSF: granulocyte colony-stimulating factor; HD-AraC: high-dose cytarabine; LDH: lactate dehydrogenase; LIPEG: lipegfilgrastim; MATRix: methotrexate-cytarabine-thiotepa-rituximab; PCNS: primary central nervous system; PEG: pegfilgrastim; PLER: plerixafor; PR: partial remission; pre-auto-SCT: before autologous stem cell transplantation. | |
| Gender | |
| Female | 10 (59) |
| Male | 7 (41) |
| Age at auto-SCT, years, median (range) | 62 (45 - 73) |
| LDH serum level at diagnosisa | |
| Normal | 10 (59) |
| Elevated | 5 (29) |
| CSF protein concentration at diagnosisb | |
| Normal | 8 (47) |
| Elevated | 6 (35) |
| Involvement of deep region of the brain at diagnosis | |
| No | 7 (41) |
| Yes | 10 (59) |
| Mobilization chemotherapy | |
| BBBD | 11 (64) |
| Bonn block | 2 (12) |
| MATRix | 2 (12) |
| HD-AraC | 1 (6) |
| DHAP | 1 (6) |
| G-CSF used in mobilization | |
| FIL | 11 (65) |
| PEG | 4 (23) |
| LIPEG | 2 (12) |
| Disease status pre-auto-SCT | |
| I CR | 14 (82) |
| I PR | 3 (18) |
| PLER use, n (%) | 7 (41) |
| Variables | n = 17 |
|---|---|
| PCNS: primary central nervous system; WBC: white blood cell. | |
| WBC count (× 109/L) at the time of the first apheresis, median (range) | 32.9 (4.8 - 116.2) |
| Blood CD34+ cell count (× 106/L) at the first apheresis, median (range) | 43 (7 - 139) |
| Peak blood CD34+ cell count (× 106/L), median (range) | 43 (11 - 139) |
| CD34+ cell yield (× 106/kg) of the first apheresis, median (range) | 2.5 (0.6 - 6.9) |
| Total collected CD34+ cell yield (× 106/kg), median (range) | 3.7 (2.3 - 6.9) |
| Number of aphereses (%) | |
| 1 | 9 (53) |
| 2 | 3 (19) |
| 3 | 2 (12) |
| 4 | 3 (19) |
| Variables (× 106/kg), median (range) | n = 10 |
|---|---|
| PCNS: primary central nervous system; 7-AAD: 7-aminoactinomycin; NK: natural killer. | |
| CD34+ cells without 7-AAD | 3.6 (1.5 - 5.3) |
| CD34+ cells with 7-AAD | 2.1 (0.9 - 5.1) |
| CD34+CD133+CD38- cells | 0.07 (0.011 - 0.17) |
| CD3+ cells | 113.2 (67.5 - 253.4) |
| CD3+CD4+ cells | 69.6 (39.5 - 164.1) |
| CD3+CD8+ cells | 41.5 (25.2 - 187.2) |
| CD4+/CD8+ | 1.6 (0.4 - 4.3) |
| CD19+ cells | 0 (0 - 0.3) |
| NK cells | 8.5 (1 - 22.9) |
| Variables | CD34+ w 7-AAD | CD3+ | CD3+CD4+ | CD3+CD8+ | CD34+CD38 | NK | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r | P | r | P | r | P | r | P | r | P | r | P | |
| *P values are statistically significant (< 0.05). CD34+ w 7-AAD: CD34+ with 7-aminoactinomycin; r: Spearman correlation coefficient; PLT: platelet; WBC: white blood cell; auto-SCT: autologous stem cell transplantation; NK: natural killer. | ||||||||||||
| Blood count 15 days after auto-SCT | ||||||||||||
| WBCs (× 109/L) | 0.491 | NS | -0.430 | NS | -0.236 | NS | -0.685 | 0.029* | 0.624 | NS | 0.006 | NS |
| Neutrophils (× 109/L) | 0.714 | 0.047* | -0.762 | 0.028 | -0.476 | NS | -0.905 | 0.002* | 0.643 | NS | 0.048 | NS |
| Lymphocytes (× 109/L) | 0.486 | NS | -0.429 | NS | -0.600 | NS | -0.314 | NS | 0.600 | NS | -0.771 | NS |
| PLTs (× 109/L) | 0.685 | 0.029* | -0.709 | 0.022* | -0.503 | NS | -0.818 | 0.004* | 0.418 | NS | 0.067 | NS |
| Blood count 1 month after auto-SCT | ||||||||||||
| Hb (g/L) | 0.455 | NS | -0.745 | 0.013* | -0.685 | 0.029* | -0.806 | 0.005* | 0.539 | NS | -0.212 | NS |
| WBCs (× 109/L) | 0.164 | NS | 0.091 | NS | -0.115 | NS | -0.115 | NS | 0.139 | NS | 0.006 | NS |
| Neutrophils (× 109/L) | 0.576 | NS | -0.685 | 0.029* | -0.600 | NS | -0.661 | 0.038* | 0.006 | NS | -0.030 | NS |
| Lymphocytes (× 109/L) | -0.285 | NS | 0.628 | NS | 0.310 | NS | 0.485 | NS | 0.033 | NS | -0.042 | NS |
| PLTs (× 109/L) | 0.467 | NS | -0.406 | NS | -0.430 | NS | -0.442 | NS | 0.091 | NS | 0.200 | NS |
| Blood count 3 months after auto-SCT | ||||||||||||
| Hb (g/L) | 0.326 | NS | -0.603 | NS | -0.803 | 0.009* | -0.494 | NS | 0.142 | NS | -0.452 | NS |
| WBCs (× 109/L) | 0.418 | NS | -0.326 | NS | -0.326 | NS | -0.176 | NS | -0.025 | NS | 0.159 | NS |
| Neutrophils (× 109/L) | 0.335 | NS | -0.109 | NS | 0.025 | NS | -0.075 | NS | 0.084 | NS | 0.385 | NS |
| Lymphocytes (× 109/L) | -0.067 | NS | 0.008 | NS | -0.293 | NS | 0.377 | NS | -0.226 | NS | -0.151 | NS |
| PLTs (× 109/L) | 0.533 | NS | -0.433 | NS | -0.383 | NS | -0.583 | NS | -0.050 | NS | 0.317 | NS |
| Blood count 6 months after auto-SCT | ||||||||||||
| Hb (g/L) | 0.217 | NS | -0.167 | NS | -0.400 | NS | -0.317 | NS | 0.217 | NS | -0.433 | NS |
| WBCs (× 109/L) | 0.217 | NS | -0.117 | NS | -0.267 | NS | -0.217 | NS | -0.200 | NS | 0.083 | NS |
| Neutrophils (× 109/L) | 0.288 | NS | 0.198 | NS | 0.036 | NS | 0.090 | NS | -0.090 | NS | 0.505 | NS |
| Lymphocytes (× 109/L) | 0.174 | NS | -0.696 | NS | -0.464 | NS | -0.551 | NS | -0.290 | NS | -0.058 | NS |
| PLTs (× 109/L) | 0.183 | NS | -0.183 | NS | -0.400 | NS | -0.217 | NS | -0.517 | NS | -0.100 | NS |
| Blood count 12 months after auto-SCT | ||||||||||||
| Hb (g/L) | 0.552 | NS | -0.494 | NS | -0.603 | NS | -0.226 | NS | 0.636 | NS | -0.075 | NS |
| WBCs (× 109/L) | -0.050 | NS | 0.133 | NS | -0.117 | NS | 0.017 | NS | -0.333 | NS | -0.283 | NS |
| Neutrophils (× 109/L) | 0.195 | NS | 0.366 | NS | 0.073 | NS | 0.342 | NS | 0.122 | NS | 0.024 | NS |
| Lymphocytes (× 109/L) | -0.539 | NS | 0.946 | < 0.001* | 0.743 | 0.035* | 0.431 | NS | -0.431 | NS | -0.228 | NS |
| PLTs (× 109/L) | 0.233 | NS | 0.050 | NS | -0.017 | NS | -0.167 | NS | -0.367 | NS | 0.333 | NS |