| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website http://www.thejh.org |
Case Report
Volume 8, Number 1, March 2019, pages 29-33
Intracranial Involvement in Multiple Myeloma Presenting as a Cranial Nerve Palsy
Eilis Fitzgeralda, Patrick Kielyb, d, Hilary O Learyc
aUniversity Hospital Limerick, Limerick, Ireland
bDepartment of Radiology, University Hospital Limerick, Limerick, Ireland
cDepartment of Haematology, University Hospital Limerick, Limerick, Ireland
dCorresponding Author: Patrick Kiely, Department of Radiology, University Hospital Limerick, Limerick V94 YVH0, Ireland
Manuscript submitted October 30, 2018, accepted November 26, 2018
Short title: Intracranial Multiple Myeloma
doi: https://doi.org/10.14740/jh468
| Abstract | ▴Top |
Multiple myeloma (MM) is characterized by the neoplastic proliferation of plasma cells producing a monoclonal immunoglobulin. Neurological complications in MM most frequently occur due to spinal cord compression by bony lesions, paraprotein-related neuropathy, hypercalcemia, hyperviscosity, or amyloidosis. Intracranial involvement is a rare complication of MM occurring in only 1% of patients. It can manifest as a solitary cerebral lesion, intra-parenchymal infiltration, or diffuse leptomeningeal disease. We present a case of a leptomeningeal myeloma in a 71-year-old woman with known relapsed MM presenting with a right sixth nerve palsy. Our patient was receiving spinal irradiation for a paraspinal plasmacytoma when she complained of double vision. Clinical exam revealed a right sixth nerve palsy. MRI revealed diffuse abnormal leptomeningeal thickening and enhancement typical for diffuse leptomeningeal infiltration. She was treated with whole brain irradiation and intrathecal methotrexate combined with a lenalidomide and dexamethasone chemotherapeutic regimen but unfortunately she passed away 5 weeks after onset of visual symptoms. MM involving the central nervous system (CNS) is a rare complication of MM and carries a poor prognosis with an average survival of 3 months. Due to its rarity, treatment of CNS MM is very heterogeneous. Thus case reporting is important to accumulate data on this rare presentation.
Keywords: Multiple myeloma; Intracranial; Leptomeningeal; Cranial nerve; Hematology; Oncology
| Introduction | ▴Top |
Multiple myeloma (MM) is a disease characterized by the neoplastic proliferation of plasma cells producing a monoclonal immunoglobulin. MM accounts for approximately 1% of all cancers and slightly more than 10% of hematologic malignancies in the USA [1]. It is an incurable disease and the cause of about 20% of deaths from hematologic malignancy and 2% of deaths from all cancers [2].
MM is thought to evolve from an asymptomatic pre-malignant stage of clonal plasma cell proliferation termed monoclonal gammopathy of undetermined significance (MGUS). MGUS is present in over 3% of the population above the age of 50 and progresses to myeloma or a related malignancy at a rate of 1% per year [3]. The malignant plasma cells produce immunoglobulin heavy chains plus light chains, light chains alone or neither. IgG and kappa are the most common immunoglobulin and light chain produced, respectively.
The diagnosis of MM requires evidence of either clonal bone marrow plasma cells or a plasmacytoma, in addition to documented organ impairment or presence of a biomarker associated with progression to end-organ damage. While MGUS is asymptomatic, MM is characterized by end-organ damage, specifically hypercalcemia, renal dysfunction, anemia or lytic bone lesions.
Central nervous system (CNS) and leptomeningeal involvement in MM
Neurological complications in MM most frequently occur due to spinal cord compression by bony lesions, paraprotein-related neuropathy, hypercalcemia, hyperviscosity, or amyloidosis [4]. Intracranial structures are rarely involved by MM themselves [5]. CNS involvement is thought to occur either as a result of direct extra-osseous spread from adjacent plasmacytomas or via hematogenous dissemination of extra-medullary disease via the cerebrospinal fluid (CSF) and has a varied interval from original diagnosis of MM to CNS involvement of between 0 and 120 months [5]. CNS involvement may manifest as either a solitary cerebral lesion, intra-parenchymal infiltration, or diffuse leptomeningeal disease such as CNS myelomatosis [6]. The most common presenting symptoms in cases of intracranial involvement include extremity weakness, change of mental status, and cranial nerve palsies. The prognosis is poor, with a median overall survival (OS) from time of diagnosis of CNS involvement of between 1.5 - 3 months [4, 6]. More updated references would suggest that this time period is now longer.
| Case Report | ▴Top |
We present the case of a 71-year-old woman referred to the hematology department with a 6-month history of back pain and generalized fatigue in August 2013. At the time of presentation she had evidence of osteopenic fractures, anemia (hemoglobin (Hb) 8.7 g/dL) and raised IgG levels (61.39 g/dL), all highly suggestive of MM. Subsequently, her skeletal survey showed multiple lucencies with occipital lytic lesions, a moth-eaten mandible and multiple levels of vertebral height loss. Serum beta-2-microglobulin was elevated at 5.59 mg/L. Bone marrow aspirate and trephine biopsy showed 80-90% infiltration by abnormal large plasma cells. She was therefore diagnosed with stage III MM, as per the International Staging System (ISS). She was commenced on bortezomib, melphalan and prednisolone (VMP) chemotherapy, but was switched to melphalan and prednisolone (MP) after two cycles due to the development of peripheral neuropathy, likely due to bortezomib. She then underwent five cycles of MP followed by five cycles of melphalan, prednisolone and thalidomide (MPT). Remission was achieved 1 year after diagnosis. Maintenance therapy of thalidomide 50 mg daily was continued.
In January 2015, the IgG paraprotein level rose so thalidomide was increased to 100 mg daily. Despite this, levels continued to rise and thalidomide was replaced with cyclophosphamide 500 mg weekly. Subsequently, the IgG paraprotein level dropped and the dose was decreased to 300 mg daily. In July 2015, the paraprotein rose again and the patient also reported a pain radiating down her right leg. Magnetic resonance imaging (MRI) revealed a soft tissue mass in the right sacral ala involving the L5/S1 formina as well as iliopsoas and paraspinal muscles. Spinal radiotherapy was commenced and her chemotherapy was switched to lenalidomide and dexamethasone (LD).
In September 2015, the patient reported new onset double vision on the background of this recent MM relapse. Clinical exam was suggestive of a right sixth nerve palsy. MRI showed diffuse abnormal leptomeningeal thickening and enhancement indicating diffuse leptomeningeal infiltration (Figs. 1, 2). There was extensive involvement of the right medial petrous apex involving the cavernous sinus with nodular enhancing leptomeninges, pathological restricted diffusion pattern and apparent diffusion coefficient correlate (Figs. 3-5). She received one dose of intrathecal methotrexate followed by whole brain radiotherapy. Unfortunately, CSF analysis was not performed. Once the whole brain radiotherapy was completed, the patient was due to recommence the third cycle of LD in October 2015. Weekly intrathecal methotrexate was also planned, but was deemed inappropriate due to the progressive decline in her performance status. She died 5 weeks after the initial onset of double vision.
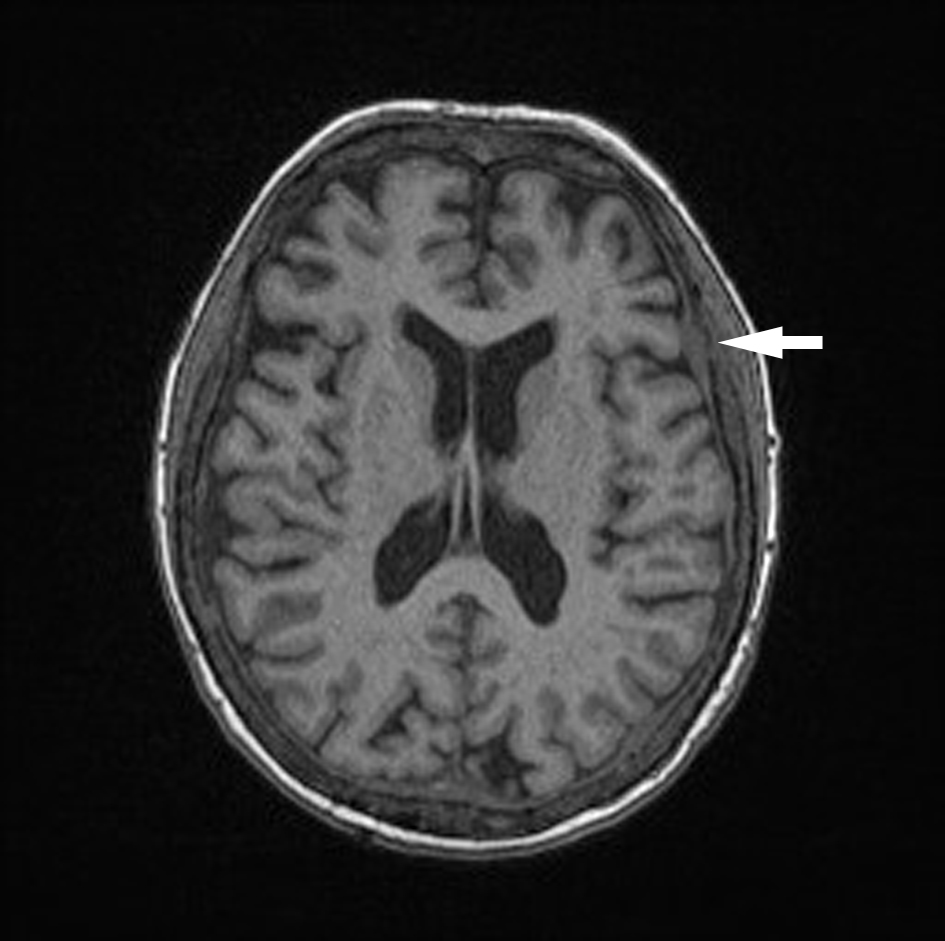 Click for large image | Figure 1. Unenhanced axial T1 MPR brain. Dural thickening diffusely. |
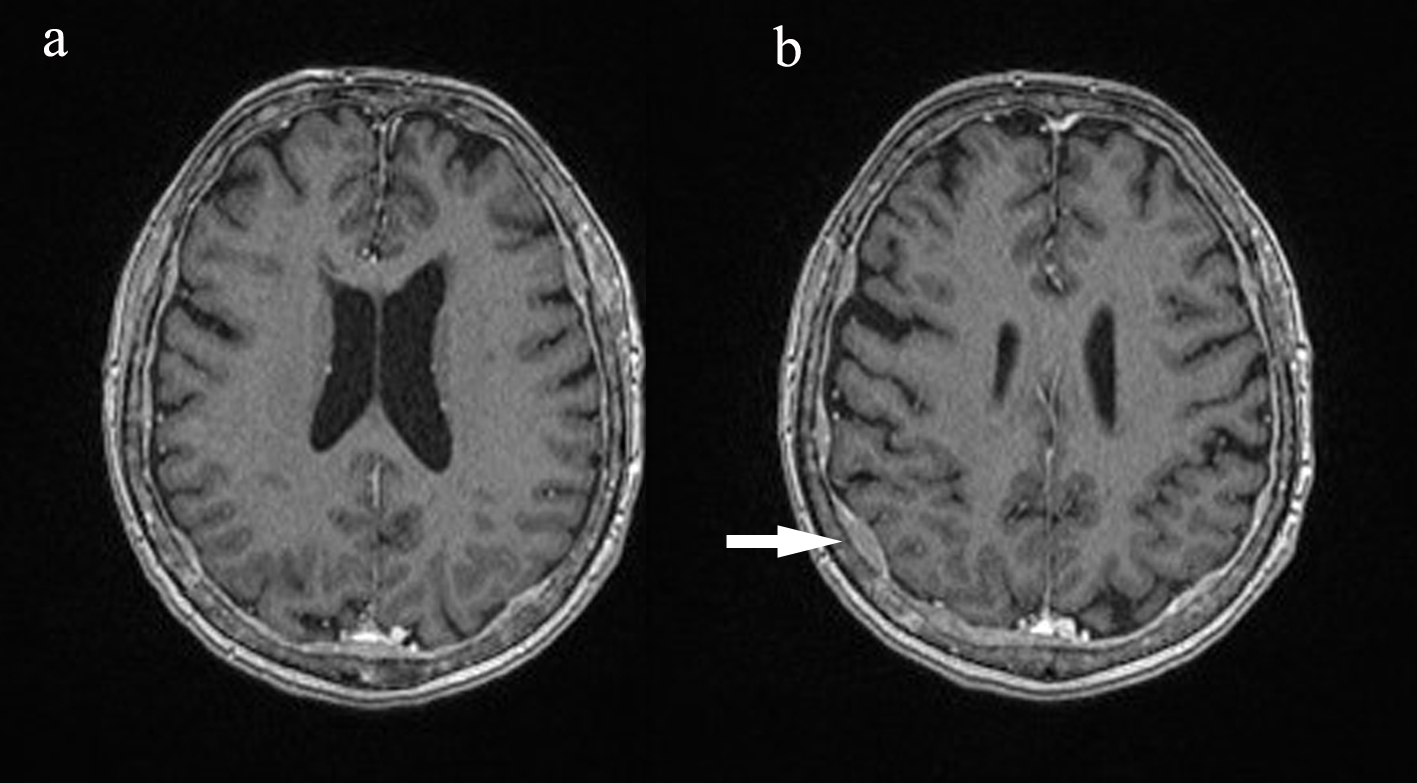 Click for large image | Figure 2. Post-contrast axial T1 MPR brain. Dural thickening and enhancing diffusely. |
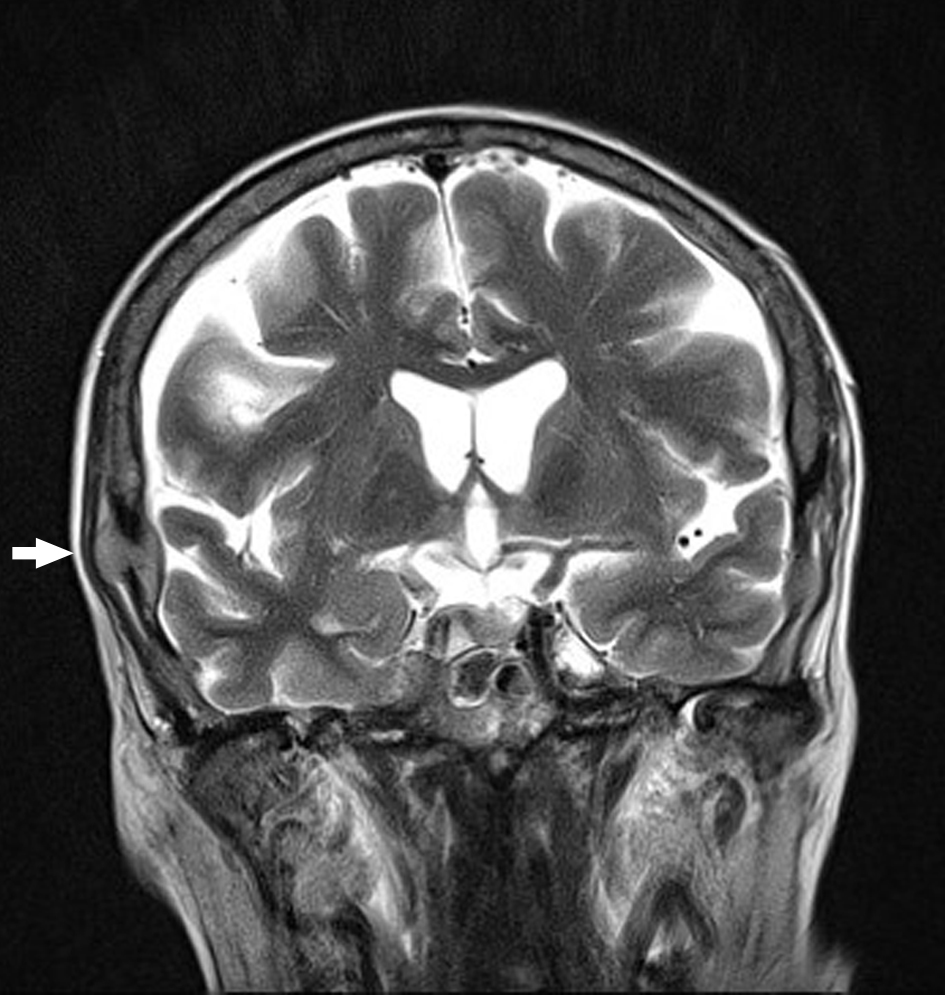 Click for large image | Figure 3. Coronal T2 BLADE MRI brain with calvarial lesion elevating the dura (arrow). |
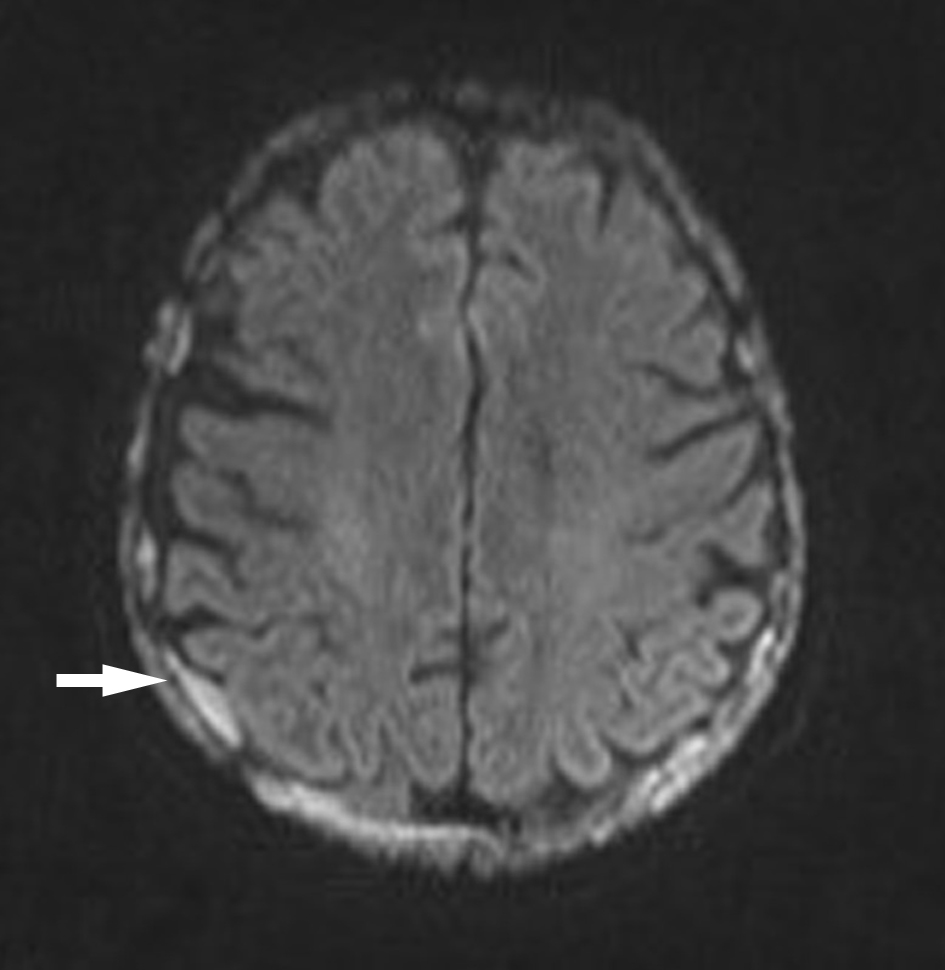 Click for large image | Figure 4. Axial diffusion-weighted image-brain with areas of dural diffusion restriction. |
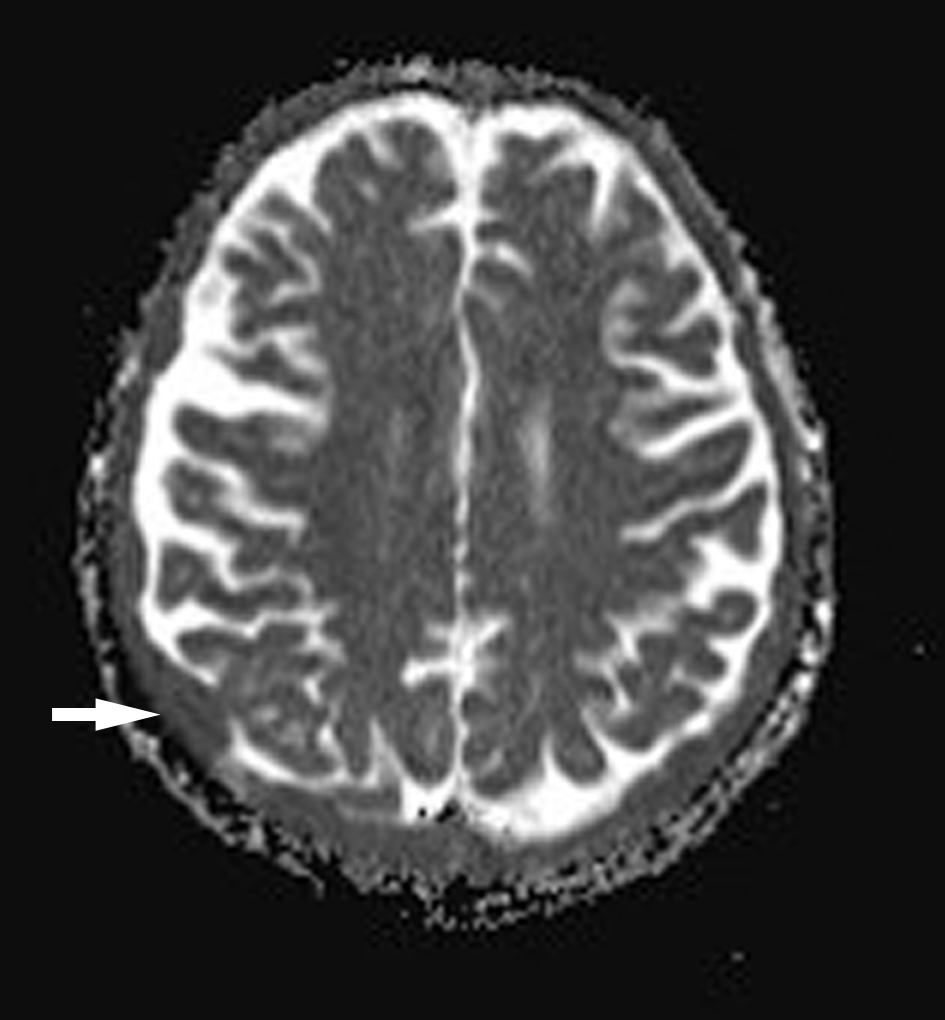 Click for large image | Figure 5. Axial apparent diffusion coefficient (ADC) map of brain with areas of dural low signal. |
| Discussion | ▴Top |
Involvement of the CNS in MM is very uncommon and has only been reported in 1% of MM patients [5]. It presents similarly to other leptomeningeal malignant neoplasms with symptoms ranging from mental status changes, gait disturbances, visual disturbances to cranial nerve palsies [4]. Leptomeningeal involvement, as seen in our patient, is one manifestation of CNS MM and its appearance is very similar to other leptomeningitides. It is differentiated from other meningeal diseases via imaging and CSF analysis is an important step in its management. Of the different modes of CNS involvement, leptomeningeal myeloma is thought to have the worst prognosis [7-9] with the median survival ranging from 1.5 - 3 months [4, 6].
Pathogenesis
The exact etiology of CNS MM remains unknown although there are several hypotheses being considered. Dural involvement is the most common intracranial manifestation of MM and usually occurs due to direct extension of a plasmacytoma of the skull into the CNS [10]. Similarly, lytic lesions of the skull may spread directly into the CNS. Hematogenous spread of plasma cells occurs in plasma cell leukemia and has been postulated to occur in a similar fashion in CNS MM. Lymphoid cells, considered as progenitors of plasma cells, may also spread in MM patients with a low tumor load. One of the most recent theories is that CNS involvement results from the continuous growth of plasma cells during the course and treatment of MM, as most drugs used in MM cannot cross the blood-brain barrier [5].
Risk factors
A number of risk factors for the development of CNS MM have been identified. Deletions of chromosome 17p13.1 (p53) have been found in 89% of the CNS MM patients [11]. It has also been suggested that an association exists between extra-medullary MM, plasmablastic morphology, cytogenetic abnormalities, high tumor load, increased LDH levels, circulating plasma cells [12] and CNS myeloma. The stage of disease has also been shown to be associated with one paper reporting stage III disease (Durie-Salmon staging system) in 41.3% of patients [12]. Paraspinal plasmacytomas have been postulated as a seeding source of plasma cells into CSF [13] and CD56 is often absent from the bone marrow and CSF of patients with CNS MM [14], suggesting that CD56 down-regulation may facilitate the escape of myeloma cells from adjacent plasmacytomas or bone marrow and their migration to, and growth in, the sub-arachnoid space.
Our patient had evidence of a high myeloma burden, stage III disease (by Dure-Salmon and ISS staging systems), extra-medullary MM, IgG kappa MM and the presence of a paraspinal plasmacytoma. Fluorescence in situ hybridization (FISH) analysis did not show any evidence of IGH-FGFR3 fusion product or proof of an aberration involving chromosome 17p. An unbalanced chromosome 1q gain was identified, which has been associated with an adverse prognosis in some studies. This patient would be considered at moderate to high risk of developing CNS MM, based on the research available.
Treatment
Since the introduction of novel agents such as thalidomide, lenalidomide and bortezomib into clinical practice, the median OS from the time of diagnosis for transplant-eligible patients with MM has been improved from 3 - 4 to 7 - 8 years [15]. While immunomodulators have contributed to the improvement in survival in MM, they appear to have limited activity against CNS MM and paradoxically, may contribute to the evolution of resistant MM clones capable of surviving within the CNS [16].
Treatment for CNS involvement in MM is usually unsuccessful and survival is very short. Regimens outlined in the literature show heterogeneity. However, certain modalities are slowly emerging as more successful than others. Craniospinal radiation has been shown to increase OS [15] as has intrathecal chemotherapy [16]. The choice of chemotherapeutic agent remains the area of most contention in CNS MM. The most common agents favored in the literature include thalidomide [17, 18], bendamustine [19, 20], bortezomib [21], lenalidomide [21] and other immunomodulatory drugs (IMiDs) [8], all of which have been shown to increase survival time.
Conclusions
CNS MM is a very rare complication of MM that carries a poor prognosis with OS of approximately 3 months. A variety of treatment strategies have been adopted, but prognosis remains short, except in rare case reports. We treated our patient with whole brain irradiation and intrathecal methotrexate. However she died despite this treatment 5 weeks after initial onset of symptoms. A more aggressive treatment strategy was not deemed appropriate due to her declining performance status and poor prognosis.
Abbreviations
MM: multiple myeloma; CSF: cerebrospinal fluid; CNS: central nervous system; MGUS: monoclonal gammopathy of undetermined significance; ISS: International Staging System; VMP: bortezomib, melphalan and prednisolone; MP: melphalan and prednisolone; MPT: melphalan, prednisolone and thalidomide; LD: lenalidomide and dexamethasone; OS: overall survival; FISH: fluorescence in situ hybridization; IMiDs: immunomodulatory drugs; Hb: hemoglobin; MRI: magnetic resonance imaging; ADC: apparent diffusion coefficient
| References | ▴Top |
- Becker N. Epidemiology of multiple myeloma. Recent Results Cancer Res. 2011;183:25-35.
doi pubmed - Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5-29.
doi pubmed - Uptodate: <https://www.uptodate.com/contents/overview-of-the-management-of-multiple-myeloma?search=intracranial%20myeloma&source=search_result&selectedTitle=1∼150&usage_type=default&display_rank=1> accessed online 29/10/2018.
- Kyle RA. Multiple myeloma: review of 869 cases. Mayo Clin Proc. 1975;50(1):29-40.
pubmed - Nieuwenhuizen L, Biesma DH. Central nervous system myelomatosis: review of the literature. Eur J Haematol. 2008;80(1):1-9.
pubmed - Yi HJ, Hwang HS, Moon SM, Shin IY, Choi YH. A case of multiple myeloma with brain parenchyme involvement. Brain Tumor Res Treat. 2013;1(2):103-106.
doi pubmed - Lee D, Kalff A, Low M, Gangatharan S, Ho P, Bajel A, Ritchie D, et al. Central nervous system multiple myeloma - potential roles for intrathecal therapy and measurement of cerebrospinal fluid light chains. Br J Haematol. 2013;162(3):371-375.
doi pubmed - Chen CI, Masih-Khan E, Jiang H, Rabea A, Cserti-Gazdewich C, Jimenez-Zepeda VH, Chu CM, et al. Central nervous system involvement with multiple myeloma: long term survival can be achieved with radiation, intrathecal chemotherapy, and immunomodulatory agents. Br J Haematol. 2013;162(4):483-488.
doi pubmed - Maghfoor I, Perry MC. Malignancy: Meningeal Myeloma: A Case Report and Review of Literature. Hematology. 2000;5(1):47-52.
doi pubmed - Lasocki A, Gangatharan S, Gaillard F, Harrison SJ. Intracranial involvement by multiple myeloma. Clin Radiol. 2015;70(8):890-897.
doi pubmed - Chang H, Sloan S, Li D, Keith Stewart A. Multiple myeloma involving central nervous system: high frequency of chromosome 17p13.1 (p53) deletions. Br J Haematol. 2004;127(3):280-284.
doi pubmed - Velasco R, Petit J, Llatjos R, Juan A, Bruna J. Can leptomeningeal myelomatosis be predicted in patients with IgD multiple myeloma? J Clin Neurosci. 2010;17(8):1071-1072.
doi pubmed - Vicari P, Ribas C, Sampaio M, Arantes AM, Yamamoto M, Filho JB, Segreto RA, et al. Can thalidomide be effective to treat plasma cell leptomeningeal infiltration? Eur J Haematol. 2003;70(3):198-199.
doi pubmed - Chang H, Bartlett ES, Patterson B, Chen CI, Yi QL. The absence of CD56 on malignant plasma cells in the cerebrospinal fluid is the hallmark of multiple myeloma involving central nervous system. Br J Haematol. 2005;129(4):539-541.
doi pubmed - Kumar SK, Rajkumar SV, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, Zeldenrust SR, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111(5):2516-2520.
doi pubmed - Nahi H, Svedmyr E, Lerner R. Bendamustine in combination with high-dose radiotherapy and thalidomide is effective in treatment of multiple myeloma with central nervous system involvement. Eur J Haematol. 2014;92(5):454-455.
doi pubmed - Muscal JA, Sun Y, Nuchtern JG, Dauser RC, McGuffey LH, Gibson BW, Berg SL. Plasma and cerebrospinal fluid pharmacokinetics of thalidomide and lenalidomide in nonhuman primates. Cancer Chemother Pharmacol. 2012;69(4):943-947.
doi pubmed - Hattori Y, Iguchi T. Thalidomide for the treatment of multiple myeloma. Congenit Anom (Kyoto). 2004;44(3):125-136.
doi pubmed - Chamberlain MC, Johnston SK. Salvage therapy with single agent bendamustine for recurrent glioblastoma. J Neurooncol. 2011;105(3):523-530.
doi pubmed - Renfrow JJ, Detroye A, Chan M, Tatter S, Ellis T, McMullen K, Johnson A, et al. Initial experience with bendamustine in patients with recurrent primary central nervous system lymphoma: a case report. J Neurooncol. 2012;107(3):659-663.
doi pubmed - Gozzetti A, Cerase A, Lotti F, Rossi D, Palumbo A, Petrucci MT, Patriarca F, et al. Extramedullary intracranial localization of multiple myeloma and treatment with novel agents: a retrospective survey of 50 patients. Cancer. 2012;118(6):1574-1584.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


