| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Case Report
Volume 9, Number 4, December 2020, pages 140-146
Molecular Genetic Analysis With Flow Cytometry Sorting Identifies Angioimmunoblastic T-Cell Lymphoma and Concomitant De Novo Myelodysplastic Syndrome Arising From the Same Hematopoietic Progenitor
Ken Naganumaa, Alexander Chanb, Yanming Zhangc, Natasha Lewisb, Wenbin Xiaob, Mikhail Roshalb, Ahmet Doganb, Masahiro Kizakia, Caleb Hob, d, Mariko Yabeb, e
aDepartment of Hematology, Saitama Medical Center, Saitama Medical University, Saitama, Japan
bHematopathology Service, Department of Pathology, Memorial Sloan Kettering Cancer Center, New York, NY, USA
cCytogenetics Laboratory, Department of Pathology, Memorial Sloan Kettering Cancer Center, New York, NY, USA
dDiagnostic Molecular Pathology Service, Department of Pathology, Memorial Sloan Kettering Cancer Center, New York, NY, USA
eCorresponding Author: Mariko Yabe, Hematopathology Service, Department of Pathology, Memorial Sloan Kettering Cancer Center, 1275 York Avenue, S-618, New York, NY 10065, USA
Manuscript submitted September 28, 2020, accepted October 23, 2020, published online November 6, 2020
Short title: AITL and MDS Arising From the Same Precursor
doi: https://doi.org/10.14740/jh760
| Abstract | ▴Top |
A 75-year-old man with no prior history of cytotoxic therapy presented with increasing fatigue and shortness of breath. He was found to have a new onset of pancytopenia, and chest X-ray showed severe pneumonia. Additional radiology exam revealed pan-lobar pneumonia, pleural effusion, generalized lymphadenopathy and mild splenomegaly. Bone marrow and mediastinal lymph node biopsy from the bilateral level 4 lymph nodes were performed to evaluate the cause of pancytopenia and generalized lymphadenopathy, respectively. Histologic sections of lymph nodes were consistent with angioimmunoblastic T-cell lymphoma (AITL), and bone marrow biopsy showed low level involvement by AITL. Background trilineage hematopoiesis showed features suggestive of myelodysplastic syndrome (MDS) with karyotyping showing deletion 20q; however, interpretation of dysplasia and exclusion of reactive process was difficult due to the presence of severe infection, administration of multiple medications and multiorgan failure. Therefore, to further evaluate the possibility of concomitant myeloid neoplasm, we performed flow cytometry sorting of bone marrow aspirate to isolate the myeloid cell population from the abnormal T-cell population, and comprehensive genomic profiling was performed in each population separately. Flow-sorted myeloid population showed three somatic mutations involving DNMT3A and BCORL1, supporting the diagnosis of MDS in conjunction with the presence of deletion 20q. Flow sorted abnormal T-cell population showed six somatic mutations consistent with AITL, involving Ras homolog gene family member A (RHOA), TET2, DNMT3A, NOTCH2 and XPO1. These two sorted populations shared the DNMT3A p.N612Rfs*26 mutation, and the variants unique to one sorted population were confirmed to be completely absent in another sorted population by manual review of the sample. These findings suggested that the two neoplasms were clonally related and were sharing a common hematopoietic progenitor precursor, but underwent clonal divergence over time, leading to the development of two distinct neoplastic processes of T and myeloid lineages. This illustrates a rare case of concurrent diagnosis of AITL and de novo MDS and reliable genomic assessment was performed at the time of diagnosis to detect mutations in each neoplastic process without contamination. Further studies are needed to assess hypomethylating agents as potential therapy options for these patients.
Keywords: Angioimmunoblastic T-cell lymphoma; Myelodysplastic syndrome; Clonal hematopoiesis; Flow cytometry; Comprehensive genomic profiling
| Introduction | ▴Top |
Angioimmunoblastic T-cell lymphoma (AITL) is one of the most common specific types of peripheral T-cell lymphoma (PTCL) [1]. The cell of origin for AITL is mature T-follicular helper cells (TFHs) [2, 3]. AITL shows unique genomic characteristics and frequently harbors mutations in the epigenetic regulators TET2, DNMT3A and IDH2, TCR signaling pathway and a loss-of-function mutation in the Ras homolog gene family member A (RHOA) [4-7]. Among these genes, TET2 and DNMT3A, and to a lesser extent, IDH2, mutations are also commonly seen in clonal hematopoiesis (CH) and myeloid neoplasms, and it has been shown that these mutations in AITL originated in hematopoietic progenitor cells [8-11]. Recent study demonstrates that CH is prevalent in patients with AITL and the divergent evolution of a CH clone may give rise to both AITL and myeloid neoplasms [12]. Although prior literature has reported cases of AITL and subsequent therapy-related myeloid neoplasm in the context of post-chemotherapy treatments for AITL, it is uncommon to see the development of myeloid neoplasms among AITL patients without any prior therapies. Here we report a case of AITL and concomitant de novo myelodysplastic syndrome (MDS) which were identified to originate from the same hematopoietic progenitor precursor by comprehensive genomic evaluation along with flow cytometry cell sorting.
| Case Report | ▴Top |
A 75-year-old man with a history of lung adenocarcinoma status post right lower lobe lobectomy, and on active surveillance without any prior history of chemotherapy or radiation therapy, presented to a local hospital with increasing fatigue and shortness of breath in 2020. He was found to have a new onset of pancytopenia and chest X-ray showed pneumonia. He was started with empiric antibiotics and was transferred to our institution for further workup. Radiology exam revealed pan lobar pneumonia, pleural effusion, generalized lymph adenopathy and mild splenomegaly (15 cm in greatest dimension). Complete blood count (CBC) showed normocytic normochromic anemia (hemoglobin (Hb) 9.0 g/dL), thrombocytopenia (platelet 52,000/µL) and leukopenia (white blood cell (WBC) 2,400/µL). Microbiology exam was notable for H. influenzae bacteremia and molecular test with sputum specimen detected rhinovirus and Pneumocystis jiroveci. His clinical condition deteriorated rapidly into multiorgan failure, requiring intensive care unit (ICU) admission with intubation, vasopressor and diuresis. Bone marrow biopsy and mediastinal lymph node biopsy from the bilateral level 4 lymph nodes were performed to evaluate the cause of pancytopenia and generalized lymphadenopathy, respectively.
Histologic sections of bilateral level 4 lymph nodes biopsy showed lymphoid tissue with effacement of normal nodal architecture, composed of intermediate sized atypical lymphoid cells with irregular nuclear contours, condensed chromatin, occasional small nucleoli and moderate amounts of pale to clear cytoplasm (Fig. 1a, b). Small lymphocytes, histiocytes and vascular proliferation were present in a background (Fig. 1c). Immunohistochemical studies showed that neoplastic cells express CD2, CD3, CD4, CD5, PD1, ICOS, CXCL13 (subset), and aberrant loss of CD7 (Fig. 1d-g). CD21 and CD23 highlighted follicular dendritic cell meshworks associated with neoplastic cells. Epstein-Barr encoding region (EBER) in situ hybridization highlighted many cells throughout the specimen (Fig. 1h). Flow cytometric analysis performed with the lymph node biopsy sample identified an abnormal T-cell population with abnormal expression of CD2 (bright), surface CD3 (absent), CD4 (dim), CD7 (absent) and PD1 (uniform bright), normal expression of CD5, CD45 and CD52, and without CD8, CD30, or CD56 (Fig. 1i). Based on these findings, the diagnosis of AITL was rendered. Flow cytometric analysis performed with bronchoalveolar lavage and pleural fluid also detected abnormal T cells showing the same immunophenotype as the AITL in lymph node biopsy. Morphologically and immunophenotypically, carcinoma was not identified in the lymph node, bronchoalveolar lavage, or pleural fluid. Bone marrow core biopsy showed hypercellular marrow for age (50-60% cellularity) with erythroid-predominant trilineage hematopoiesis with dysplastic features in megakaryocytic lineage (Fig. 2a). PD1 immunohistochemistry performed on the core biopsy highlighted scattered PD1-positive cells (Fig. 2b). Bone marrow aspirate smears showed mild dysplastic features in erythroid and megakaryocytic lineages. Erythroid precursors occasionally showed karyorrhexis and megaloblastoid changes (Fig. 2c). Small mono/hypo-lobated megakaryocytes and forms with nuclear lobes separation were noted (Fig. 2a inset). Myeloid cells showed orderly maturation without definitive dysplasia. Blasts are not increased. Corresponding flow cytometry identified a small abnormal T-cell population (2.1% of total WBC) showing the same immunophenotype as the AITL in mediastinal lymph nodes (Fig. 2d). Overall morphologic and immunophenotypic findings were consistent with low-level bone marrow involvement by AITL, but the dysplastic features seen were also highly concerning for the presence of low-grade MDS. However, bone marrow biopsy was performed while the patient was in critical clinical condition, with severe pneumonia, multiorgan failure and multiple drug administration, making it difficult to rule out the possibility that the dysplastic features are reactive; therefore, we performed cytogenetic and molecular analysis to further evaluate the possibility of concomitant myeloid neoplasm. Cytogenetic analysis (karyotype) showed deletion of the long arm of chromosome 20 in 14 out of 20 metaphases. To confirm this finding, we performed fluorescent in situ hybridization (FISH) for deletion 20q (del(20q)) in the bone marrow sample. Del(20q) was detected in 36.3% of cells. These findings suggested that this chromosomal abnormality is present in the majority of marrow hematopoietic elements and cannot be explained by the low level of marrow involvement by AITL. For molecular analysis, due to the presence of marrow involvement by AITL, we performed flow cytometry cell sorting of bone marrow aspirate sample to isolate the myeloid cell population and the abnormal T-cell population. Cell sorting was performed on BD FACS Aria Fusion (BD Biosciences, San Jose, CA) by four-way sorting using an 85-µm nozzle. The abnormal T-cell population was sorted using CD3-/CD5+ gate into RPMI-1640 (Corning Inc., Corning, NY) with 20% fetal bovine serum (Thermo Fisher Scientific, Waltham, MA) in 5-mL flow tube and chilled at 4 °C via water recirculator. Sorting for the myeloid population was performed with the same methodology using the CD45 and side scatter gate, and CD3+ or CD5+ population were further excluded. Subsequently, comprehensive genomic evaluation was performed in each population separately (Fig. 2e). A hybridization capture-based, next-generation sequencing (NGS) targeted panel (MSK-IMPACT Heme panel) was performed, a clinically validated assay that targets 400 genes relevant to hematologic malignancies, for detection of mutations, insertion/deletions, as well as chromosomal level copy number changes and loss of heterozygosity (LOH). As per the standard reporting protocol, the threshold for variant calling is ≥ 2.0% variant frequencies for known hotspot, frameshift and nonsense mutations, and ≥ 5.0% variant frequencies for other variants. Flow-sorted myeloid population showed three somatic mutations: DNMT3A p.N612Rfs*36 (variant allele frequency (VAF) 4.4%), BCORL1 p.Y484* (VAF 3.0%) and BCORL1 p.Q929* (VAF 14.7%) (Table 1). In addition, allele-specific copy number analysis with this sorted myeloid population showed LOH and low-level copy number loss in 20q11-13. In light of these genomic mutations and deletion 20q, findings were most consistent with MDS. Flow-sorted abnormal T-cell population showed six somatic mutations consistent with AITL: RHOA p.G17V (VAF: 8.1%), TET2 p.R1440Tfs*38 (VAF: 8.8%), DNMT3A p.N612Rfs*36 (VAF: 4.1%), DNMT3A p.P904L (VAF: 3.9%), NOTCH2 p.Q2341* (VAF: 8.3%) and XPO1 p.E571K (VAF: 5.8%) (Table 1). In addition, allele-specific copy number analysis with this sorted abnormal T-cell population showed no LOH nor copy number loss in 20q. We also performed FISH with the formalin-fixed paraffin-embedded (FFPE) lymph node biopsy sample to evaluate the presence of del(20q) in AITL. We observed few cells showing single copy of 20q (below cut-off), and this was more likely due to nuclear truncation caused by FFPE section preparation. Therefore, these two sorted populations shared a DNMT3A p.N612Rfs*36 mutation. Manual review of the sequencing data of the samples was done, and the variants unique to one sorted population were confirmed to be completely absent in the other sorted population. Since all the detected variants were found at relatively low allele frequencies, they can be safely inferred to be all somatic in nature. The observation that the two sorted populations shared the DNMT3A p.N612Rfs*26 mutation, while each also exhibited multiple unique mutations, supported our suspicion that the AITL and MDS shared a common hematopoietic progenitor precursor, but underwent clonal divergence over time, leading to the development of two distinct neoplastic processes of T and myeloid lineages (Fig. 3). Finally, the lack of TET2 and RHOA mutations in the sorted myeloid sample suggest that these mutations arose during later time points during disease evolution, subsequent to the acquisition of DNMT3A p.N612Rfs*26 mutation by the common progenitor. Of note, retrospective manual review of the sequencing data from other samples available from this patient also found this DNMT3A mutation at extremely low level (VAF: < 1.0%) in the lung adenocarcinoma specimen in early 2019, but at a higher level in a peripheral blood sample obtained at around the same time (VAF: 3.4%). The presence of the DNMT3A p.N612Rfs*20 mutation at a significantly higher level in the blood than the lung adenocarcinoma suggested that it was present in the myeloid elements in form of clonal hematopoiesis, rather than a subclonal mutation in the lung adenocarcinoma sample. This contradicts earlier studies that concluded that TET2 mutations tended to occur antecedent to DNMT3A mutations in AITL [5, 6].
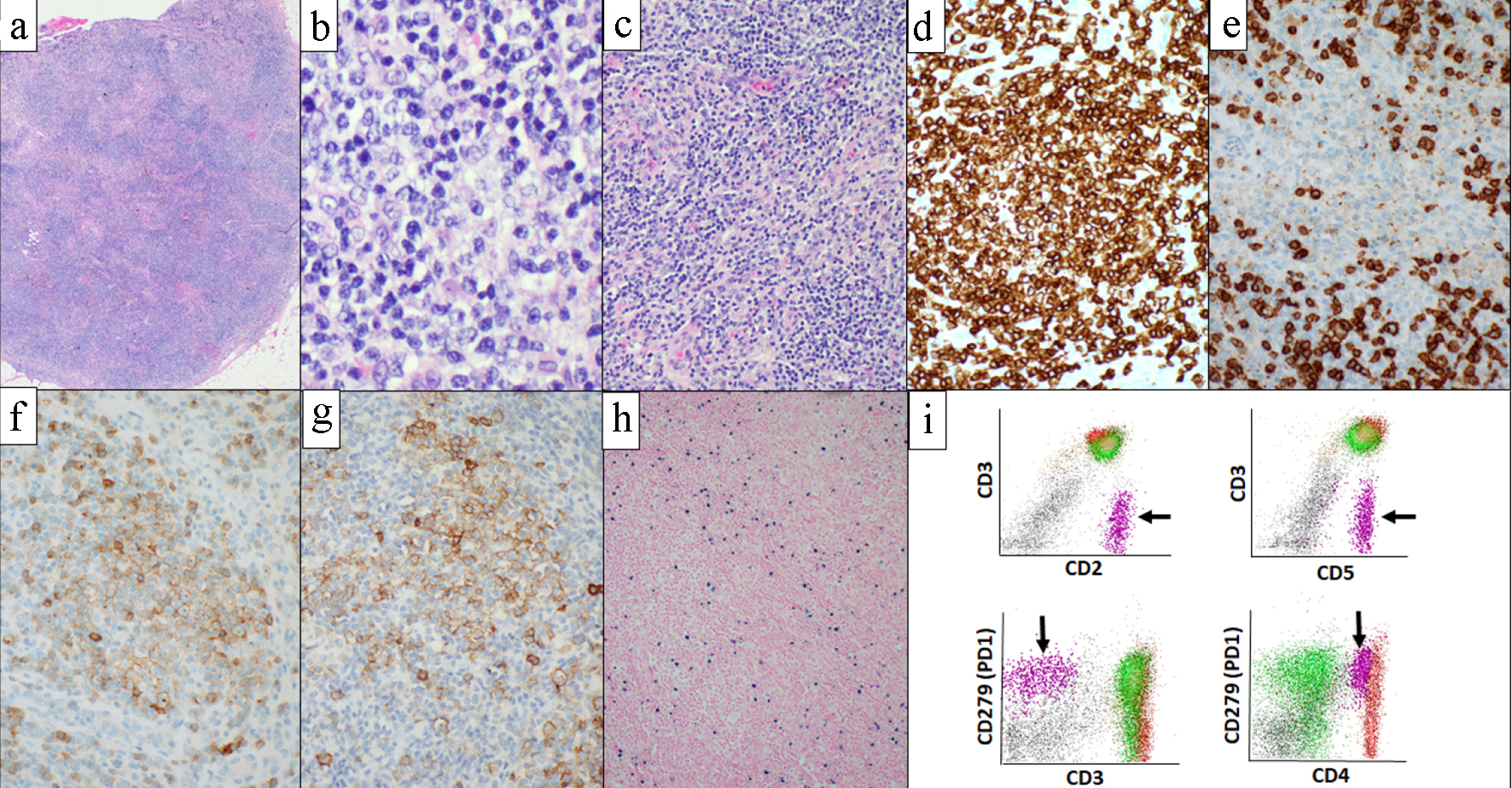 Click for large image | Figure 1. Level 4 lymph nodes biopsy and corresponding flow cytometric analysis. Histologic section showed lymphoid tissue with effacement of normal nodal architecture (a). Abnormal lymphocytes had irregular nuclear contours, condensed chromatin, occasional small nucleoli and moderate amount of clear cytoplasm (b). Small lymphocytes, histiocytes and vascular proliferation were present in a background (c). Lymphoma cells are highlighted with CD3 (d), show aberrant loss of CD7 (e) and express TFH markers; PD1 (f) and ICOS (g). EBER in situ hybridization highlighted many cells throughout the specimen (h). Flow cytometric analysis performed with the level 4 lymph nodes biopsy identified abnormal T-cell population (black arrow) showing loss of surface CD3 and bright expression of PD1 (i). (a-c) Hematoxylin and eosin; (d) CD3 immunohistochemistry; (e) CD7 immunohistochemistry; (f) PD1 immunohistochemistry; (g) ICOS immunohistochemistry; (h) EBER in situ hybridization. EBER: Epstein-Barr encoding region. |
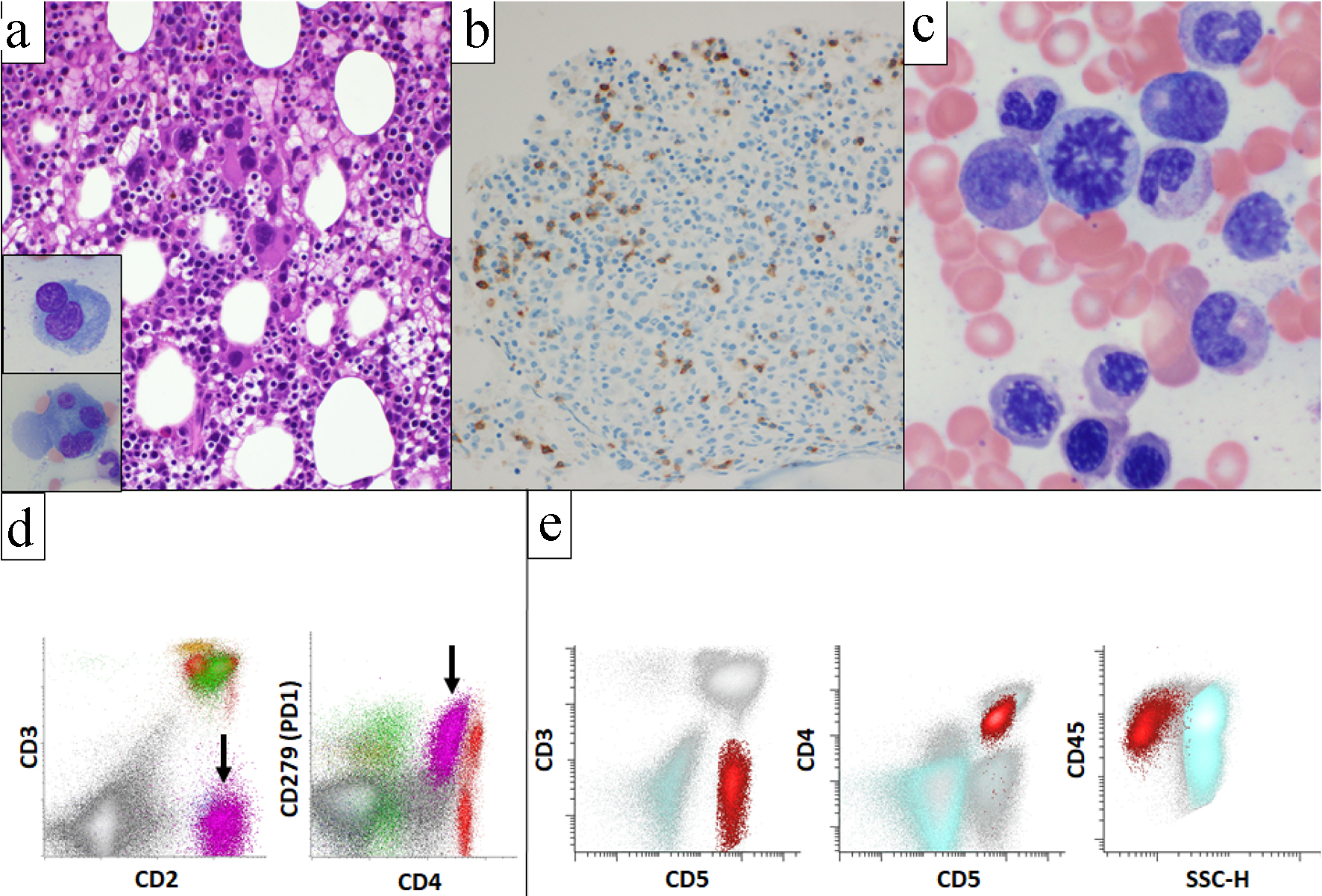 Click for large image | Figure 2. Bone marrow biopsy, aspirate, and corresponding flow cytometric analysis. Bone marrow was hypercellular for age (50-60% cellularity) and showed erythroid-predominant trilineage hematopoiesis with dysplastic features in megakaryocytes (a). PD1 immunohistochemistry highlighted scattered PD1-positive cells (b). Erythroid precursors occasionally showed megaloblastoid changes (c). Flow cytometric analysis identified an abnormal T-cell population (black arrow) showing the same immunophenotype with the AITL in mediastinal lymph nodes (d). Flow sorting was performed to separate abnormal T-cell population (red) from myeloid cell population (aqua) (e). AITL: angioimmunoblastic T-cell lymphoma. |
 Click to view | Table 1. Results of Comprehensive Genomic Evaluation in Flow Sorted Samples |
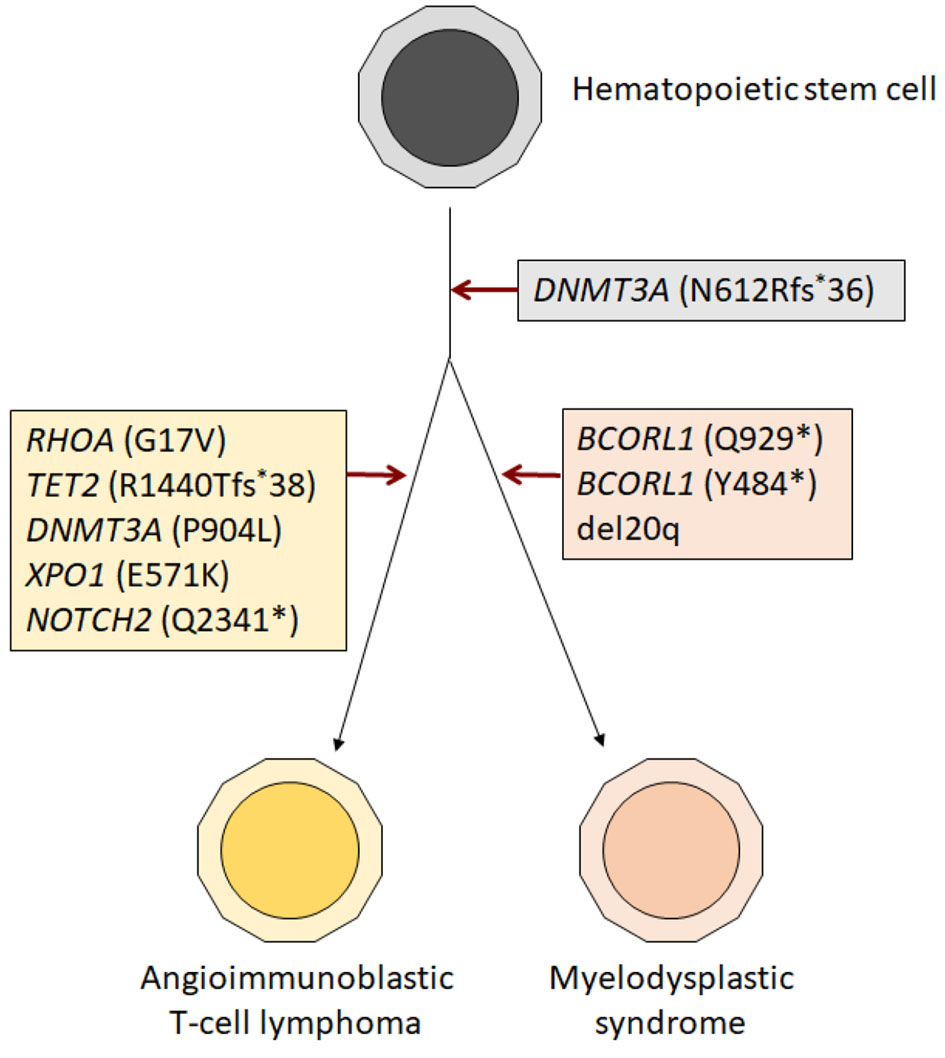 Click for large image | Figure 3. Schematic diagram showing steps in the development of AITL and MDS from the common hematopoietic progenitor precursor. The observation that the two sorted populations shared the DNMT3A p.N612Rfs*26 mutation, supporting that the AITL and MDS shared the common precursor. It underwent clonal divergence over time, leading to the development of two distinct neoplastic processes. AITL: angioimmunoblastic T-cell lymphoma; MDS: myelodysplastic syndrome. |
Based on the diagnosis of AITL, he was started on etoposide, prednisolone, oncovin, cyclophosphamide, hydroxydaunorubicin (EPOCH) with omission of anthracycline (daunorubicin) and 20% dose reduction for other drugs due to right ventricular dysfunction and deterioration of systemic condition. However, in spite of full supportive measures, the patient’s condition continued to deteriorate, and he expired 9 days after the initiation of EPOCH. Autopsy was not performed.
| Discussion | ▴Top |
AITL is one of the most common specific types of T-cell lymphoma in western nations, representing 15-20% of all cases of PTCL [1]. The cell of origin for AITL is mature TFH, supported by gene expression profiling studies of AITL demonstrating similarity of TFH to AITL cells [2]. The cellular derivation of AITL from these TFH cells provides a rational model to explain several characteristic pathological and biological features seen in this disease, such as immunodysregulation, expansion of B cells, the intimate association with germinal centers in early stage disease and the prominent proliferation of follicular dendritic cell meshworks in advanced stage. Immunophenotypic studies of AITL have shown that these tumors are of mature T-cell lineage, expressing a variety of pan-T-cell antigens, showing an immunophenotype closely akin to that of normal follicular T-helper cells [2, 13, 14].
Genomic characteristics of AITL are unique. It does not have mutations commonly found in other mature T-cell neoplasms, and harbors mutations in the epigenetic regulators (TET2, DNMT3A and IDH2), TCR signaling pathway and RHOA [4-7, 15]. TET2, DNMT3A and IDH2 mutations, which are more often seen in myeloid neoplasms, and TET2 and DNMT3A are also frequently identified in the setting of CH. In fact, CH is prevalent in patients with AITL, and in many cases, the TET2 and DNMT3A mutations detected in AITL have been demonstrated to originate from the hematopoietic stem/progenitor cells, supported by studies identifying these mutations in bone marrow myeloid-derived cells of AITL patients [12, 16-19]. Clinically, AITL is a disease of elderly population, and a median age of the patients with AITL is in the sixth decade [1, 20]. Similarly, the prevalence of CH increases with age. Although, no consistent risk factors or etiological agents have yet been identified in AITL, the finding of shared hematopoietic progenitor in patients affected by both CH and AITL suggests the two processes may share a common initiating factor for pathogenesis.
There are a few studies as well as sporadic case reports depicting patients with AITL and myeloid neoplasms. Clonal relatedness between AITL and myeloid neoplasm by molecular analysis was shown in some of the cases. However, the majority of these cases are patients with AITL who developed therapy-related myeloid neoplasm after treatment for AITL [12, 18]. It remains unclear if patients with AITL have a higher risk for developing therapy-related myeloid neoplasms, as compared to patients with other types of PTCL. Large-scale studies for comparison will be necessary to further assess this issue, but definitive conclusions may be difficult due to confounding factors including different treatment regimens and patient demographics for different types of PTCL. This case of 75-year-old man had a history of lung adenocarcinoma status post lobectomy, and he never received chemotherapy or radiation therapy for his lung adenocarcinoma. There are rare reported cases of clonally related AITL and myeloid neoplasms arising prior to therapy for AITL, like this case. However, in these reports, comprehensive genetic assessment was performed after the initiation of chemotherapy, and not on flow cytometry-sorted samples, potentially affecting the results [4, 16, 17, 19, 21]. Evaluation of clonal relatedness of AITL and myeloid neoplasm can be problematic, due to high probability of contamination of cells from one neoplasm in the other, particularly in the bone marrow. In our experience, when assessed with sensitive flow cytometry, AITL is universally systemic, showing at least low-level involvement of peripheral blood and bone marrow [22]. In this case, we performed comprehensive genomic assessment with flow sorted sample at the time of diagnosis to avoid all potential factors affecting the reliability of molecular findings. In this case, DNMT3A N612Rfs*36 was identified as the common mutation for AITL and myeloid neoplasm. TET2 mutation and another DNMT3A mutation were only present in AITL and were not identified in myeloid neoplasm. Previous studies suggest that TET2 mutation occurs earlier than DNMT3A mutation based on the fact that DNMT3A mutated cases also harbor TET2 mutation [5, 6]. However, a case of AITL with DNMT3A mutation without TET2 mutation is also reported [4]. The findings in our case cast further doubt on the belief that TET2 mutations always precede DNMT3A mutations during pathogenesis of AITL.
Karyotype on the marrow detected del(20q) in 14 of 20 metaphases, and FISH detected del(20q) in 36.3% of cells. These findings suggest the high level of the neoplastic clone. However, in this study, all the detected VAFs were relatively low. This could be due to the contamination of non-neoplastic cells in sorted population (Fig. 2e). The shared DNMT3A p.N612Rfs*36 in the two sorted populations seems to be at a lower level than some other mutations present, and this argues against our hypothesis that this mutation is the earliest mutation acquired in the common progenitor cell. BCORL1 is on chromosome X, and, since this is a male patient, we will expect the VAF to be double that of mutations in genes in other chromosomes. In addition, these could have been influenced by copy number gain/loss, or copy-neutral LOH which increase the VAF more than what we would otherwise expect based on tumor content. Due to a lack of matched normal control, evaluation of copy number changes and LOH is limited in our patient’s samples.
There are anecdotal reports in the literature of patients with AITL and myeloid neoplasms who underwent therapy with hypomethylating agent with good clinical responses [17, 19, 21]. Unfortunately, our patient expired soon after the diagnosis of AITL and MDS due to the deterioration of general condition. Further evaluation of potential therapy with hypomethylating agents to patients with AITL and myeloid neoplasm is needed.
Conclusions
In conclusion, we experienced a case of 75-year-old man with clonally related AITL and de novo MDS, with a shared DNMT3A mutation indicating that these two neoplasms originated from the same hematopoietic progenitor. Reliable genomic assessment was performed on bone marrow flow cytometry-sorted populations at the time of concurrent diagnoses of AITL and MDS, to reliably detect mutations in each neoplastic process without contamination. Further studies are needed to assess hypomethylating agents as potential therapy options for these patients.
Acknowledgments
We would like to acknowledge the multidisciplinary team at Memorial Sloan Kettering Cancer Center who were involved in the care of this patient.
Financial Disclosure
KN, none to declare; AC owned equity/stock in Abbvie and Bristol Myers Squibb; YZ, none to declare; NL received consultant fees from and is on the board of directors of United States Drug Testing Laboratories; WX received research support from Stemline Therapeutics; MR received personal fees from Agios, Cellgene and Physicians’ Education Resource, research grants from Agios, Cellularity, and Roche, serves as an advisor and owns equity in Auron Pharmaceuticals; AD received personal consultancy fees from Roche, Corvus Pharmaceuticals, Physicians’ Education Resource, Seattle Genetics, Peerview Institute, Oncology Specialty Group, Pharmacyclics, Celgene, Novartis, Takeda, EUSA Pharma and research grants from National Cancer Institute and Roche; MK received Honoraria from Sumitomo Dainippon Pharma, Celgene, Bristol Myers Squibb, Janssen, Ono Pharmaceutical, Novartis, MSD, Research grant from Takeda, Scholarship funds from Takeda, Ono Pharmaceutical, Daiichi Sankyo, Chugai Pharmaceutical, Kyowa Kirin; CH received Honorarium from Invivoscribe, Inc.; MY received personal consultancy fees from Janssen Research and Development, LLC.
Conflict of Interest
None to declare.
Informed Consent
The manuscript has been sufficiently de-identified to protect the patient.
Author Contributions
KN, CH and MY designed the study, analyzed the data, reviewed the literature and wrote the paper; AC and MR analyzed the data and conducted the flow cytometry analysis; YZ performed cytogenetic analysis. All authors contributed to the editing of the manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Federico M, Rudiger T, Bellei M, Nathwani BN, Luminari S, Coiffier B, Harris NL, et al. Clinicopathologic characteristics of angioimmunoblastic T-cell lymphoma: analysis of the international peripheral T-cell lymphoma project. J Clin Oncol. 2013;31(2):240-246.
doi pubmed - de Leval L, Rickman DS, Thielen C, Reynies A, Huang YL, Delsol G, Lamant L, et al. The gene expression profile of nodal peripheral T-cell lymphoma demonstrates a molecular link between angioimmunoblastic T-cell lymphoma (AITL) and follicular helper T (TFH) cells. Blood. 2007;109(11):4952-4963.
doi pubmed - Grogg KL, Attygalle AD, Macon WR, Remstein ED, Kurtin PJ, Dogan A. Angioimmunoblastic T-cell lymphoma: a neoplasm of germinal-center T-helper cells? Blood. 2005;106(4):1501-1502.
doi pubmed - Couronne L, Bastard C, Bernard OA. TET2 and DNMT3A mutations in human T-cell lymphoma. N Engl J Med. 2012;366(1):95-96.
doi pubmed - Odejide O, Weigert O, Lane AA, Toscano D, Lunning MA, Kopp N, Kim S, et al. A targeted mutational landscape of angioimmunoblastic T-cell lymphoma. Blood. 2014;123(9):1293-1296.
doi pubmed - Sakata-Yanagimoto M, Enami T, Yoshida K, Shiraishi Y, Ishii R, Miyake Y, Muto H, et al. Somatic RHOA mutation in angioimmunoblastic T cell lymphoma. Nat Genet. 2014;46(2):171-175.
doi pubmed - Palomero T, Couronne L, Khiabanian H, Kim MY, Ambesi-Impiombato A, Perez-Garcia A, Carpenter Z, et al. Recurrent mutations in epigenetic regulators, RHOA and FYN kinase in peripheral T cell lymphomas. Nat Genet. 2014;46(2):166-170.
doi pubmed - Genovese G, Kahler AK, Handsaker RE, Lindberg J, Rose SA, Bakhoum SF, Chambert K, et al. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N Engl J Med. 2014;371(26):2477-2487.
doi pubmed - Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG, Lindsley RC, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014;371(26):2488-2498.
doi - Xie M, Lu C, Wang J, McLellan MD, Johnson KJ, Wendl MC, McMichael JF, et al. Age-related mutations associated with clonal hematopoietic expansion and malignancies. Nat Med. 2014;20(12):1472-1478.
doi pubmed - Papaemmanuil E, Gerstung M, Malcovati L, Tauro S, Gundem G, Van Loo P, Yoon CJ, et al. Clinical and biological implications of driver mutations in myelodysplastic syndromes. Blood. 2013;122(22):3616-3627; quiz 3699.
- Lewis NE, Petrova-Drus K, Huet S, Epstein-Peterson ZD, Gao Q, Sigler AE, Baik J, et al. Clonal hematopoiesis in angioimmunoblastic T-cell lymphoma with divergent evolution to myeloid neoplasms. Blood Adv. 2020;4(10):2261-2271.
doi pubmed - Piccaluga PP, Fuligni F, De Leo A, Bertuzzi C, Rossi M, Bacci F, Sabattini E, et al. Molecular profiling improves classification and prognostication of nodal peripheral T-cell lymphomas: results of a phase III diagnostic accuracy study. J Clin Oncol. 2013;31(24):3019-3025.
doi pubmed - de Leval L, Gisselbrecht C, Gaulard P. Advances in the understanding and management of angioimmunoblastic T-cell lymphoma. Br J Haematol. 2010;148(5):673-689.
doi pubmed - Sandell RF, Boddicker RL, Feldman AL. Genetic Landscape and Classification of Peripheral T Cell Lymphomas. Curr Oncol Rep. 2017;19(4):28.
doi pubmed - Quivoron C, Couronne L, Della Valle V, Lopez CK, Plo I, Wagner-Ballon O, Do Cruzeiro M, et al. TET2 inactivation results in pleiotropic hematopoietic abnormalities in mouse and is a recurrent event during human lymphomagenesis. Cancer Cell. 2011;20(1):25-38.
doi pubmed - Cheminant M, Bruneau J, Kosmider O, Lefrere F, Delarue R, Gaulard P, Radford I, et al. Efficacy of 5-azacytidine in a TET2 mutated angioimmunoblastic T cell lymphoma. Br J Haematol. 2015;168(6):913-916.
doi pubmed - Tiacci E, Venanzi A, Ascani S, Marra A, Cardinali V, Martino G, Codoni V, et al. High-risk clonal hematopoiesis as the origin of AITL and NPM1-Mutated AML. N Engl J Med. 2018;379(10):981-984.
doi pubmed - Tobiasson M, Pandzic T, Cavelier L, Sander B, Wahlin BE. Angioimmunoblastic T-cell lymphoma and myelodysplastic syndrome with mutations in TET2, DNMT3 and CUX1 - azacitidine induces only lymphoma remission. Leuk Lymphoma. 2019;60(13):3316-3319.
doi pubmed - Mourad N, Mounier N, Briere J, Raffoux E, Delmer A, Feller A, Meijer CJ, et al. Clinical, biologic, and pathologic features in 157 patients with angioimmunoblastic T-cell lymphoma treated within the Groupe d'Etude des Lymphomes de l'Adulte (GELA) trials. Blood. 2008;111(9):4463-4470.
doi pubmed - Saillard C, Guermouche H, Derrieux C, Bruneau J, Frenzel L, Couronne L, Asnafi V, et al. Response to 5-azacytidine in a patient with TET2-mutated angioimmunoblastic T-cell lymphoma and chronic myelomonocytic leukaemia preceded by an EBV-positive large B-cell lymphoma. Hematol Oncol. 2017;35(4):864-868.
doi pubmed - Yabe M, Gao Q, Ozkaya N, Huet S, Lewis N, Pichardo JD, Moskowitz AJ, et al. Bright PD-1 expression by flow cytometry is a powerful tool for diagnosis and monitoring of angioimmunoblastic T-cell lymphoma. Blood Cancer J. 2020;10(3):32.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


