| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website http://www.thejh.org |
Case Report
Volume 8, Number 3, September 2019, pages 132-136
Combined Modality Treatment With Brentuximab Vedotin and Radiation Therapy for Primary Cutaneous Anaplastic Large Cell Lymphoma: A Case Report
Erin G. Floyda, Timothy F. Burnsb, Konstantinos Linosc, Robert E. LeBlancc, Joi B. Carterd, Lesley A. Jarvise, Frederick Lansiganf, g
aGeisel School of Medicine at Dartmouth, Dartmouth-Hitchcock Medical Center, Zimmerman Lounge Box 47, 1 Medical Center Drive, Lebanon, NH 03766, USA
bDepartment of Medicine, Division of Medical Oncology and Hematology, Mayo Clinic Health System, 1221 Whipple St., Eau Claire, WI, 54703, USA
cDepartment of Pathology and Laboratory Medicine, Dartmouth-Hitchcock Medical Center, 1 Medical Center Drive, Lebanon, NH 03766, USA
dSection of Dermatology, Dartmouth-Hitchcock Medical Center, 1 Medical Center Drive, Lebanon, NH 03766, USA
eDepartment of Radiation Oncology, Dartmouth-Hitchcock Medical Center, 1 Medical Center Drive, Lebanon, NH 03766, USA
fDepartment of Hematology and Oncology, Dartmouth-Hitchcock Medical Center, 1 Medical Center Drive, Lebanon, NH 03766, USA
gCorresponding Author: Frederick Lansigan, Department of Hematology and Oncology, Dartmouth-Hitchcock Medical Center, 1 Medical Center Drive, Lebanon, NH 03766, USA
Manuscript submitted July 3, 2019, accepted August 14, 2019
Short title: Combined Therapy for CD30+ T-Cell Lymphoma
doi: https://doi.org/10.14740/jh534
| Abstract | ▴Top |
Primary cutaneous anaplastic large cell lymphoma (pcALCL) is a rare form of non-Hodgkins lymphoma. Current frontline treatments for pcALCL include surgical resection, anthracycline-based chemotherapy, and/or radiation therapy (RT) depending on disease severity. While brentuximab vedotin (BV) has been used for refractory/relapsed cases, it recently received Food and Drug Administration (FDA) approval for use in combination with chemotherapy for peripheral T-cell lymphomas. In this case report, we utilized a combined modality therapy of RT and BV for a limited stage aggressive pcALCL presentation for which routine management is contraindicated. A 59-year-old man with a history of peripheral vascular disease (PVD) presented with an aggressive pcALCL involving the left inferior eyelid and small ipsilateral level II hypermetabolic lymph nodes at stage IIE. Due to the patient’s history of PVD, the tumor’s rapid growth, possible lymph node involvement, and eye proximity, BV was chosen as the initial chemotherapy treatment followed by RT. Complete metabolic resolution of the primary cutaneous lesion and lymphadenopathy was reached after BV treatment alone; complete clinical response of the primary tumor was reached following radiation therapy. Relapse occurred within 7 months. Salvage cyclophosphamide, vincristine, etoposide, and prednisone were not effective. Retreatment with BV + RT is currently being used to treat the new lesions. Our case illustrates that a combination of BV and RT can be a safe and effective initial treatment in patients with limited stage pcALCL who cannot tolerate anthracycline-based chemotherapy. Our patient had a complete response but ultimately relapsed; thus larger clinical trials are needed to better understand early-stage disease.
Keywords: Non-Hodgkins lymphoma; CD30+ lymphoproliferative disorders; Brentuximab vedotin; Radiation therapy
| Introduction | ▴Top |
Primary cutaneous anaplastic large cell lymphoma (pcALCL) is a rare form of non-Hodgkins lymphoma (NHL) characterized by cutaneous and subcutaneous nodules of neoplastic CD30+ lymphocytes. Initial treatment strategies include surgical excision or radiation therapy (RT) for solitary lesions, and chemotherapy such as cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) for advanced disease [1]. In limited stage disease, RT alone is effective [2-5]. Combined modality treatment (CMT) with chemotherapy and radiation is effective for more advanced disease [6]. Overall, treatment responses are excellent, but relapses are frequent. For relapsed/refractory CD30+ lymphomas, brentuximab vedotin (BV) is highly effective with improved survival outcomes [7-9]. Its efficacy in the frontline setting has yet to be well described in pcALCL, but BV is approved for frontline use in combination with chemotherapy for peripheral T-cell lymphomas [10]. Here we present a patient with an aggressive limited stage pcALCL who was not a candidate for anthracycline-based chemotherapy but successfully achieved a complete response to upfront BV followed by radiation and continued to respond to BV after relapse.
| Case Report | ▴Top |
A 59-year-old man with a past medical history of peripheral vascular disease (PVD) presented with a rapidly growing 1.5 cm erythematous plaque on the left medial canthus. Full skin exam was negative for pre-existing patches or other plaques. Shave biopsy revealed a dense, nodular lymphoid infiltrate effacing the dermis. The cells were large with irregular, lobulated nuclei, nucleoli, and mitoses. All malignant cells expressed CD30, CD4, and CD2 by immunohistochemistry and were negative for anaplastic lymphoma kinase (ALK), CD20, CD56 and Epstein-Barr virus-encoded small RNA (EBER) (Fig. 1). Peripheral blood flow cytometry was negative for circulating abnormal T cells. The patient was diagnosed with a CD30+ lymphoproliferative disorder and referred to Hematology for staging.
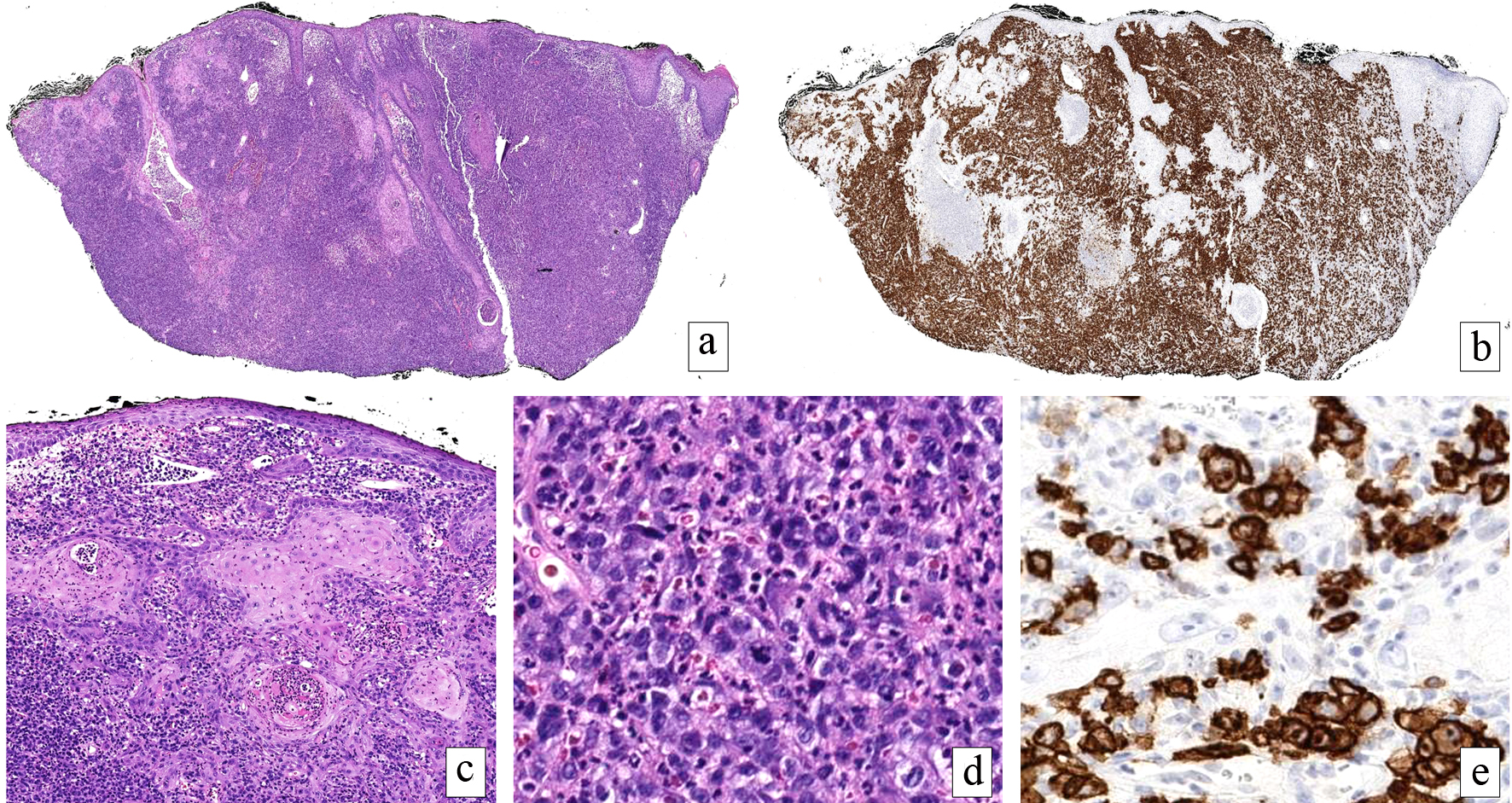 Click for large image | Figure 1. Primary cutaneous anaplastic large cell lymphoma. A dense, cellular infiltrate fills the dermis (H&E, × 20) (a) comprised of cells with uniform CD30 expression (× 20) (b). The overlying epidermis and follicular epithelium exhibit a reactive pseudoepitheliomatous hyperplasia mimicking squamous cell carcinoma (H&E, × 100) (c). Large, pleomorphic and mitotically active cells with bizarre nuclear contours predominate below the epidermal changes (H&E, × 200) (d). The markedly atypical cells exhibited an immunophenotype consistent with lymphoma. Higher power magnification of CD30 highlighting lesional cells (× 200) (e). The tumor cells were negative for ALK, CD20 and CD56, EBER, cytokeratin, 34BetaE12, and p63 (not shown). H&E: hematoxylin-eosin; ALK: anaplastic lymphoma kinase; EBER: Epstein-Barr virus-encoded small RNA. |
Positron emission tomography/computed tomography (PET/CT) scan was performed approximately 4 weeks after presentation and revealed a 2.5 × 4.1 cm hypermetabolic mass involving the left inferior eyelid and small ipsilateral level II hypermetabolic lymph nodes, clinically stage IIE. The clinical and histopathologic findings were consistent with a diagnosis of pcALCL. Given the rapid tumor growth, close proximity to the eye (Fig. 2a), and probable lymph node involvement, prompt treatment with BV was recommended.
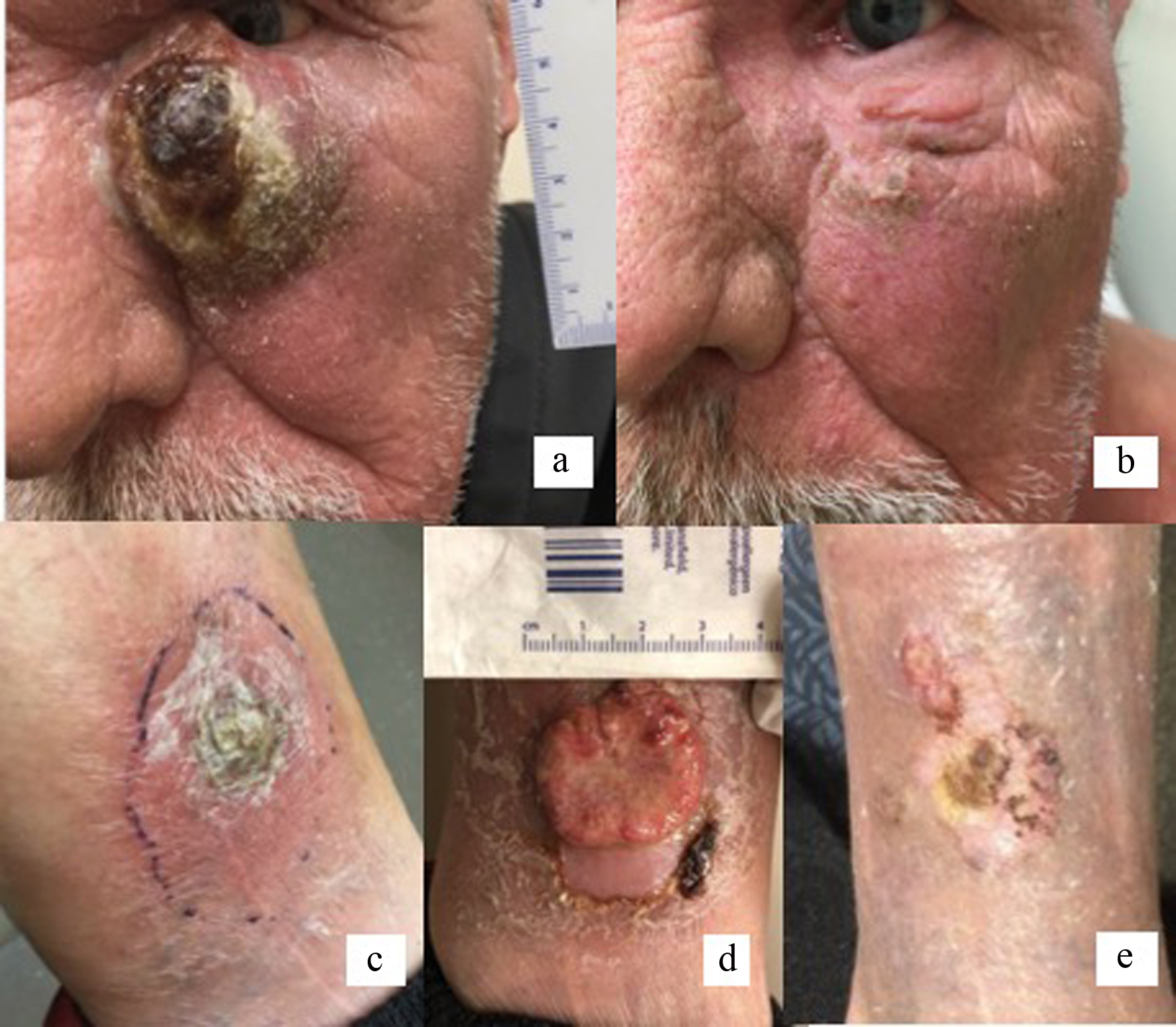 Click for large image | Figure 2. Rapidly growing tumor extending to left medial canthus, 1 week prior to first cycle of brentuximab vedotin (a). Resolution of primary tumor following three cycles of brentuximab vedotin and 20 fractions of radiation therapy (b). Relapse on left distal leg within 7 months of achieving complete clinical response with CMT (c). Progression of tumor following three cycles of CEOP (d). Left distal leg lesion following six cycles of brentuximab vedotin and radiation therapy (e). CMT: combined modality treatment; CEOP: cyclophosphamide, etoposide, vincristine and prednisone. |
The patient received BV at a dose of 1.8 mg/kg every 3 weeks for a total of three cycles. Treatment was well tolerated. After three cycles, the primary lesion had significantly reduced in size to 1.2 × 0.8 cm. Restaging PET/CT, obtained 68 days after starting treatment, showed a complete metabolic resolution of the primary cutaneous lesion and lymphadenopathy. The patient subsequently underwent consolidative radiation therapy with 20 fractions of 180 cGy (total dose 36 Gy) to complete definitive therapy. Post-radiation follow-up showed complete clinical response of the primary tumor (Fig. 2b).
He was followed clinically but approximately 7 months after CMT presented with a new ulcerated lesion of the right forearm and two left lower extremity lesions, one distal (Fig. 2c) and the other proximal. Biopsy of the left leg proximal lesion showed about 80% of cells with CD30+ expression, confirming relapse of pcALCL. Restaging PET/CT showed no other areas of uptake. His case was discussed at multidisciplinary tumor board, and systemic therapy was recommended, specifically an anthracycline sparing regimen given his history of cardiovascular disease. He began salvage treatment with three cycles of cyclophosphamide, vincristine, etoposide, and prednisone (CEOP) chemotherapy [11, 12], which he tolerated well without significant side effects. The patient displayed significant clinical regression of cutaneous lesions of the right forearm. However, there was progression of the left lower extremity lesion (Fig. 2d). Based on the recently published data showing efficacy of retreatment with BV in relapsed CD30+ hematologic malignancies [13], and his prior complete response to BV, the decision was made to retreat with BV and radiation therapy, which again led to significant clinical regression of both lower extremity lesions (Fig. 2e). He is currently receiving maintenance BV with a plan to complete 6 - 12 months of therapy.
| Discussion | ▴Top |
pcALCL represents a rare form of NHL with heterogeneous cutaneous characteristics, and despite a favorable overall survival for most patients, relapses occur in most cases, indicating that new therapies are needed. CHOP chemotherapy is often included when CMT is given, but this regimen carries higher toxicity, and patients with comorbidities may not be good candidates for CHOP. In our case, the patient had limited stage disease but displayed an aggressive clinical course with a rapidly expanding lesion and local lymph node involvement. Surgical resection was deemed highly morbid given the proximity to the eye. Additionally, his history of PVD contraindicated the use of anthracycline-based chemotherapy, forcing us to proceed to a more tolerable regimen in BV.
BV is an antibody-drug conjugate that binds to CD30+ cells and delivers the microtubule disrupting agent monomethyl auristatin E [14]. Based on activity in relapsed/refractory CD30+ lymphomas [9], BV was felt to be the safest and most effective systemic therapy to use in combination with RT for our patient. BV has been approved for relapsed or refractory CD30+ disease [8, 10]. Recently, frontline use of BV in combination with systemic chemotherapy has been approved for the treatment of CD30+ peripheral T-cell lymphoma, and of note, pcALCL was excluded in the ECHELON-2 study [10]. Frontline use of BV has not yet been adequately described in limited stage pcALCL, although it is recommended by the National Comprehensive Cancer Network guidelines.
In our patient, frontline therapy with BV provided a radiographic and clinical complete response after just three cycles of BV followed by RT. Unfortunately, as is typical of this disease, the patient relapsed with two distant areas of cutaneous lesions 7 months after completing radiation therapy. Salvage combination chemotherapy that did not include an anthracycline was subsequently used, but unfortunately, he did not respond adequately as evident by progression of the larger tumor on the leg. Retreatment with BV has been shown to be effective in systemic ALCL [13]. Bartlett et al showed that 88% responded when retreated including 63% complete remission (CR) in systemic ALCL patients. The estimated median duration of response for patients with an objective response was 9.5 months. Thus, BV was resumed as a third-line therapy in addition to radiation with good response.
There are many treatment options for pcALCL, but the optimal strategy is not well described. Incorporating a targeted agent such as BV is worthwhile, and currently approved for salvage therapy. Our case indicates that frontline use of BV can induce a complete remission, but further studies are needed to verify its use in this manner. Additionally, our patient did not respond as well to combination chemotherapy in the salvage setting but did respond to retreatment with BV. Given the tendency for pcALCL to relapse, further studies are needed to identify optimal treatment strategies both in the frontline and salvage settings.
Conclusions
Our case illustrates that a combination of BV and RT can be a safe and effective first-line treatment in patients with limited stage disease pcALCL with contraindications to anthracycline-based chemotherapy; however, relapse can still occur. Incorporation of CD30-directed therapy to current practices for limited stage pcALCL should be investigated in the context of a clinical trial.
Acknowledgments
Authors would like to acknowledge and thank the patient to whom we had the privilege of providing care.
Financial Disclosure
This research was supported by the National Center for Complementary and Integrative Health (NCCIH) grant: T32AT003997 (Hecht/Adler). This research did not receive any additional funding from agencies in the public, commercial, or not-for-profit sectors.
Conflict of Interest
Dr. Frederick Lansigan has been a consultant for Seattle Genetics. The other authors have stated that they have no conflict of interest.
Informed Consent
Patient’s informed consent for publication of this report was obtained.
Author Contributions
All authors had substantial contributions to the conception or design of the manuscript or the acquisition, analysis, or interpretation of data. EF and TB drafted the manuscript. All authors revised it critically for important intellectual content, approved the final version to be published, and are in agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
| References | ▴Top |
- Kempf W, Pfaltz K, Vermeer MH, Cozzio A, Ortiz-Romero PL, Bagot M, Olsen E, et al. EORTC, ISCL, and USCLC consensus recommendations for the treatment of primary cutaneous CD30-positive lymphoproliferative disorders: lymphomatoid papulosis and primary cutaneous anaplastic large-cell lymphoma. Blood. 2011;118(15):4024-4035.
doi pubmed - Million L, Yi EJ, Wu F, Von Eyben R, Campbell BA, Dabaja B, Tsang RW, et al. Radiation therapy for primary cutaneous anaplastic large cell lymphoma: an international lymphoma radiation oncology group multi-institutional experience. Int J Radiat Oncol Biol Phys. 2016;95(5):1454-1459.
doi pubmed - Melchers RC, Willemze R, Daniels LA, Neelis KJ, Bekkenk MW, de Haas ERM, Horvath B, et al. Recommendations for the optimal radiation dose in patients with primary cutaneous anaplastic large cell lymphoma: a report of the Dutch Cutaneous Lymphoma Group. Int J Radiat Oncol Biol Phys. 2017;99(5):1279-1285.
doi pubmed - Smith GL, Duvic M, Yehia ZA, Allen P, Garg N, Suki T, Milgrom SA, et al. Effectiveness of low-dose radiation for primary cutaneous anaplastic large cell lymphoma. Adv Radiat Oncol. 2017;2(3):363-369.
doi pubmed - Yu JB, McNiff JM, Lund MW, Wilson LD. Treatment of primary cutaneous CD30+ anaplastic large-cell lymphoma with radiation therapy. Int J Radiat Oncol Biol Phys. 2008;70(5):1542-1545.
doi pubmed - Hapgood G, Pickles T, Sehn LH, Villa D, Klasa R, Scott DW, Gerrie AS, et al. Outcome of primary cutaneous anaplastic large cell lymphoma: a 20-year British Columbia Cancer Agency experience. Br J Haematol. 2017;176(2):234-240.
doi pubmed - Younes A, Bartlett NL, Leonard JP, Kennedy DA, Lynch CM, Sievers EL, Forero-Torres A. Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. N Engl J Med. 2010;363(19):1812-1821.
doi pubmed - Prince HM, Kim YH, Horwitz SM, Dummer R, Scarisbrick J, Quaglino P, Zinzani PL, et al. Brentuximab vedotin or physician's choice in CD30-positive cutaneous T-cell lymphoma (ALCANZA): an international, open-label, randomised, phase 3, multicentre trial. Lancet. 2017;390(10094):555-566.
doi - Pro B, Advani R, Brice P, Bartlett NL, Rosenblatt JD, Illidge T, Matous J, et al. Five-year results of brentuximab vedotin in patients with relapsed or refractory systemic anaplastic large cell lymphoma. Blood. 2017;130(25):2709-2717.
doi pubmed - Horwitz S, O'Connor OA, Pro B, Illidge T, Fanale M, Advani R, Bartlett NL, et al. Brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma (ECHELON-2): a global, double-blind, randomised, phase 3 trial. Lancet. 2019;393(10168):229-240.
doi - Bartlett NL, Petroni GR, Parker BA, Wagner ND, Gockerman JP, Omura GA, Canellos GP, et al. Dose-escalated cyclophosphamide, doxorubicin, vincristine, prednisone, and etoposide (CHOPE) chemotherapy for patients with diffuse lymphoma: Cancer and Leukemia Group B studies 8852 and 8854. Cancer. 2001;92(2):207-217.
doi - Schmitz N, Trumper L, Ziepert M, Nickelsen M, Ho AD, Metzner B, Peter N, et al. Treatment and prognosis of mature T-cell and NK-cell lymphoma: an analysis of patients with T-cell lymphoma treated in studies of the German High-Grade Non-Hodgkin Lymphoma Study Group. Blood. 2010;116(18):3418-3425.
doi pubmed - Bartlett NL, Chen R, Fanale MA, Brice P, Gopal A, Smith SE, Advani R, et al. Retreatment with brentuximab vedotin in patients with CD30-positive hematologic malignancies. J Hematol Oncol. 2014;7:24.
doi pubmed - Deng C, Pan B, O'Connor OA. Brentuximab vedotin. Clin Cancer Res. 2013;19(1):22-27.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


