| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Review
Volume 10, Number 3, June 2021, pages 89-97
Role of Venetoclax in the Treatment of Relapsed and Refractory Multiple Myeloma
Hamid Ehsana, g, Ahsan Wahabb, Zunairah Shahc, Muhammad Khawar Sanad, g , Adeel Masoodb, Abdul Rafaee, Hamza Hashmif
aGeorgetown University, Washington, DC, USA
bUniversity of Alabama at Birmingham, Birmingham, AL, USA
cWeiss Memorial Hospital, Chicago, IL, USA
dDepartment of Internal Medicine, King Edward Medical University, Lahore, Pakistan
eDepartment of Internal Medicine, McLaren Flint-Michigan State University, MI, USA
fDivision of Hematology Oncology, Medical University of South Carolina, Charleston, SC, USA
gCorresponding Author: Hamid Ehsan, Department of Medicine, MedStar Good Samaritan Hospital, MedStar Union Memorial Hospital, Baltimore, MD, USA; Muhammad Khawar Sana, Department of Internal Medicine, King Edward Medical University, Lahore, Pakistan
Manuscript submitted April 21, 2021, accepted May 4, 2021, published online June 16, 2021
Short title: Venetoclax in RRMM
doi: https://doi.org/10.14740/jh844
| Abstract | ▴Top |
Biomarker-driven targeted therapies have been an area of exploration for innovative therapeutic options in oncology. B-cell lymphoma-2 (BCL-2) protein is an anti-apoptotic protein expressed on the clonal plasma cells in patients with multiple myeloma (MM). MM subsets with t (11;14) have overexpression of BCL-2 and can benefit from venetoclax (VEN) when used either alone or in combination with other chemotherapeutic agents with an overall response rate (ORR) ranging from 40% to 100%. The most commonly reported grade ≥ 3 adverse effects include cytopenias and gastrointestinal side effects. This review highlights the meaningful efficacy and tolerable safety of VEN monotherapy and its combination regimens in the treatment of relapsed refractory MM.
Keywords: Venetoclax; Multiple myeloma; Relapsed; Refractory
| Introduction | ▴Top |
Multiple myeloma (MM) is a chronic, incurable hematological malignancy of clonal plasma cells. It is usually a disease of the elderly, with a median age of 65 years at the time of diagnosis [1, 2]. It is characterized by the uncontrolled proliferation of malignant plasma cells and is accompanied by the accumulation of abnormal immunoglobulin (Ig) M proteins that are often detectable in the serum and urine. Accumulation of abnormal proteins and malignant plasma cells can cause end-organ damage and eventually bone marrow failure [1, 2]. Significant improvements in overall response rate (ORR) and progression-free survival (PFS) have been seen with combinations of drugs such as immunomodulatory drugs (IMiDs), proteasome inhibitors (PIs), monoclonal antibodies, and dexamethasone (Dex) [3-5]. Despite recent advances in drug therapies, treatment of relapsed refractory MM (RRMM) continues to be challenging [5]. New mutations and genetic alterations acquired during the disease course often lead to chemotherapy resistance and progressively shorter duration of response (DOR) to subsequent therapy [6]. Hence, the use of advanced genetic analysis to explore targeted therapies with improved efficacy and toxicity profiles is an area of great interest in the treatment landscape of RRMM [7]. Venetoclax (VEN or ABT-199), a B-cell lymphoma-2 (BCL-2) homology 3 (BH3)-mimetic small molecule capable of binding to and antagonizing protein BCL-2, is one such example. As BCL-2 is an anti-apoptotic protein, inhibition of BCL-2 by VEN leads to the activation of apoptosis and induction of malignant plasma cell death as shown in Figure 1 [8]. VEN has shown anti-tumor effects in patients with relapsed and refractory acute myeloid leukemia (AML), follicular lymphoma (FL), diffuse large B-cell lymphoma (DLBCL), acute lymphoblastic leukemia (ALL), mantle cell lymphoma (MCL) and chronic lymphocytic leukemia (CLL) with promising results [8]. About 20% of the patients with MM have t (11;14) with overexpression of BCL-2 [9]. In preclinical studies, VEN has shown activity against MM cell lines, especially in subsets with t (11;14) [10]. The co-treatment of human myeloma cells with Dex and VEN significantly increased cell death when compared with VEN alone in four of the five cell lines involved, as well as the patient samples tested [11]. The underlying mechanism appears to be an increase in the expression of two pro-apoptotic molecules BCL-2 and its mediator with the addition of Dex, leading to striking efficacy seen with this combination [11]. When studied in human subjects, there is increasing clinical evidence of efficacy and safety of VEN in RRMM patients, with a superior depth and DOR in patients with t (11:14). Given superior efficacy and tolerable safety of monotherapy, VEN is currently being investigated in combination regimens with IMiDs, PIs, monoclonal antibodies and Dex.
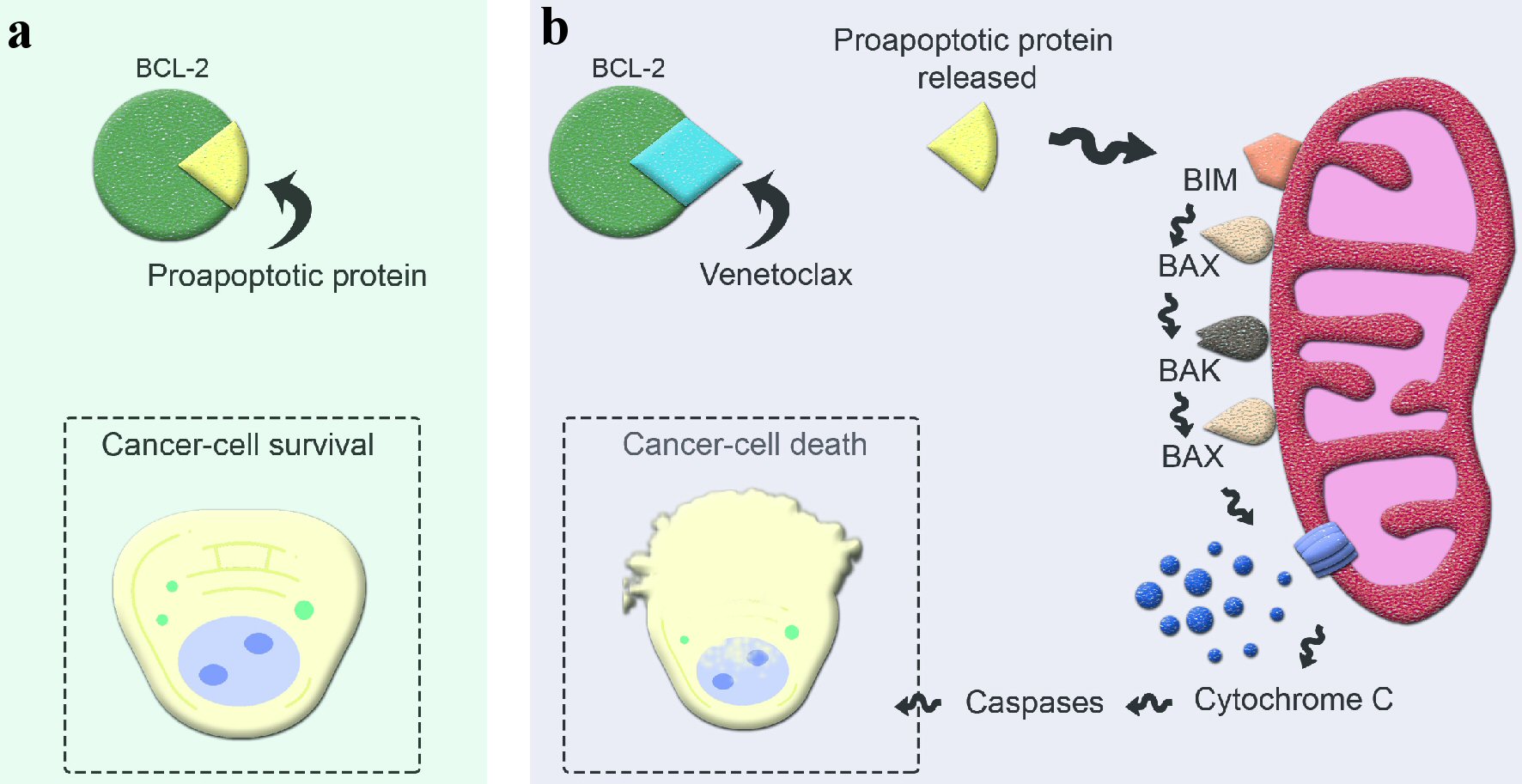 Click for large image | Figure 1. Mechanism of action of venetoclax in apoptosis. (a) Excessive BCL-2 production by cancer cells sequesters proapoptotic protein and evades apoptosis. (b) Venetoclax, a selective BCL-2 inhibitor, binds to BCL-2 and releases the proapoptotic protein, initiating the apoptosis cascade. BCL-2: B-cell lymphoma-2; BIM: BCL-2 interacting mediator; BAX: BCL-2 associated X protein; BAK: BCL-2 antagonist/killer protein. |
In this review, we discuss the emerging role of VEN in RRMM and ongoing clinical trials. The search for the relevant articles was conducted in PubMed, Medline and Clinicaltrials.gov by combining the medical subject headings (MeSHs) terms for “multiple myeloma”, “ABT-199” and their entry terms. The articles retrieved from the search were then screened for retrospective observational studies and completed/ongoing clinical trials investigating VEN either alone or in combination with other antineoplastic agents in RRMM. Tables 1, 2, and 3 [12-20] provide details on the study characteristics, regimen efficacy, and regimen toxicity for published clinical trials and retrospective studies. Table 4 highlights ongoing clinical trials from https://clinicaltrials.gov/ that are investigating VEN in various combinations.
 Click to view | Table 1. Study Characteristics of the Included Articles |
 Click to view | Table 2. Efficacy of Venetoclax-Based Regimens in the Treatment of Multiple Myeloma |
 Click to view | Table 3. Safety of Venetoclax-Based Regimens in the Treatment of Multiple Myeloma |
 Click to view | Table 4. Ongoing Clinical Trials of Venetoclax-Based Regimens in Relapsed Refractory Multiple Myelomaa |
| Clinical Trials | ▴Top |
VEN monotherapy
Kumar et al evaluated the safety, pharmacokinetics, and maximum tolerated dose (MTD) of VEN in an open-label phase I (P-1) trial (n = 66, median age 63 years (yrs), range 31 - 79). All patients had measurable disease at baseline and had an Eastern Cooperative Oncology Group (ECOG) performance status of 0 - 1. A total of 30 patients (46%) in this trial had t (11;14). Thirty patients were in the dose-escalation cohort (3 + 3 design, VEN at doses of 300 mg, 600 mg, 900 mg, or 1,200 mg), while 36 patients were in the safety expansion cohort (VEN dose 1,200 mg). All the patients in the dose-escalation cohort had at least one prior line of therapy and the majority of the patients (61%) were refractory to both bortezomib and lenalidomide. In the safety expansion cohort, all patients had received treatment with both PIs and IMiDs. For the entire cohort, ORR was 21% (n = 14) and very good partial response (VGPR) of 15%. ORR among the subset of patients with t (11;14) was 40% whereas VGPR was 27%. The median DOR was similar among all patients versus those with t (11;14), i.e., 9.7 months. However, time to progression (TTP) was longer for t (11;14) subset vs. entire cohort, 6.6 months (95% confidence interval (CI): 3.9 - 10.2 months) vs. 2.6 months (95% CI: 1.9 - 4.7), respectively. Gastrointestinal toxicity, notably nausea (47%), was the most common adverse event (AE) whereas the most common grade (G)-3 or 4 AEs were hematologic in nature, including thrombocytopenia (26%), neutropenia (21%), anemia/leukopenia (14% each) [12].
VEN combination therapies
VEN and Dex
An open-label ongoing P-1/P-2 study reported by Kaufman et al comprised of two phases, P-1 with dose-escalation of VEN + Dex and P-2 with dose expansion of the same regimen. The investigators enrolled 20 patients with RRMM in P-1 (85% males, median age of 63 yrs, range 46 - 77) and 31 patients with RRMM in P-2 (58% males, with median age of 65 yrs, range 48 - 80). All patients were positive for t (11;14) as determined by fluorescence in situ hybridization (FISH). The median number of prior lines of therapy in P-1 and P-2 was 2.5 (range 1 - 7) and 5 (range 1 - 9), respectively. Oral VEN 800 mg/day and oral Dex 40 mg on days 1, 8, and 15 of each 21-day cycle were given to patients in both phases. ORR was 65% (n = 13) in P-1 and 45% (n = 16) in P-2, whereas VGPR was 30% (n = 6) and 26% (n = 8) for P-1 and P-2, respectively. The median time to the first response was 1.4 months and 0.7 months in the P-1 and P-2, respectively. The most common AEs were insomnia (45%) and hypophosphatemia (40%) in P-1, and diarrhea/lymphopenia (32% for each), and nausea (26%) in P-2. Tumor lysis syndrome and sepsis were the most serious AEs for P-1 and P-2, respectively. Neutropenia, lymphopenia, thrombocytopenia, and hypophosphatemia were the most common G-3/4 AEs as a whole. Median PFS was 12.4 months (95% CI: 3.6 - 20.9) for P-1 but was not reached for P-2. At a median of 9 months, PFS for P-2 was 57% (95% CI: 19% - 82%) [13].
VEN, bortezomib and Dex
P-1b trial by Moreau et al enrolled 66 patients with RRMM (median age = 64 yrs), 54 in the dose-escalation cohort and 12 in the safety expansion cohort [14]. All patients had received a median of 3 (1 - 13) prior lines of therapies. About 53% (n = 35) of the patients were refractory to prior bortezomib therapy, and 39% (n = 26) were refractory to lenalidomide. Patients were given VEN once daily (50 - 1,200 mg in designated dose cohort), subcutaneous bortezomib (1.3 mg/m2, days 1, 4, 8, and 11 during cycles 1 - 8 and days 1, 8, 15, and 22 during cycles 9 - 11), and oral Dex (20 mg, days 1, 2, 4, 5, 8, 9, 11, 12 during cycles 1 - 8 and on days 1, 8, 15, and 22 during cycles 9 - 11 ) for 11 cycles. Patients who remained on the study beyond 11 cycles received VEN monotherapy. ORR was 67% (n = 44) and VGPR or better was 42% (n = 28). Overall, 21% of the patients (n = 14) had disease progression. Median TTP and median DOR was 9.5 months and 9.7 months, respectively. Bortezomib non-refractory patients had much better ORR (90% vs. 31%) and VGPR (64% vs. 8%) when compared to bortezomib refractory patients. Correspondingly, median TTP (11.3 months vs. 1.8 months) and median DOR (10.2 months vs. 4.2 months) were longer for bortezomib non-refractory vs. refractory patients. On the other hand, lenalidomide refractoriness had less impact on ORR, with ORR of 60% in refractory vs. 72% in the non-refractory group. Gastrointestinal side effects were most common, including diarrhea (46%), constipation (41%) and nausea (38%). Common G-3/4 AEs included thrombocytopenia (29%) and anemia (15%) [14]. BELLINI, a multicenter P-3 double-blind randomized trial, evaluated the role of VEN vs. placebo (Pbo) + bortezomib-Dex in 291 patients with RRMM patients having received 1 - 3 prior lines of therapies [15]. With 2:1 randomization, 194 patients (median age = 66 yrs, 16% high-risk) were randomized to the VEN (800 mg) + bortezomib-Dex arm, and 97 patients (median age = 65 yrs, 19% high-risk) to Pbo + bortezomib-Dex. The VEN and the Pbo arm had 10% and 15% of the patients positive for t (11;14) by FISH, respectively. A high BCL-2 expression (measured by immunohistochemical analysis on core biopsies) was seen in 78% of patients in the VEN arm vs. 81% in the Pbo arm. The dose of VEN could be reduced in a stepwise fashion in case of excessive toxicity. Bortezomib (1.3 mg/m2) was given subcutaneously or intravenously on days 1, 4, 8 and 11 during cycles 1 - 8 (21-day cycle), and days 1, 8, 15 and 22 during cycles 9 - 11 (35-day cycle). Dex (20 mg) was given orally on days 1, 2, 4, 5, 8, 9, 11, 12 during cycles 1 - 8 and on days 1, 2, 8, 9, 15, 16, 22 and 23 during cycles 9 - 11. At a median follow-up of 28.6 months, 33% (n = 64) deaths were reported in the VEN arm vs. 25% in the Pbo (n = 24) arm. Median PFS at 18.7 months (median) was 22.4 months (95% CI: 15.3 - not estimable) with VEN vs. 11.5 months (95% CI: 9.6 - 15.0) with Pbo arm, hazard ratio (HR): 0.63 (95% CI: 0.44 - 0.90). Median OS was not reached in either of the arms, but the proportion of survival events was worse for the VEN arm vs. Pbo arm, 21% vs. 11%, HR: 2.03 (95% CI: 1.04 - 3.95). Median PFS among patients with high BCL-2 expression was 22.4 months in the VEN arm vs. 9.9 months in the Pbo arm, HR: 0.24 (95% CI: 0.12 - 0.48). Median PFS among those with t (11;14) was not reached for VEN arm vs. 9.5 months for the Pbo arm, HR: 0.11, 95% CI: 0.02 - 0.56. The common AEs due to VEN were diarrhea (59%), nausea (37%), and constipation (35%). Most common G-3/4 AEs (VEN/Pbo) were neutropenia (21%/8%), thrombocytopenia (15%/30%), anemia (16%/15%), diarrhea (15%/12%) and pneumonia (18%/13%) [15].
VEN, carfilzomib and Dex
A P-2 study published by Costa et al in 2018, enrolled 42 patients with RRMM (median age = 67 yrs, range 37 - 69 yrs) with ECOG ≤ 2 to study the safety and tolerability of VEN, carfilzomib and Dex combination. Part 1 was a dose-escalation phase and part 2 was a dose-expansion phase with selected doses. Patients had received 1 - 3 prior lines of therapy and those with prior refractoriness to PIs were allowed. Patients in cohort 1 (n = 4) received treatment with VEN 400 mg/day plus carfilzomib 27 mg/m2 on days 1, 2, 8, 9, 15, 16 and Dex 40 mg on days 1, 8, 15, 22. Cohort 2 (n = 3) received VEN 800 mg/day but the schedule and doses of carfilzomib and Dex were the same as in cohort 1. In cohort 3/expansion cohort (n = 20), patients received VEN 800 mg/day plus carfilzomib 70 mg/m2 on days 1, 8, 15 and Dex 40 mg on days 1, 8, 15, 22. Cohort 4 (n = 3) received VEN 800 mg/day plus carfilzomib 56 mg/m2 on days 1, 2, 8, 9, 15, 16 and Dex 20 mg on days 1, 2 , 8, 9, 15, 16, 22, 23. In this trial, 29% of the patients (n = 12) discontinued treatment, 7% (n = 3) due to progressive disease, 2% (n = 1) due to AEs, 5% (n = 2) due to withdrawal of consent, 7% (n = 3) due to lack of efficacy and 7% (n = 3) due to death. ORR was 83% in 30 evaluable patients (stringent complete response (sCr) 7%, CR 17% and VGPR 33%). Major AEs due to this therapy were diarrhea in 57% of the patients (n = 24) and fatigue in 41% of the patients (n = 17). Major G-3/4 AE was lymphopenia (24%, n = 10) [16].
VEN, daratumumab and Dex
An ongoing P-2 study reported by Kaufman et al enrolled 48 patients with RRMM. In part 1, 24 patients (median age = 63, range 51 - 76) with t (11;14) RRMM who had received at least one prior line of therapy (IMiDs and PI) were treated with VEN + daratumumab + Dex (VEN-Dd). In part 2, 24 patients (median age = 65, range 41 - 80) irrespective of t (11;14) status and non-refractory to PIs received VEN + daratumumab + bortezomib + Dex (VEN-DVd). Median follow-ups with VEN-Dd and VEN-DVd were 10 and 9 months, respectively. ORR and VGPR or better in VEN-Dd group were 96% for both, whereas ORR and ≥ VGPR in VEN-DVd group were 92% and 79%, respectively. The median DOR and PFS were not reached for either group. Fatigue was the most commonly reported AE in the VEN-Dd group (75%) whereas nausea and insomnia were the most commonly reported AEs in the VEN-DVd group. Neutropenia was the most common G-3 or higher AE (17%) in the VEN-Dd group. Other AEs are outlined in Table 3 [17].
| Retrospective Studies | ▴Top |
In a retrospective study, investigators evaluated 56 patients with RRMM (75% with t (11:14), n = 42), who received VEN as monotherapy or in combination (not specified) between December 2016 to March 2019 at the Mayo Clinic. The median number of previous therapies was 6 (range 1 - 15), and 79% (n = 44) of the patients had a history of prior autologous stem cell transplantation (ASCT). VEN alone or in combination with Dex was used in 55% of the patients (n = 31), whereas 45% (n = 25) of the patients received VEN in combination with PIs, IMiDs, daratumumab as triplet or quadruplet therapy. Amongst 52 evaluable patients, ORR was 44%. Among patients with t (11;14), ORR was 49% compared to 31% in patients without t (11;14). Those with high BCL-2 expression had a superior ORR compared to those without high expression, i.e., 59% vs. 8%. Median OS was not reached (95% CI: 12 - not reached (NR)) but median PFS was 5.6 months (95% CI: 4.8 - 9.9). PFS and OS were better among those with the presence of t (11;14) vs. those without t (11;14); median PFS 5.7 months vs. 4.2 months, and median OS not reached vs. 10.8 months for t (11;14) positive and negative patients, respectively. At the last follow-up, 14 (25%) patients had died. All of those patients had received VEN as the last anti-myeloma agent [18].
Kambhampati et al performed a retrospective study of 47 patients (high-risk, n = 8; t (11;14), n = 18) with RRMM who had a median of 7 prior lines of therapy. Forty-one (87%) patients received VEN + PIs ± Dex. VEN dose was 800 mg in 68% of the patients (n = 32), < 800 mg in 30% of the patients (n = 14) and > 800 mg in only 2% of the patient (n = 1). ORR was 39% with a VGPR 13%. In the t (11;14) cohort, ORR was 71%, with a VGPR of 24%. The median PFS was 2.1 months with an OS of 15.6 months. Two patients (4%) experienced a serious AE including pneumonia and cytomegalovirus (CMV) pneumonitis. There was one treatment-related death due to infection [19].
In a small retrospective study, Basali et al reported the safety and efficacy of VEN-based therapy in 10 RRMM patients with t (11:14), who had received a median of 6 (2 - 19) prior lines of therapy. Fifty percent of the patients (n = 5) had undergone prior ASCT. The most common regimen was VEN-bortezomib-Dex (6/10). Amongst nine evaluable patients, ORR was 78% (n = 7) with VGPR and CR of 11% each. As calculated by the Kaplan-Meier method, 6-month OS was 77%, and 6-month PFS was 28% [20].
| Discussion | ▴Top |
VEN has emerged as the first targeted therapeutic option in patients with RRMM, both as a single agent and in combination with other drugs. While VEN appears to be particularly effective in patients with t (11;14), which is seen in about 20% of newly diagnosed myeloma patients and is associated with high BCL-2 expression [9], several important questions remain unanswered about the potential role of this agent in the treatment of RRMM.
Based on the published data highlighted in this review article, it appears that patients with or without t (11;14) seem to gain benefit from VEN-based therapy, although more in patients with the translocation. It seems obvious that combination therapy with VEN yields deeper responses with ORRs of 78-100% than VEN monotherapy (ORR of 40%) [13-16]. However, there remains a major concern that patients without t (11;14) appear to have an increased risk of mortality and inferior overall survival [15]. In the P-3 BELLINI trial, the addition of VEN to bortezomib and Dex reported increased mortality mainly in the VEN arm [21]. The detailed mechanism and underlying etiologies for this increased mortality are not clearly known at this time. Whether it is related to the increased risk of infection with BCL-2 inhibition or change in the biological behavior of the disease at the time of disease relapse, or both, remains unknown. Hence, it is not advisable to use VEN-based therapy for patients without t (11;14), especially as patients with heavily pretreated RRMM have severely compromised immunity and continue to remain at the risk of life-threatening infections as a major cause of mortality. Besides this increased risk of infections and mortality, the most common toxicities are gastrointestinal and hematological in nature and the overall toxicity profile for VEN appears to be reasonable and easily manageable. Tumor lysis syndrome that is typically seen in patients with CLL receiving VEN-based therapy has not been seen in patients with RRMM. However, the use of VEN should be cautiously pursued in patients with the extramedullary disease and plasma cell leukemia, as they are at higher risk of tumor lysis syndrome. Similarly, optimal dosing for VEN when used in monotherapy or combination with other chemotherapeutic agents has not been clearly defined. Clinical trials have used doses of VEN ranging from 400 to 1,200 mg/day. VEN at higher doses is associated with more significant myelosuppression, especially in patients with AML [22]. Significant myelosuppression in heavily pretreated RRMM patients amplifies the risk of life-threatening infections, as suggested by the BELLINI trial. Although these toxicities may be mitigated by using lower doses of VEN, it is not entirely clear whether lower doses will be as effective as the higher doses that have been typically explored in clinical trials. It is important to highlight that currently, there are no clinical guidelines on any additional supportive care with VEN use including surveillance for infections, use of antimicrobial and antifungal prophylaxis and dose modifications for major drug-drug interactions. Hence, it is important to analyze real-world data on dosing and toxicity management of VEN-based therapy to safely administer this very effective therapy in a selected patient population. This will be an important step before continuing to explore the role of VEN in future clinical trials.
Given remarkable efficacy in patients with t (11;14), it is imperative that FISH panel testing for t (11;14) become a standard practice for newly diagnosed MM. Although, t (11;4) is considered a primary cytogenetic abnormality that can be seen in about 20% of the patients with newly diagnosed MM patients [9], whether patients should undergo bone marrow biopsies to evaluate for this translocation at that time of disease relapse is not entirely clear, especially if they were not found to have the translocation at the time of initial diagnosis. It is important to highlight that several biomarkers may be able to predict sensitivity to VEN. In vitro and in vivo sensitivity to VEN has been observed in plasma cells harboring t (11;14), which is associated with high BCL-2 expression and a low myeloid cell leukemia-1 (MCL-1) or BCL-XL expression (high BCL-2 to MCL-1 ratio) [10]. The expression of BCL-2 family members in MM is variable, with upregulation of either MCL-1 or BCL-XL that can lead to VEN resistance [10]. In a clinical study, a high BCL2/BCL2L1 gene expression ratio was associated with improved ORR of 80% and median TTP of 11.5 months in patients with t (11:14) when treated with VEN monotherapy [12]. The underlying mechanism of this association between t (11;14) and BCL-2 expression remains unknown. Although FISH testing for t (11;14) continues to remain the surrogate marker for high BCL-2 expression, whether laboratory assays of BCL-2 expression or BCL-2/MCL-1 ratio can serve as readily available real-time biomarkers (with or without t (11;14)) predictive of sensitivity to VEN in clinical practice needs to be determined. Gupta et al studied a panel of 31 myeloma cell lines and using ribonucleic acid (RNA) sequencing analysis identified an enhanced expression of B-cell genes in patients sensitive to VEN. Moreover, a panel of cell surface markers (i.e., cluster of differentiation (CD)20, CD79a) correlated well with the ex vivo sensitivity of myeloma cell lines to VEN. The authors concluded that B-cell gene expression and cell surface markers may serve as better biomarkers for predicting response to VEN than t (11;14) alone [23].
The concept of minimal residual disease (MRD) status is currently being utilized more often due to advancement in next-generation sequencing, flow cytometry and polymerase chain reaction (PCR) techniques [24]. The response criteria based on MRD status is now considered one of the most important prognostic factors and is predictive of subsequent relapse. There is improved PFS and OS in MM patients who achieve MRD-negative or low-level MRD status with upfront therapy with or without ASCT. In the BELLINI trial, the addition of VEN to bortezomib was associated with deep and durable responses, including higher rates of MRD negativity. This MRD negativity was associated with prolonged PFS in patients with RRMM [21]. With ongoing clinical trials (Table 4), we will have more data on VEN in regards to an optimal dose that has maximal efficacy with minimal side effects. Utilizing advances in the genomic analysis will help us better understand the synergistic effect when VEN is given in combination with other chemotherapeutic agents. With better understanding from P-1/2 studies, P-3 clinical trials comparing VEN-based therapy with other novel chemotherapeutic options for their efficacy and safety endpoints will help guide appropriate timing and duration of use of VEN-based therapy. With the increasing use of VEN in clinical practice, the “real-world experience” will guide dosing, schedule and supportive care for clinicians in the community-based practice.
| Conclusions | ▴Top |
VEN is an active and well-tolerated agent in patients with heavily pretreated RRMM with t (11:4), and can produce durable responses as a single agent and in combination regimens. Major toxicities include hematological and gastrointestinal side effects that are overall manageable. Prospective randomized controlled trials, as well as real-world data on the use of VEN as monotherapy and in combination with other chemotherapeutic agents in a carefully selected patient population, will help us better determine its role as a targeted therapy in the armamentarium for patients with RRMM where the options are limited.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Author Contributions
HE, AW, ZS contributed to the conception of the idea and the acquisition of data. MKS, AM, AR, HH contributed to the interpretation of data for the work. All authors contributed to drafting the work, revising it critically for important intellectual content, final approval of the version to be published, agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
BCL-2: B-cell lymphoma-2; MM: multiple myeloma; VEN: venetoclax; ORR: overall response rate; Ig: immunoglobulin; PFS: progression-free survival; OS: overall survival; IMiDs: immunomodulatory drugs; PIs: proteasome inhibitors; Dex: dexamethasone; RRMM: relapsed refractory multiple myeloma; DOR: duration of response; BH3: BCL-2 homology 3; AML: acute myeloid leukemia; FL: follicular lymphoma; DLBCL: diffuse large B-cell lymphoma; ALL: acute lymphoblastic leukemia; MCL: mantle cell lymphoma; CLL: chronic lymphocytic leukemia; MTD: maximum tolerated dose; ECOG: Eastern Cooperative Oncology Group; VGPR: very good partial response; sCR: stringent complete response; CR: complete response; TTP: time to progression; AE: adverse event; G: grade; P-1: phase I; P-2: phase II; FISH: fluorescence in situ hybridization; Pbo: placebo; Yrs: years; HR: hazard ratio; CI: confidence interval; VEN-Dd: venetoclax + daratumumab + dexamethasone; VEN-DVd: venetoclax + daratumumab + bortezomib + dexamethasone; ASCT: autologous stem cell transplantation; NR: not reached; CMV: cytomegalovirus; MCL-1: myeloid cell leukemia-1; BCL2L1: BCL2-like 1; RNA: ribonucleic acid; CD: cluster of differentiation; MRD: minimal residual disease
| References | ▴Top |
- Bataille R, Harousseau JL. Multiple myeloma. N Engl J Med. 1997;336(23):1657-1664.
doi pubmed - Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364(11):1046-1060.
doi pubmed - Moreau P. How I treat myeloma with new agents. Blood. 2017;130(13):1507-1513.
doi pubmed - Kumar SK, Dispenzieri A, Lacy MQ, Gertz MA, Buadi FK, Pandey S, Kapoor P, et al. Continued improvement in survival in multiple myeloma: changes in early mortality and outcomes in older patients. Leukemia. 2014;28(5):1122-1128.
doi pubmed - Chim CS, Kumar SK, Orlowski RZ, Cook G, Richardson PG, Gertz MA, Giralt S, et al. Management of relapsed and refractory multiple myeloma: novel agents, antibodies, immunotherapies and beyond. Leukemia. 2018;32(2):252-262.
doi pubmed - Egan JB, Shi CX, Tembe W, Christoforides A, Kurdoglu A, Sinari S, Middha S, et al. Whole-genome sequencing of multiple myeloma from diagnosis to plasma cell leukemia reveals genomic initiating events, evolution, and clonal tides. Blood. 2012;120(5):1060-1066.
doi pubmed - Morgan GJ, Rasche L. Maintaining therapeutic progress in multiple myeloma by integrating genetic and biological advances into the clinic. Expert Rev Hematol. 2018;11(7):513-523.
doi pubmed - Bose P, Gandhi V, Konopleva M. Pathways and mechanisms of venetoclax resistance. Leuk Lymphoma. 2017;58(9):1-17.
doi pubmed - Wu H, Zhang H, He HY, Jiang H, Zhao YY, An R, He J, et al. [Cytogenetic abnormalities and prognosis of 532 patients with multiple myeloma]. Zhonghua Xue Ye Xue Za Zhi. 2017;38(9):739-743.
- Punnoose EA, Leverson JD, Peale F, Boghaert ER, Belmont LD, Tan N, Young A, et al. Expression profile of BCL-2, BCL-XL, and MCL-1 predicts pharmacological response to the BCL-2 selective antagonist venetoclax in multiple myeloma models. Mol Cancer Ther. 2016;15(5):1132-1144.
doi pubmed - Matulis SM, Gupta VA, Nooka AK, Hollen HV, Kaufman JL, Lonial S, Boise LH. Dexamethasone treatment promotes Bcl-2 dependence in multiple myeloma resulting in sensitivity to venetoclax. Leukemia. 2016;30(5):1086-1093.
doi pubmed - Kumar S, Kaufman JL, Gasparetto C, Mikhael J, Vij R, Pegourie B, Benboubker L, et al. Efficacy of venetoclax as targeted therapy for relapsed/refractory t (11;14) multiple myeloma. Blood. 2017;130(22):2401-2409.
doi pubmed - Kaufman JL, Gasparetto C, Schjesvold FH, Moreau P, Touzeau C, Facon T, et al. Phase I/II study evaluating the safety and efficacy of venetoclax in combination with dexamethasone as targeted therapy for patients with t (11;14) relapsed/refractory multiple myeloma. Blood. 2019;134(Supplement_1):926.
doi - Moreau P, Chanan-Khan A, Roberts AW, Agarwal AB, Facon T, Kumar S, Touzeau C, et al. Promising efficacy and acceptable safety of venetoclax plus bortezomib and dexamethasone in relapsed/refractory MM. Blood. 2017;130(22):2392-2400.
doi pubmed - Kumar SK, Harrison SJ, Cavo M, de la Rubia J, Popat R, Gasparetto C, Hungria V, et al. Venetoclax or placebo in combination with bortezomib and dexamethasone in patients with relapsed or refractory multiple myeloma (BELLINI): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2020;21(12):1630-1642.
doi - Costa LJ, Stadtmauer EA, Morgan G, Monohan G, Kovacsovics T, Burwick N, et al. Phase 2 Study of venetoclax plus carfilzomib and dexamethasone in patients with relapsed/refractory multiple myeloma. Blood. 2018;132(Supplement 1):303.
doi - Kaufman JL, Baz RC, Harrison SJ, Quach H, Ho S-J, Vangsted AJ, et al. Updated analysis of a phase I/II study of venetoclax in combination with daratumumab and dexamethasone, +/- bortezomib, in patients with relapsed/refractory multiple myeloma. J Clin Oncol. 2020;38(15_suppl):8511.
doi - Sidiqi MH, Al Saleh A, Lee J, Jevremovic D, Fonseca R, Gertz M, et al. Venetoclax for the treatment of multiple myeloma: outcomes outside of clinical trials. Clinical Lymphoma Myeloma and Leukemia. 2019;19:e278-e279.
doi - Kambhampati S, Galligan D, Huang CY, Wong S, Wolf J, Martin T, Shah N. A single-center retrospective cohort analysis of venetoclax in relapsed/refractory multiple myeloma. Leuk Lymphoma. 2020;61(5):1211-1219.
doi pubmed - Basali D, Chakraborty R, Rybicki L, Rosko N, Reed J, Karam M, Schlueter K, et al. Real-world data on safety and efficacy of venetoclax-based regimens in relapsed/refractory t (11;14) multiple myeloma. Br J Haematol. 2020;189(6):1136-1140.
doi pubmed - Kumar S, Harrison SJ, Cavo M, de La Rubia J, Popat R, Gasparetto C, et al. Updated results from BELLINI, a phase III study of venetoclax or placebo in combination with bortezomib and dexamethasone in relapsed/refractory multiple myeloma. J Clin Oncol. 2020;38(15_suppl):8509.
doi - DiNardo CD, Jonas BA, Pullarkat V, Thirman MJ, Garcia JS, Wei AH, Konopleva M, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. 2020;383(7):617-629.
doi pubmed - Gupta VA, Barwick BG, Matulis SM, Shirasaki R, Jaye DL, Keats J, Oberlton B, et al. Venetoclax sensitivity in multiple myeloma is associated with B cell gene expression. Blood. 2021.
doi pubmed - Sanchez R, Ayala R, Martinez-Lopez J. Minimal residual disease monitoring with next-generation sequencing methodologies in hematological malignancies. Int J Mol Sci. 2019;20(11):2832.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


