| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website http://www.thejh.org |
Original Article
Volume 8, Number 3, September 2019, pages 102-110
Distinct Origins of Restricted Progenitors in Postnatal Mouse Blood
Wuyi Konga, b, Xiujuan Hana, Hong Wanga, Xiaoping Zhua
aBeijing Khasar Medical Technology Co., Beijing, China
bCorresponding Author: Wuyi Kong, Beijing Khasar Medical Technology Co., Jing-Meng A101, Shangdi East Street 5-1, Haidian District, Beijing, China
Manuscript submitted June 29, 2019, accepted August 27, 2019
Short title: Restricted Progenitors in Mouse Blood
doi: https://doi.org/10.14740/jh540
| Abstract | ▴Top |
Background: Most researchers have accepted that unipotent progenitors are the predominant components in bone marrow for tissue regeneration. However, the unipotent progenitors for blood components are still unclear. We previously found that erythrocytes are derived from a distinct unipotent progenitors, or erythrocyte sacs.
Methods: In the current study, we investigated if the other types of unipotent blood cell progenitors existed, what was their original morphologies, and the mechanism of their generation in mouse blood.
Results: We found two morphologically distinct structures that released spore-like small progenitors in mouse blood. One structure was filamentary-like, contained inclusions, widened due to differentiation of the inclusions, and eventually, released spore-like DNA+ and cluster of differentiation 34 (CD34)+ spore-like small progenitors. Another structure was bud-like, contained inclusion, enlarged from less than 10 µm to more than 30 µm, and also released many spore-like small progenitors. Each type of these spore-like progenitors was approximately 1 µm in diameter and could continue to transdifferentiate in circulation.
Conclusions: Our data provide evidence that two types of blood cell restricted progenitors are produced from either filamentary structures or bud-like structures. Both filamentary and bud-like structures were originally released from morphologically distinct, or lineage predetermined tube-shaped structures, or specific niches. Thus, distinct lineages of blood unipotent progenitors are newly produced.
Keywords: Spore-like progenitors; Filaments; Bud-like; Tube-shaped structures; CD34
| Introduction | ▴Top |
The classical consensus about the hematopoietic stem cells (HSCs) is that in postnatal life, hierarchical differentiation occurs in the bone marrow from HSCs that are defined by a self-renewal ability to stably reconstitute life-long blood components [1-5]. The hierarchical differentiation of HSCs first results in common myeloid progenitors and common lymphoid progenitors. Common myeloid progenitors then differentiate into granulocyte monocyte progenitors and megakaryocyte erythroid progenitor, the latter further segregating into erythrocyte and megakaryocyte progenitors [6, 7].
A new concept debated that platelet-biased stem cells or megakaryocytes reside at the apex of the HSC hierarchy [8, 9]. The recent investigation describes that the differentiation of HSCs to other blood progenitors has a two-tier hierarchy; that is, HSCs or megakaryocytes directly differentiate to unipotent progenitors that are the predominant components in bone marrow [9]. However, this new theory could not explain how HSC transition through the multipotency to the restricted progenitor stages. Another recent report claims that the restricted lineage segregation has occurred during the early embryonic development [10], indicating that cell lineage has been carefully categorized prenatally, or cells are switched from multipotent to unipotent in embryonic development. Moreover, some reports indicate that HSCs are able to differentiate into restricted progenitors before cell division, or HSC transition to restricted blood progenitors is via transdifferentiation procedures [11]. Although these investigations have provided great details about the restricted progenitors, the entire picture about the production of restricted blood progenitors is still unclear.
Here we show that two groups of small spore-like progenitors of 1 µm were released from either thin filaments we called “progenitor-releasing filaments” (CRFs) or bud-like structures. The spore-like small progenitors are two types of restricted progenitors that may undergo transdifferentiation to become two distinct nucleated cells in the blood. Both CRFs and bud-like structures originate from tube-shaped structures that are lineage predetermined.
| Materials and Methods | ▴Top |
Animals and materials
Male BALB/c and C57BL6 mice of 8 to 16 weeks old were purchased from Vital River Laboratories (Beijing). Green fluorescent protein (GFP)-transgenic (FVB.Cg-Tg(ACTB-EGFP)B5Nagy/J) mice were from the Jackson Lab (Bar Harbor, ME, USA) and bred in the animal facility until 8 to 12 weeks old. Mice received food and water ad libitum. The animal committee in Khasar Medical Technology had approved the research protocol “stem cells in the mouse blood”. All animal procedures followed the guidelines of the Animal Regulatory Office relevant to national and international guidelines. Anti-cluster of differentiation 34 (CD34) and anti-E-cadherin antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-GFP antibody was from Aves Lab (Tigard, OR, USA). Alexa Fluor goat anti-rabbit and donkey anti-mouse IgG antibodies were from Invitrogen (Carlsbad, CA, USA).
Mouse blood collection
Blood was collected from five of 8- to 10-week-old mice of each strain via cardiac puncture under sterile conditions after CO2 euthanasia. Each syringe was prefilled with 100 µL heparin (0.5%) to prevent coagulation. Blood was placed on ice for about 5 min, then pooled (about 5 mL) and transferred to a 50-mL centrifuge tube, immediately diluted with 1 × phosphate buffered saline (PBS) at a ratio of 1:5 (blood/PBS), then centrifuged at 200 × g for 5 min. After removing the supernatant, the cellular portion was diluted with PBS in a 1:1 ratio, and then 15% paraformaldehyde was slowly added to a final concentration of 4% paraformaldehyde. Blood cells were fixed at 4 °C for overnight. The next day, cells were centrifuged at 200 × g for 5 min, then washed two times with PBS and centrifuged at 100 × g for 5 min and twice at 50 × g for 5 min to remove free erythrocytes. Visible red clots were removed during washing. After a final centrifugation and removing the supernatant, the cellular portion was fixed again with 4% paraformaldehyde at a ratio of 1:5 (original blood volume/fixative volume) and dropped (200 µm/drop from about 5 cm above) onto gelatin-coated histology slides. At least 20 slides from each strain of mice were stained and examined.
Immunohistochemistry and fluorescence staining
For immunohistochemistry staining, slides were blocked for 1 h and incubated with anti-CD34 antibody overnight at 4 °C. After washing, slides were incubated with horseradish peroxidase-conjugated secondary antibody for 1 h, stained with diaminobenzidine (DAB), and then counterstained with hematoxylin before final mounting. For fluorescent staining, slides were blocked for 1 h, incubated with anti-CD34 or anti-E-cadherin and anti-GFP antibodies in blocking buffer overnight at 4 °C, then washed and incubated with a fluorescent-conjugated secondary antibody for 1 h. After the washing, cells were counterstained with 4’,6-diamidino-2-phenylindole (DAPI) and visualized by conventional fluorescence microscopy (Leica, Wetzlar, Germany) and photographed by use of a digital camera (Leica, DFC500). Control tissue was treated with non-specific antiserum of the same species.
Hematoxylin and eosin (H&E) staining
Cellular portions from each mouse strain preparation were dropped on at least 20 slides. Slides were dried overnight, washed three times with PBS, and stained with H&E. Stained slides were visualized by standard fluorescence microscopy (Leica) and photographed with use of a digital camera (Leica, DFC500).
| Results | ▴Top |
Identifying progenitor-releasing filaments in mouse blood
H&E-stained slides were examined by microscopy. Grouped thin filamentary structures were identified (Fig. 1a). Most filaments were in bundles; each of the filaments was as thin as 1 μm and more than 500 µm in length. Amplified images revealed small dot-like materials that were weakly stained by H&E and located in the lumen of each filament. Some inclusions in the filaments were stained more to eosin, or red color (Fig. 1b), which suggests that the inclusions in these filaments were relatively rich in proteins. Some filaments had branches (Fig. 1c). In addition, the diameter of some grouped filaments with node structures was wider than those without node structures (Fig. 1d). The size changes suggest that filaments can differentiate from thin filaments into those with wider nodes. Additionally, grouped DNA granules were identified near wider-sized filaments (Fig. 1e).
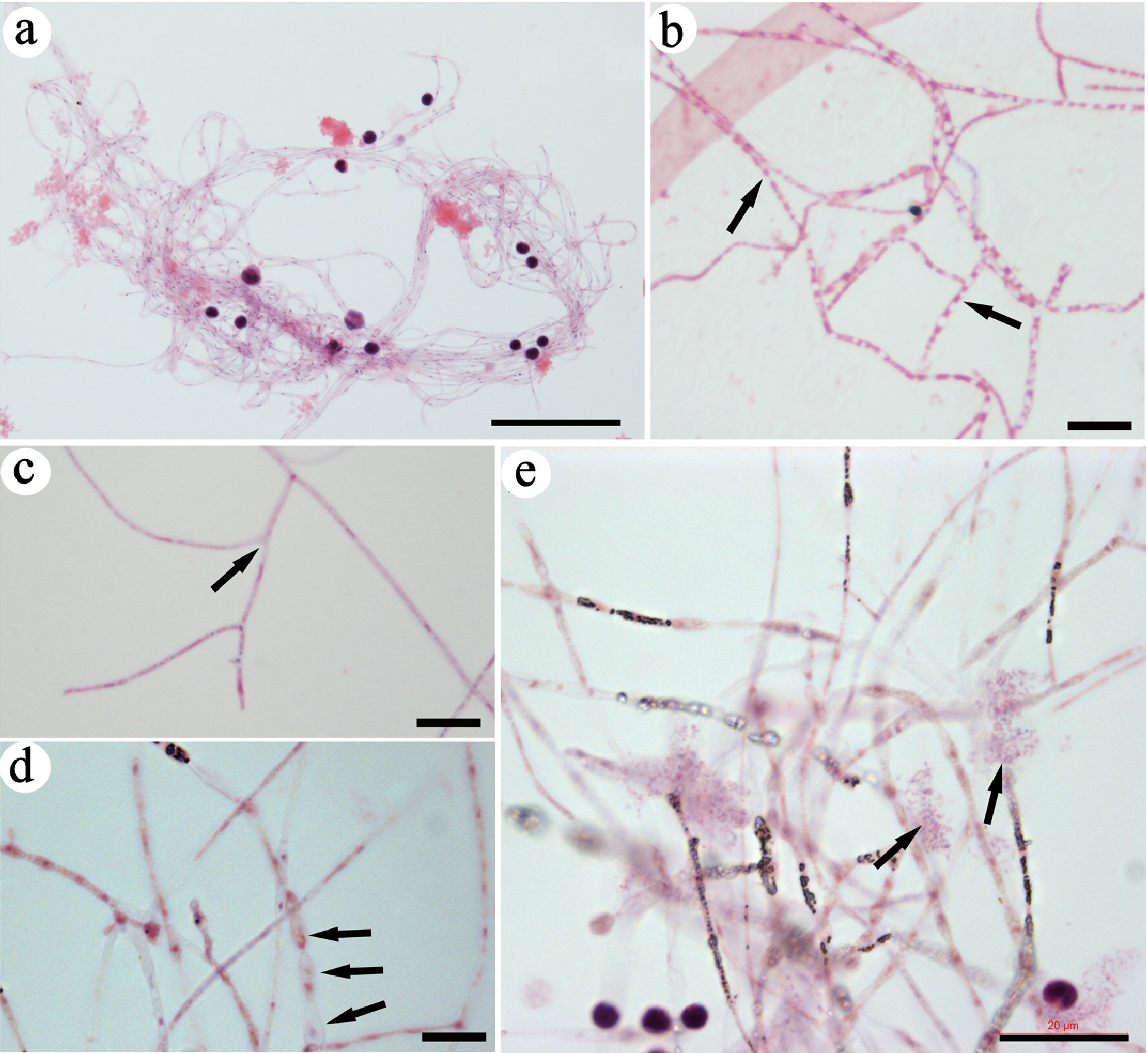 Click for large image | Figure 1. Identifying CRFs in mouse blood. Slides were stained with H&E. A group of CRFs was identified and imaged under lower magnification (a). High-magnification images revealed small eosin-stained materials in the lumen of each filament (arrows in b), filaments with branches (arrows in c), and filaments that were wider and had nodes (arrows in d). Grouped DNA fragments (arrows in e) were located close to the filaments. Bar in a = 50 µm; in b-d = 10 µm; in e = 20 µm. CRF: progenitor-releasing filament; H&E: hematoxylin and eosin. |
Tiny dot-like DNA in the lumen of the filaments
Fluorescent DAPI staining revealed that DNA was expressed in tiny dots in filaments. A bundle of filaments showed E-cadherin, GFP and DAPI staining (Fig. 2a). DAPI staining under overexposed fluorescent light revealed that numerous small nuclear dots were located in the lumen of each bundled filament, indicating that the inclusion in the lumen of the filaments contained DNA. The adjacent regular-sized (about 10 µm) cell nucleus clearly illustrated the sizes of these tiny DNA materials. DAPI staining showed other numerous, tiny, separate DNA dots along a bundled filament (Fig. 2b). In comparison to regular-sized cell nuclei, these small nucleus-like dots were stained very weakly by DAPI and were less than 1 µm in diameter.
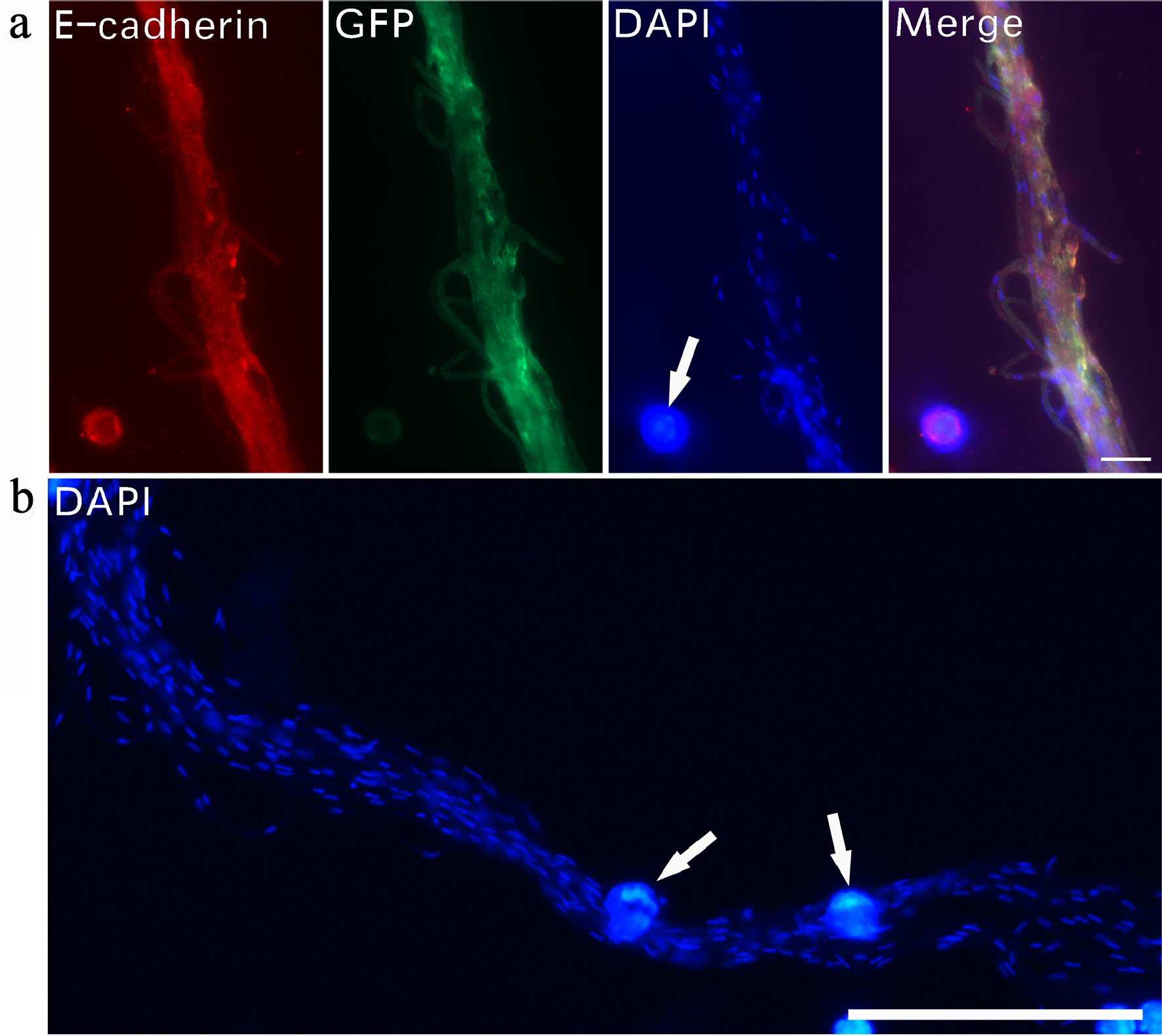 Click for large image | Figure 2. Tiny dot-like DNA in the lumen of filaments. Immunofluorescent staining was used to examine DNA expression in CRFs. A bundle of filaments stained with E-cadherin, GFP and DAPI (a). DAPI staining revealed numerous small nucleus-like DNA dots along a group of bundled filaments (b). Regular-sized cell nuclei (arrows in a, b) are included for size comparison. Bar in a = 10 µm; in b = 50 µm. CRF: progenitor-releasing filament; GFP: green fluorescent protein; DAPI: 4’,6-diamidino-2-phenylindole. |
Spore-like small progenitors released from CRFs
All the images in this figure were collected under the same magnification for size comparison. Via immunohistochemistry, we observed that CD34+ small spore-like progenitors were released from CRFs (Fig. 3a, b). In Fig. 3a, the sizes of the spore-like progenitors were slightly different, suggesting that they continue to differentiate after being released. The filaments were empty and transparent after the release. The CRFs that released small spore-like progenitors were wider and had nodes (Fig. 3c), indicating that the inclusions differentiated inside the filaments. A few small progenitors of different sizes were located adjacent to filaments, also suggesting that their sizes increased after release. Some red-stained small inclusions were still observed inside the filaments. A group of spore-like small progenitors were identified (Fig. 3d) that were strongly stained with eosin and had similar sizes as the small spore-like progenitors that were released from the filaments (Fig. 3a-c), which suggested that these small progenitors were the same type of spore-like progenitors released from CRFs. The released small progenitors in the images had similar morphologies, supporting that CRFs only released one type of small progenitors. These small spore-like progenitors were the progenitors of one-lineage blood cells, or restricted progenitors.
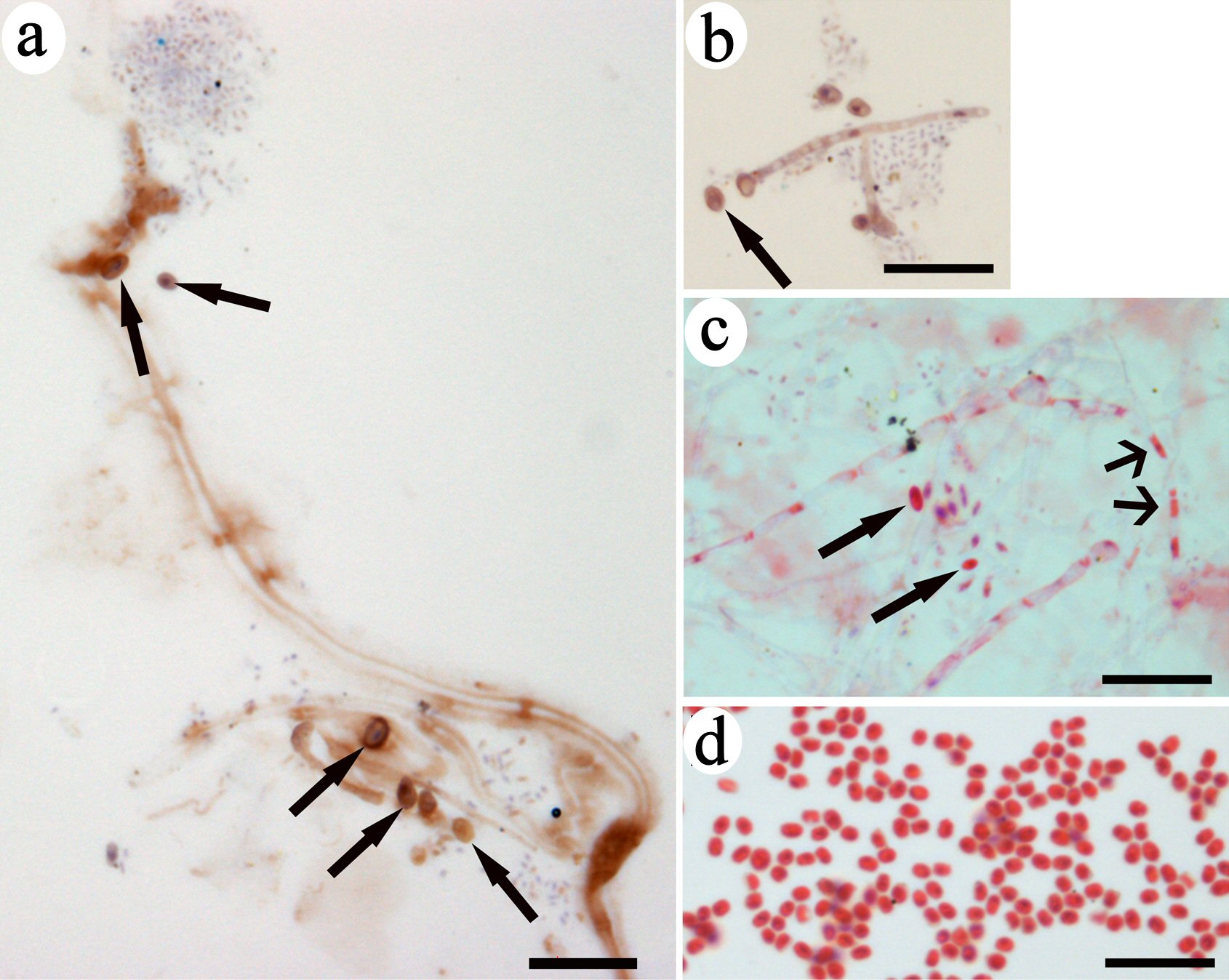 Click for large image | Figure 3. Spore-like small progenitors were released from CRFs. Immunohistochemistry of brown-colored DAB staining indicated CD34 expression on filaments and small progenitors (arrows in a, b). A group of small red-colored progenitors located near the filament with nodes had the same color as the inclusion in the lumen (wide-head arrows in c). One small progenitor (arrow in c) had the same morphology as the small eosin-stained progenitors in (d). Bars = 10 µm. CRF: progenitor-releasing filament; DAB: diaminobenzidine; CD: cluster of differentiation. |
Progenitor differentiation after release
We examined CD34 expression in these filaments. Immunofluorescence images (Fig. 4) revealed that both CD34 and GFP were irregularly but clearly expressed on the granules inside CRFs, with a cell pattern of small progenitors at the filamentary tip. CD34 and GFP expression suggests that they were expressed in the inclusions. CD34 expression was stronger and DAPI staining was dim in the small progenitor located at the end of CRFs, suggesting that this small progenitor underwent further nuclear reprogramming after being released.
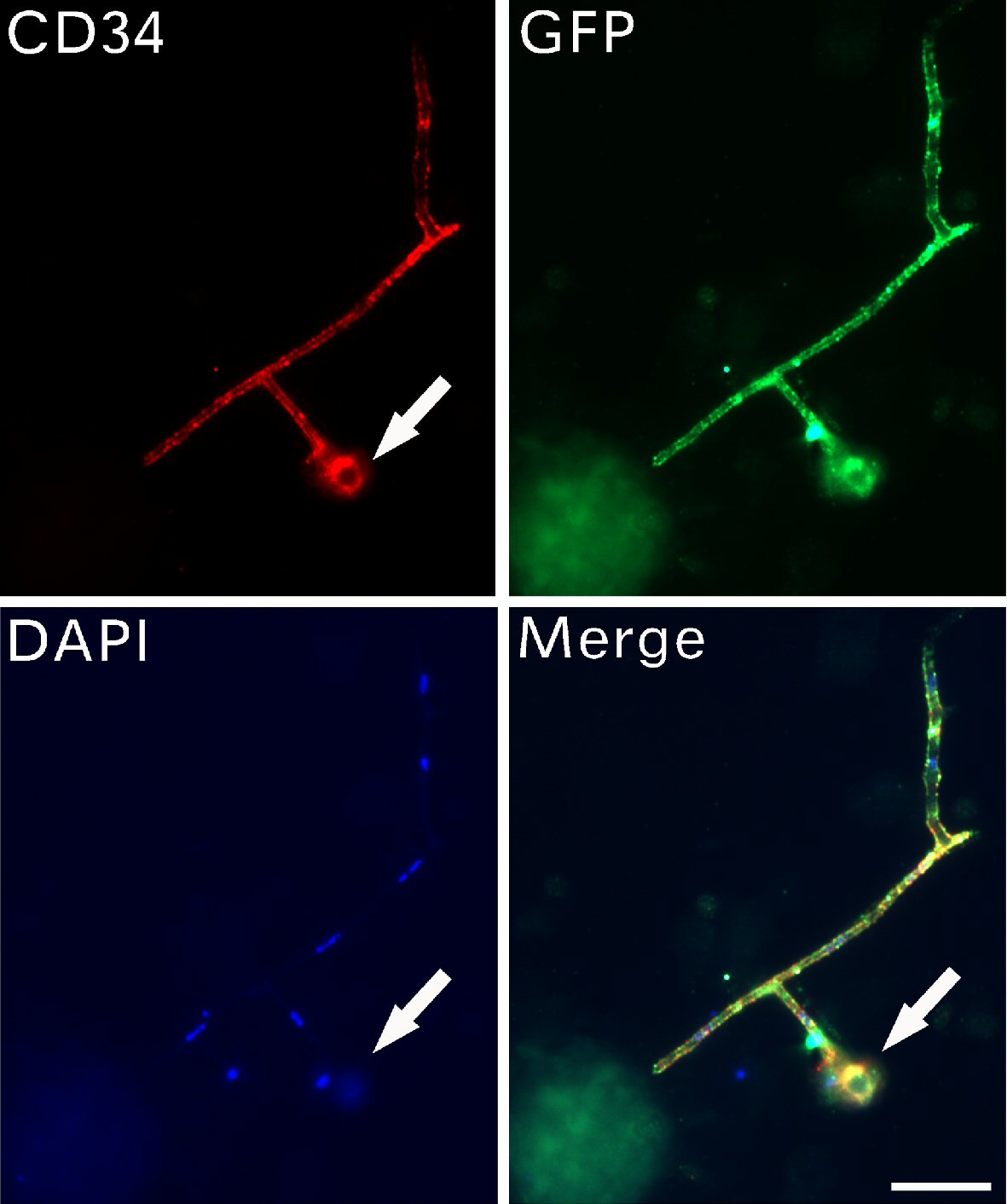 Click for large image | Figure 4. CD34-positive progenitors were larger after release. Immunofluorescent assays detected the expression of CD34, GFP and DAPI on filaments with branches. A small stem cell progenitor (arrows) located at the tip of the filament. Bar = 10 µm. CD: cluster of differentiation; GFP: green fluorescent protein; DAPI: 4’,6-diamidino-2-phenylindole. |
CRFs were originally bundled
The ends of some grouped filaments were even up and tightly bound (Fig. 5a, b), which suggests that CRFs were originally bundled and later became loose or unwound in circulation. Amplified images showed two groups of bundled filaments (Fig. 5c, d). The staining color on the bundle sides of these two bundled filaments was different, but the staining color on the loose sides was the same, which indicates that these filaments were the same type. This observation could explain why CRFs within the same group were almost identical to each other but differed from those in other groups. The time when the bundled filaments entered the blood was different, which determined the status of the morphology or maturation in each bundled CRF. Two nucleated cells were closely attached on the filaments (Fig. 5c), suggesting these cells were related to the filaments, or these cells determined the lineage of filament-released progenitors.
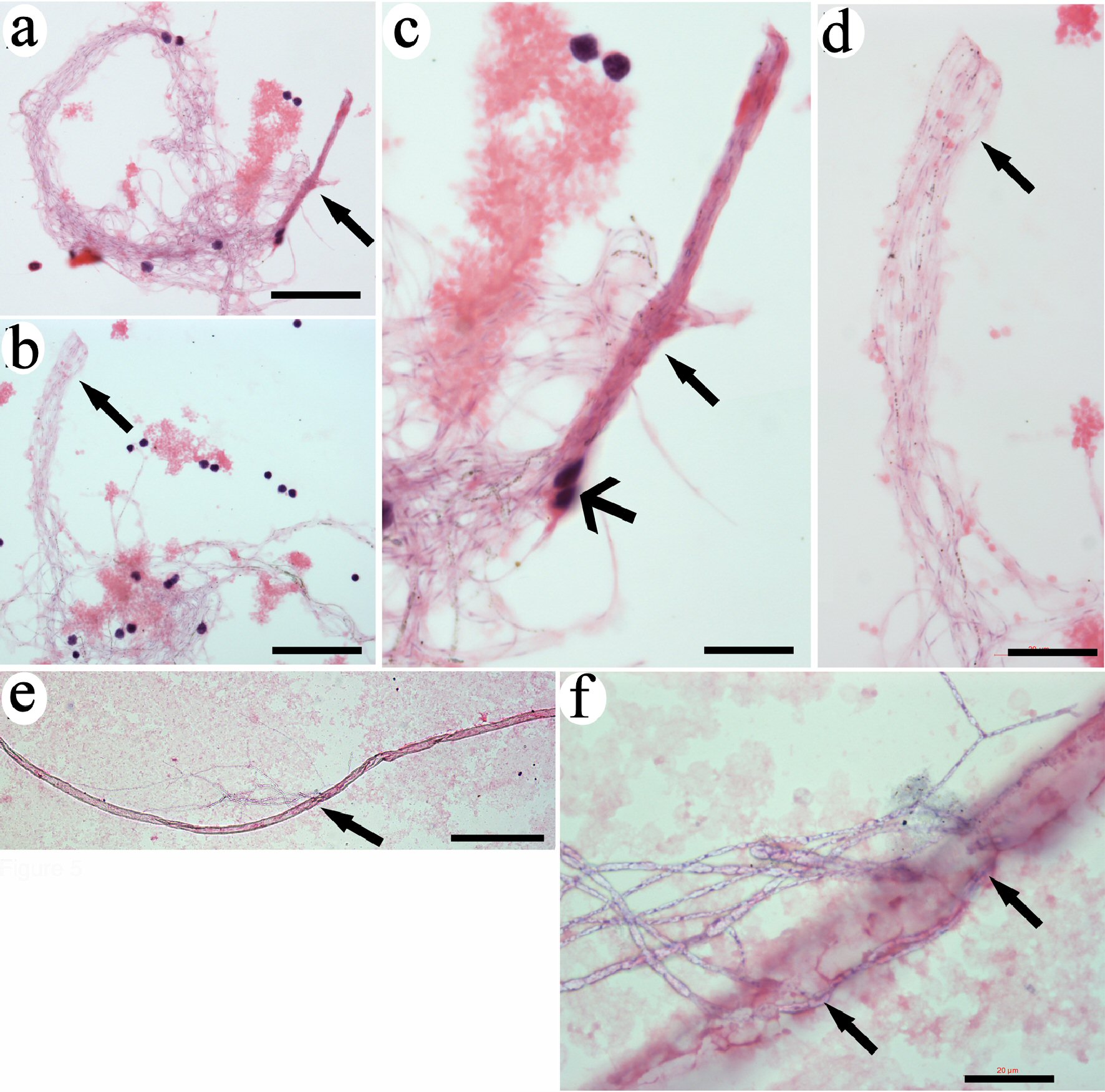 Click for large image | Figure 5. CRFs were originally released from tube-like structures. The ends of tightly bundled filaments were even and with the same length (arrows in a and b). High magnification revealed that the filaments were identical (arrows in c and d). Two monocytes were tightly attached to the filaments (wide-head arrows in c). A long tube-like structure was detected (e). High magnification revealed that filamentary structures were released from the opening in the middle of the tube (arrows in f). Bars in a, b = 50 µm; in c = 10 µm; in d, f = 20 µm; in e = 200 µm. CRF: progenitor-releasing filament. |
CRFs were released from tube-like structures
We further identified tube-like structures in the blood. These tube-like structures were more than 1,000 µm long and had a diameter of 30 µm (Fig. 5e). A group of filaments were released from the middle openings of the tube (Fig. 5f). These observations suggest that these tube-like structures were the original locations of CRFs. We believe that the thick outer layer of the tube-like structures can be further degraded after filament release, similar to the outer layers of the “erythrocyte sacs” reported previously [12].
Identification of bud-like structure-released spore-like progenitors
We also found bud-like structures that were stained a copper color stain by H&E (Fig. 6). All the images were collected under the same magnification for size comparison. Some bud-like structures that were less than 10 µm in size had similar morphologies and color, and were located close to each other (Fig. 6a). Larger-sized bud-like structures were identified. They had inclusions that could be observed through the semi-transparent membranes (Fig. 6b, c). Few small progenitors extruded through the membrane (Fig. 6c). The leaf-like membrane of the bud-like structures opened to release numerous small spore-like progenitors (Fig. 6d). These small spore-like progenitors were approximately 1 µm in diameter and had an identical morphology and color, indicating that they were the same type of progenitors. Comparing to the CRF-released small progenitors that had strong stain by eosin, the bud-like structure-released small progenitors showed weaker staining by eosin but stronger staining by hematoxylin, or were stained purple. We also identified segmented tube-like structures that showed copper-color after H&E staining (Fig. 6e). Each bud-like structure may be released from one of the segment in this tube-like structure. A small bud-like structure could still be observed in the lumen through the broken area of tube-like structure. These data provide evidence of another type of spore-like small progenitors that had a distinct morphology and origin from CRF-released small progenitors.
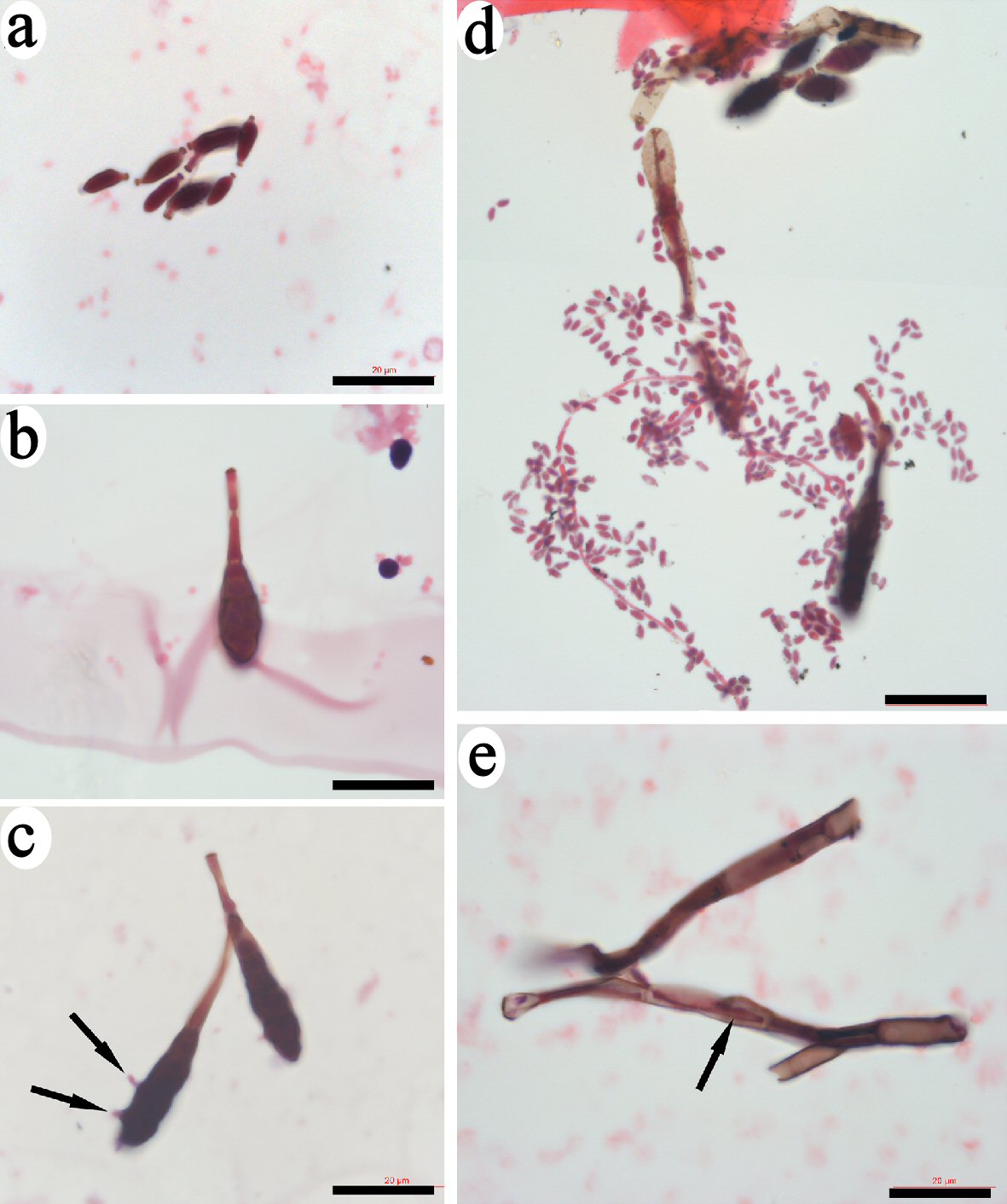 Click for large image | Figure 6. Identification of bud-like structure that released spore-like progenitors. Small bud-like structures of less than 10 µm that had similar morphologies and were stained copper-color were identified (a). Some larger-sized bud-like structures that contained dark inclusions were identified (b, c). Few small progenitors extruded through the membrane of bud-like structure (arrows in c). The leaf-like membrane of the bud-like structures opened to release numerous small spore-like progenitors (d). Bars = 20 µm. |
| Discussion | ▴Top |
We describe two distinct novel blood cell progenitors and structures, including the spore-like small progenitors, the CRFs, the bud-like structures, and the origins they are from. Although cell-sorting has extremely sped up data acquisition in many investigations, it can only identify cells that express the surface markers. Our studies found that the thick coating membranes of CRFs and the bud-like structures do not express most surface markers, and that the small spore-like small progenitors were only about 1 µm, which may explain why none of them were identified by the immunophenotypic cell-sorting method. In the previous study, we have found the progenitors of the erythrocyte, or “erythrocyte sacs” that do not express the surface marker [12]. Thus, we used classical and time-consuming methods for the current studies, which is by sorting cell morphologies under the microscope.
Although the mechanism of cellularization occurring inside CRFs and bud-like structures is still unknown, it is clear that genetic material and other proteins are needed to participate in the formation of specific progenitors. The acquisition of genetic material may occur inside CRFs or bud-like structures at very early stages or before their release from tube-shaped structures. Therefore, the genetic material should be small. We observed numerous DNA fragments that released from different cell nucleus (Supplementary figure 1, www.thejh.org). The release of DNA fragments seemed not related to cell apoptosis, and may be due to some unidentified mechanism. We also believe that the DNA granules located very close to CRFs (Figs. 1e, 3a, b) took parts in applying the genetic materials to the small progenitor production. These observations suggest that the genetic materials required for spore-like progenitor formation are from similar DNA fragments via a falling-off pattern via an unknown mechanism. Thus, the genetic material is DNA fragments and not DNA from nuclear division. In CRF-released progenitors, we believe that the genetic material was from the nuclei of cells that had the same lineage as the monocytes tightly attached on the filaments (Fig. 5c) (Supplementary figure 2, www.thejh.org). One nucleus could releases hundred DNA fragments that become the genetic materials to initiate the formation of many restricted progenitors. Our data provide evidence that the restricted progenitors are newly made, which support the report [10] that only the lineage-restricted progenitors are present in the postnatal life.
The expression of CD34, a well-known blood stem cell marker, on CRF-released small progenitors indicates that they are stem cell-related progenitors. One filament or one bud-like structure can release many similarly sized progenitors, suggesting synchronized production of progenitors in filaments or in bud-like structures. Although we could not provide data to trace the subsequent differentiation of these small progenitors after their release, we observed that the sizes of the small progenitors were not the same after release (Figs. 3a, c, 4), which suggests that these restricted progenitors undergo transdifferentiation to become restricted cells in the blood. When the nucleus of the progenitors is fully differentiated, mitotic division begins.
We also identified the tube-shaped structures that are the origins or niches of CRFs or the bud-like structures. These tube-shaped structures have distinct differences in morphologies, their contents and the staining colors to H&E, suggesting that their specificities have been predetermined. Each niche is from the specific cellular area and produces only one type of specific cell progenitors. Also, these tube-shaped niches can migrate and release their contents in the circulation. Although the formation and the components of these specialized niches remained unknown, we believe that these tube-shaped structures locate in bone-marrow sinus or perivascular areas.
Because the size of these newly released small progenitors is similar to platelets, they locate in the same density of platelets in the blood before their fully differentiation and therefore, could be mistakenly classified as platelets. Thus, they may participate in the function credited to the platelets.
Our data support the new consensus that unipotent progenitors are the predominant components in bone marrow. However, our data do not support the HSC transformation into restricted progenitors, either hierarchically or two-tiered. We believe that the lineage segregation occurs during the embryonic development, so only lineage-restricted cells exist in postnatal life. The life-long production of the restricted progenitors in postnatal life is by forming new progenitors via the combination of the lineage restricted DNA fragments, the circulation proteins and other unidentified materials. Although transdifferentiation is the procedure for producing the spore-like progenitors, it may not be the procedure for other progenitor production, since we have found that epithelial progenitors are produced by particle fusion [13]. Thus, the progenitor forming procedures may vary depending on the lineage of the progenitor, which include particle fusion, transformation and other unidentified steps.
Acknowledgments
None to declare.
Financial Disclosure
This work was supported by a grant from the Department of Technology, Inner Mongolia, China.
Conflict of Interest
None of the authors have any competing interest for the work in this manuscript.
Informed Consent
Not applicable.
Author Contributions
WK: manuscript writing, project directing and experimental designs. XH: performing experiments and data collection. HW: performing experiments and data collection. XZ: performing experiments and data collection.
| References | ▴Top |
- Seita J, Weissman IL. Hematopoietic stem cell: self-renewal versus differentiation. Wiley Interdiscip Rev Syst Biol Med. 2010;2(6):640-653.
doi pubmed - England SJ, McGrath KE, Frame JM, Palis J. Immature erythroblasts with extensive ex vivo self-renewal capacity emerge from the early mammalian fetus. Blood. 2011;117(9):2708-2717.
doi pubmed - Shizuru JA, Negrin RS, Weissman IL. Hematopoietic stem and progenitor cells: clinical and preclinical regeneration of the hematolymphoid system. Annu Rev Med. 2005;56:509-538.
doi pubmed - Wagers AJ, Christensen JL, Weissman IL. Cell fate determination from stem cells. Gene Ther. 2002;9(10):606-612.
doi pubmed - Orkin SH, Zon LI. Hematopoiesis: an evolving paradigm for stem cell biology. Cell. 2008;132(4):631-644.
doi pubmed - Nakajima H. Role of transcription factors in differentiation and reprogramming of hematopoietic cells. Keio J Med. 2011;60(2):47-55.
doi pubmed - Heffner GC, Clutter MR, Nolan GP, Weissman IL. Novel hematopoietic progenitor populations revealed by direct assessment of GATA1 protein expression and cMPL signaling events. Stem Cells. 2011;29(11):1774-1782.
doi pubmed - Sanjuan-Pla A, Macaulay IC, Jensen CT, Woll PS, Luis TC, Mead A, Moore S, et al. Platelet-biased stem cells reside at the apex of the haematopoietic stem-cell hierarchy. Nature. 2013;502(7470):232-236.
doi pubmed - Notta F, Zandi S, Takayama N, Dobson S, Gan OI, Wilson G, Kaufmann KB, et al. Distinct routes of lineage development reshape the human blood hierarchy across ontogeny. Science. 2016;351(6269):aab2116.
doi pubmed - Wuidart A, Sifrim A, Fioramonti M, Matsumura S, Brisebarre A, Brown D, Centonze A, et al. Early lineage segregation of multipotent embryonic mammary gland progenitors. Nat Cell Biol. 2018;20(6):666-676.
doi pubmed - Grinenko T, Eugster A, Thielecke L, Ramasz B, Kruger A, Dietz S, Glauche I, et al. Hematopoietic stem cells can differentiate into restricted myeloid progenitors before cell division in mice. Nat Commun. 2018;9(1):1898.
doi pubmed - Kong W, Zhu XP, Han XJ, Wang H. Erythrocytes are produced in thick membrane-like structures in adult mouse blood. J Cytol Histol. 2018;9:513.
doi - Kong W, Zhu XP, Han XJ, Nuo M, Wang H. Epithelial stem cells are formed by small-particles released from particle-producing cells. PLoS One. 2017;12(3):e0173072.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


