| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Case Report
Volume 11, Number 6, December 2022, pages 223-232
Thymoma With Triple Threat: Pure Red Cell Aplasia, Autoimmune Hemolytic Anemia, and T-Cell Large Granular Lymphocytic Leukemia
Tara Seiberta, Patrick J. Loehrerb, Andrew R.W. O’Brienb, c
aIndiana University School of Medicine, Indianapolis, IN 46202, USA
bDivision of Hematology/Oncology, Department of Medicine, Indiana University School of Medicine, Indianapolis, IN 46202, USA
cCorresponding Author: Andrew R.W. O’Brien, Division of Hematology/Oncology, Department of Medicine, Indiana University School of Medicine, Indianapolis, IN 46202, USA
Manuscript submitted September 26, 2022, accepted December 16, 2022, published online December 30, 2022
Short title: Thymoma With Triple Threat
doi: https://doi.org/10.14740/jh1061
| Abstract | ▴Top |
Thymomas are a rare neoplasm of the anterior mediastinum and often associated with paraneoplastic syndromes. Though myasthenia gravis is the most common and well-known, the list of reported paraneoplastic syndromes occurring with thymoma is extensive and ever-growing. Paraneoplastic syndromes can involve nearly every organ system, including hematologic abnormalities affecting any or all cell lines. This can present challenges to the clinician in terms of diagnosis, prognostic impact, and management. We present the case of a previously healthy 41-year-old female who was diagnosed with thymoma and three rare hematologic paraneoplastic syndromes: pure red cell aplasia (PRCA), autoimmune hemolytic anemia (AIHA), and T-cell large granular lymphocytic leukemia (T-LGLL). To the best of our knowledge, there have been only four other reported cases of PRCA and AIHA in a single patient with thymoma, all of which were treated with thymectomy. Upfront surgical resection was not possible in the present case and thus the patient was alternatively treated with corticosteroids and octreotide, which proved successful in resolving the anemia. The authors present this case to share these findings of an alternative treatment strategy for thymoma-associated PRCA and AIHA and to highlight the importance of careful monitoring with routine blood work for these complex patients.
Keywords: Thymoma; PRCA; AIHA; T-LGLL
| Introduction | ▴Top |
The thymus is responsible for the maturation and selection of functional, non-autoreactive T cells through mechanisms of positive and negative selection. Tight regulation of these processes is necessary to produce T cells capable of recognizing and reacting to foreign antigens while remaining non-reactive against self-antigens. Insults to thymic tissue architecture and consequent disruption of positive and negative selection processes is a proposed theory for the autoimmune paraneoplastic syndromes associated with thymoma [1]. An invading thymic neoplasm leading to lapse of regulation may enable autoreactive T cells to evade apoptosis, escape to the periphery, and precipitate autoimmune reactions. These autoimmune reactions often manifest as hematologic abnormalities, including bone marrow aplasia, hemolysis, and lymphoproliferative disorders.
| Case Report | ▴Top |
A 41-year-old white female with a history of psoriasis and asthma presented in February 2016 with complaints of dyspnea. Imaging revealed a 5.4-cm anterior mediastinal mass and a pleural-based soft tissue neoplasm with extensive spread throughout the right hemithorax, possible mediastinal invasion, and a large right pleural effusion (Fig. 1). CT-guided percutaneous biopsy with flow cytometry of the right pleural mass demonstrated numerous small TdT+ lymphocytes with intermixed cytokeratin-positive cells, consistent with the World Health Organization (WHO) B2 thymoma, stage IVA. Surgical excision was not recommended due to extent of disease and precarious location near the superior vena cava (SVC). The patient underwent six cycles of PAC (cisplatin, doxorubicin, and cyclophosphamide) from March to July 2016 with stable disease and persistence of pleural masses. Anticipated drops in trilineage cell counts occurred during the timeframe of active chemotherapy but normalized following completion of treatment (Fig. 2, time point A).
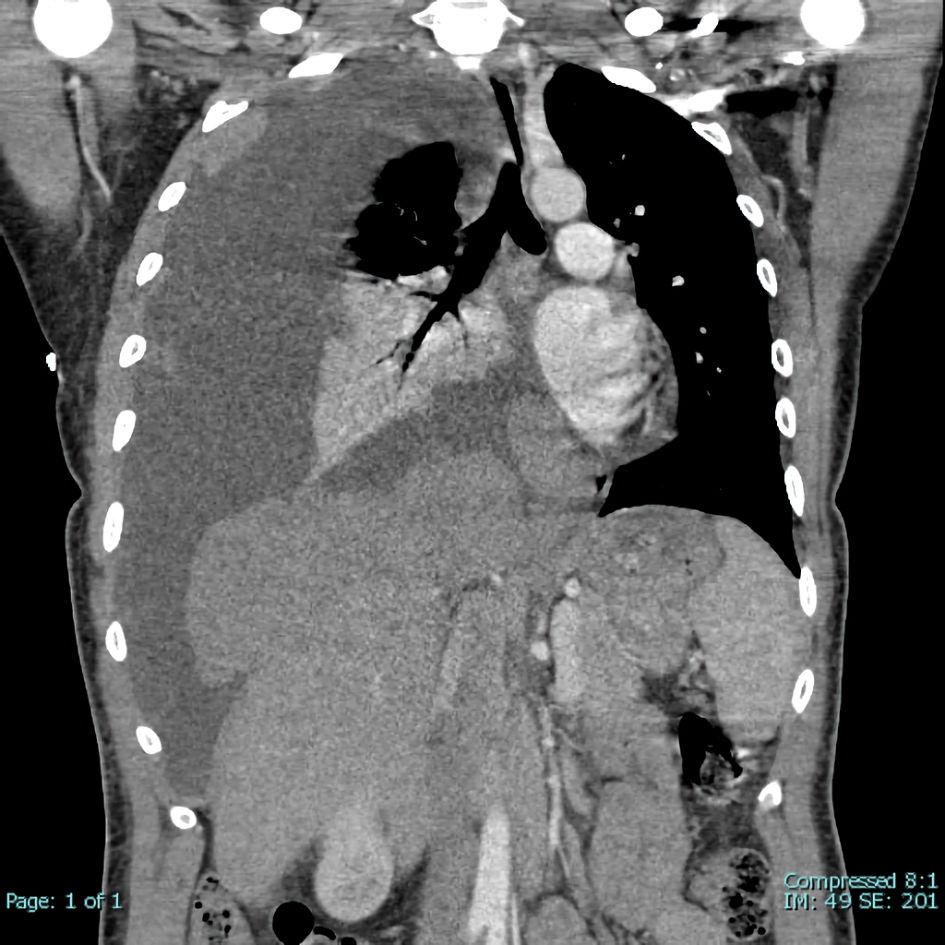 Click for large image | Figure 1. Extensive spread of right pleural-based soft tissue neoplasm. Very large right pleural effusion with secondary leftward mediastinal and tracheal shift. Partial collapse of right lung with air bronchograms. |
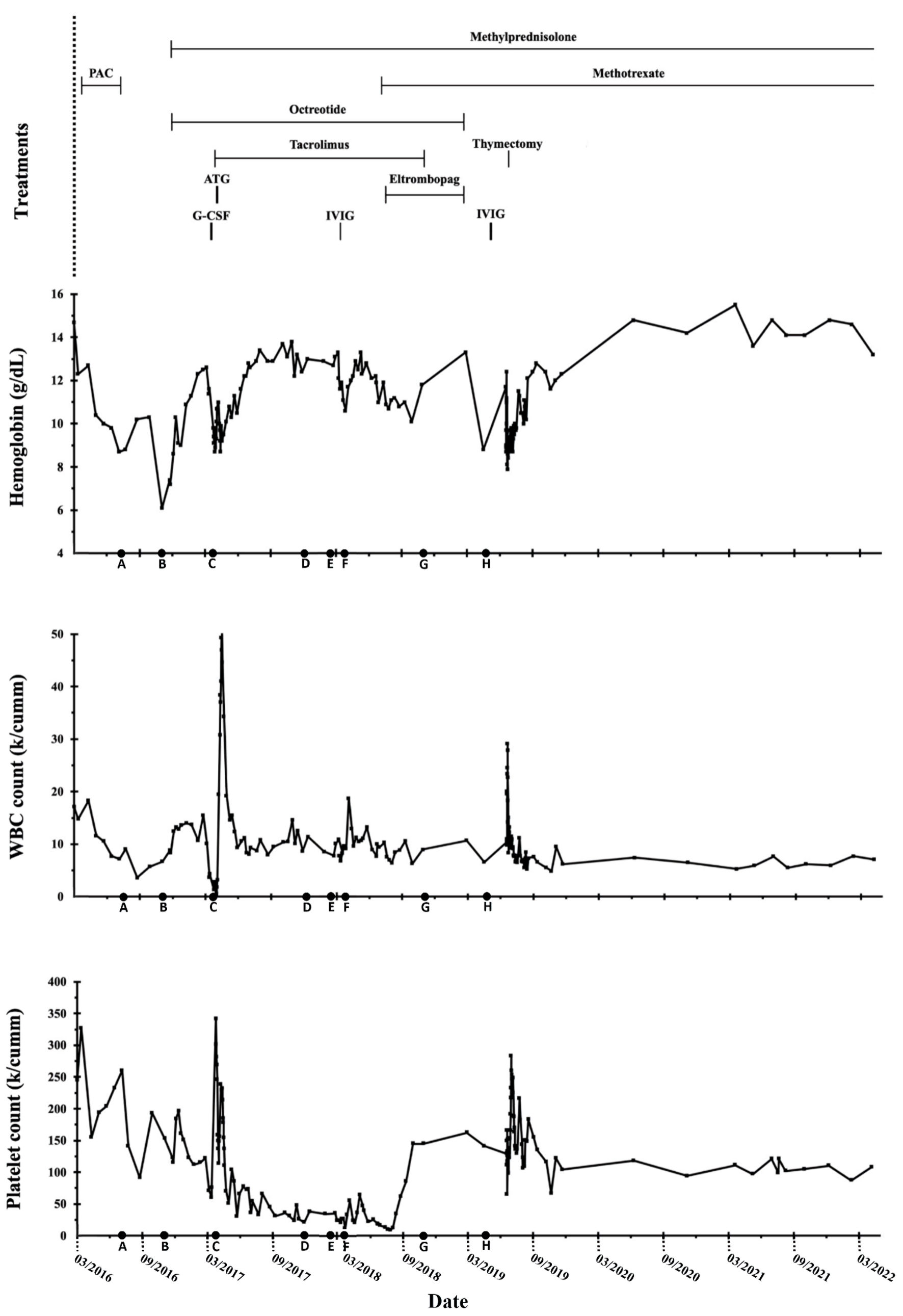 Click for large image | Figure 2. Hemoglobin, white blood cell (WBC), and platelet count trends throughout the clinical course. ATG: anti-thymocyte globulin; G-CSF: granulocyte colony-stimulating factor; IVIG: intravenous immunoglobulin; PAC: cisplatin, doxorubicin, and cyclophosphamide. |
In November 2016, the patient reported headaches, fatigue, and dizziness. Bloodwork revealed a normocytic anemia with a reticulocyte count < 0.1%, indicative of an erythroid underproduction etiology, but preserved myelopoiesis and thrombopoiesis with normal white blood cell (WBC) and platelet counts. Evidence of hemolysis was also present, represented by haptoglobin < 6.0 mg/dL, positive direct antiglobulin test (DAT), and a positive warm autoantibody. Key laboratory findings are listed in Table 1. Bone marrow biopsy was suboptimal but appeared hypocellular with an overall cellularity of approximately 20% (range 10-30%). Concurrent diagnoses of pure red cell aplasia (PRCA) and warm autoimmune hemolytic anemia (AIHA) were made (Fig. 2, time point B). Methylprednisolone 48 mg per os (PO) and octreotide 40 mg intramuscular (IM) monthly were initiated. The patient achieved transfusion independence with median hemoglobin > 10.0 g/dL after 1 - 2 months of treatment.
 Click to view | Table 1. Key Laboratory Findings at Presentation of PRCA and AIHA |
Four months later, the patient developed neutropenic fever (absolute neutrophil count (ANC) 0 × 103/µL), painful lymphadenopathy, and oropharyngeal candidiasis (Fig. 2, time point C). Parvovirus B19 and infectious cultures were negative. Bone marrow was hypocellular (cellularity 10-20%) with absent granulopoiesis and markedly decreased erythropoiesis (Fig. 3). Cytogenetics demonstrated no clonal abnormalities. These results were interpreted as continued red cell aplasia with new agranulocytosis. Treatment was initiated with granulocyte colony-stimulating factor (G-CSF), tacrolimus, and equine anti-thymocyte globulin (ATG). The patient responded well; however, leukocytosis subsequently developed consistent with G-CSF effect with increased neutrophils, lymphocytes, and eosinophils. Granulocytes normalized within a few weeks; however, lymphocytosis persisted. In addition, platelet counts steadily decreased over the next several months (Fig. 2, time point D). At the end of 2017, median hemoglobin was 11.6 g/dL (range 8.7 - 13.8 g/dL), median WBC count was 12.4 × 103/µL (range 8.0 - 50.7 × 103/µL), and median platelet count was 66 × 103/µL (range 21 - 238 × 103/µL).
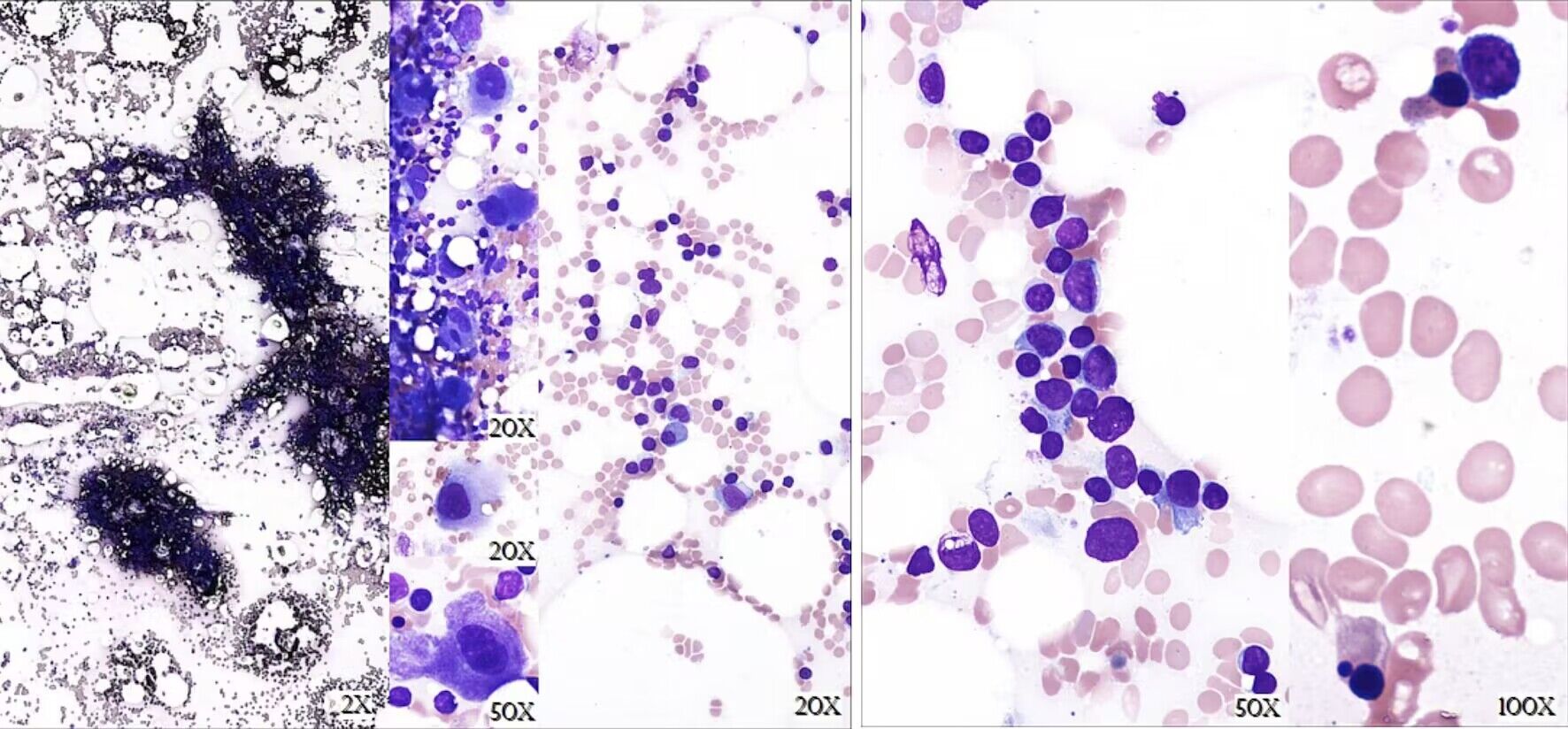 Click for large image | Figure 3. Bone marrow biopsy from March 2017. Description of bone marrow biopsy: Markedly hypocellular with cellularity 10-20%. Near absence of granulopoiesis with occasional blastic cells present but not increased. Erythropoiesis is decreased with very rare erythroids and some dysplasia. Increased small mature lymphocytes were noted. Findings were overall suggestive of a bone marrow failure syndrome. Description of bone marrow aspirate: Granulopoiesis essentially absent with a few scattered blasts seen. Erythroids are very rare, and some are dysplastic. Megakaryocytes are adequate to mildly increased with normal morphology. Lymphocytes are increased. |
In February 2018, 11 months post-ATG therapy, labs continued to show lymphocytosis with a further decline in platelet counts (Fig. 2, time point E). WBC count was 10.1 × 103/µL with 67.1% lymphocytes and an absolute lymphocyte count of 6.77 × 103/µL (reference range 1.1 - 2.7 × 103/µL). Intravenous immunoglobulin (IVIG) was trialed, but significant side effects necessitated cessation of therapy. There was no appreciable improvement in thrombocytopenia and new neutropenia was noted. Peripheral blood flow cytometry revealed an abnormal T-cell population with bright CD3, CD8, CD2, and CD7 with coexpression of CD57 and lack of CD5, CD56, and CD25 suggestive of T-cell large granular lymphocytic leukemia (T-LGLL) (Fig. 2, time point F). This was not detected on previous bone marrow biopsies. Polymerase chain reaction (PCR) confirmed presence of a clonal T-cell population with T-cell receptor gamma (TCR-γ) gene rearrangement. Bone marrow biopsy was again suboptimal but hypercellular (cellularity 70%) and consistent with T-LGLL (Fig. 4). Fluorescence in situ hybridization (FISH) for acute myelogenous leukemia (AML) and myelodysplastic syndrome (MDS) panels and cytogenetic analyses were normal. Testing for STAT3 and STAT5 gene mutations was negative. Next generation sequencing identified two genomic alterations: ASXL1 rearrangement at exon 3 and PIK3R1 at position N564D. This occurred while the patient was on maintenance therapy with tacrolimus and methylprednisolone.
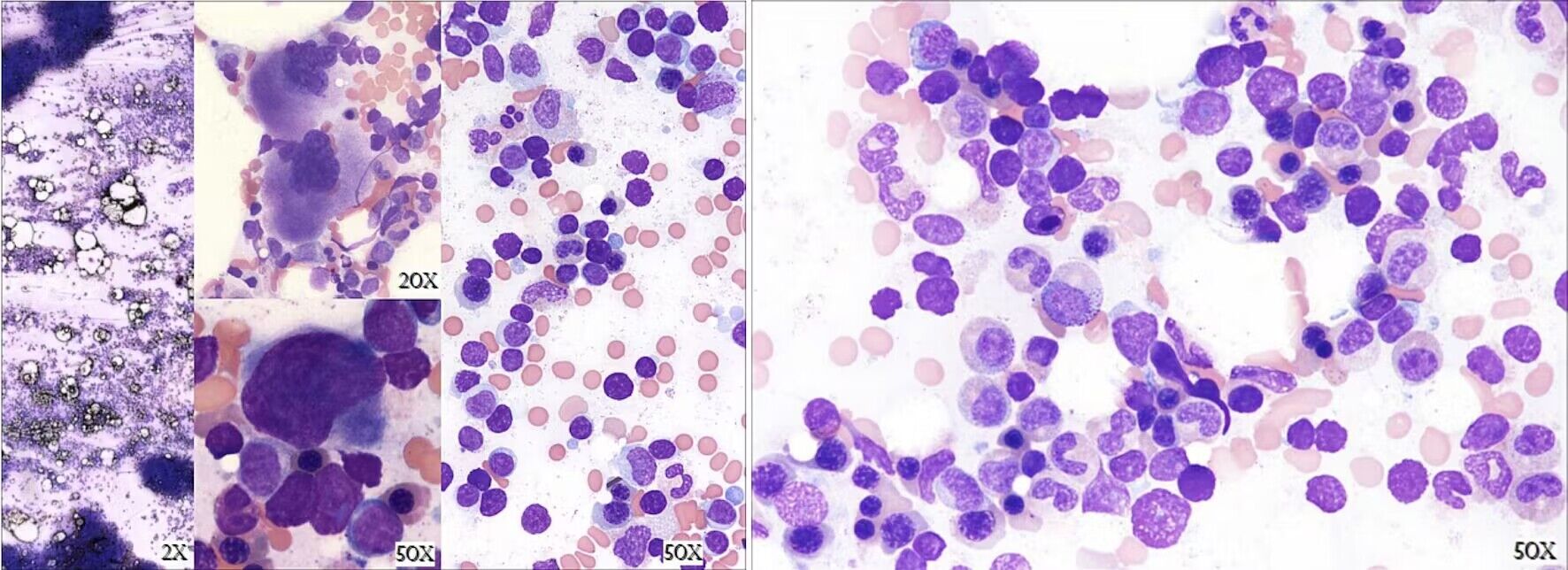 Click for large image | Figure 4. Bone marrow biopsy from June 2018. Description of bone marrow biopsy: Hypercellular with cellularity 70%. Myelopoiesis is increased with full maturation. Erythropoiesis is mildly increased with erythroid islands. Megakaryopoiesis is mildly increased with clustering and range of maturation. Interstitial lymphocytosis and scattered small lymphohistiocytic aggregates identified. Description of bone marrow aspirate smear: Lymphocytosis is present. Frequent small lymphoid cells have round to oval nuclei, compact chromatin and scant to moderately abundant cytoplasm with azurophilic granules. Granulopoiesis and erythropoiesis are normal. |
Around five months later, methotrexate and eltrombopag were added to the therapeutic regimen with subsequent improvement in all cell lines (Fig. 2, time point G). Percentage of lymphocytes significantly decreased but remained mildly elevated. Platelet counts normalized after six months of eltrombopag, and tacrolimus was tapered off in October 2018. Methotrexate and methylprednisolone were continued.
Unfortunately, imaging in February 2019 demonstrated disease progression. The patient experienced one recurrence of neutropenia (ANC 0.07 × 103/µL) and gradual return of thrombocytopenia was noted (Fig. 2, time point H). Four months later, an extensive surgical resection of thymoma was performed. Final pathology revealed thymoma WHO B2, pT1bN0M1, modified Masoako Koga stage IVA with pleural involvement. The patient recovered and subsequently underwent six weeks of proton therapy.
In June 2021, surveillance imaging demonstrated recurrent disease with enlarging right periaortic adenopathy and a right pleural-based mass. Biopsy of the mass revealed thymoma WHO B2. At most recent follow-up, hemoglobin and WBC counts remain stable and within normal limits. WBC differential revealed 30.1% lymphocytes, indicating stable-to-improved T-LGLL. Platelet counts remain mildly low with median 111 × 103/µL (range 94 - 121 × 103/µL), though significantly improved compared to prior (nadir platelet count 9 × 103/µL). The overall trend of blood counts across treatment course is shown in Figure 2. The patient continues on methotrexate alone after a prolonged steroid taper.
| Discussion | ▴Top |
The association of hematologic abnormalities with thymoma is well described in the literature; however, the pathogenesis, interrelationship between concurrent and successive hematologic abnormalities, and the optimal treatment regimens of such associations are not yet fully elucidated. The most common paraneoplastic hematologic abnormality seen with thymoma is PRCA [2]. Additional paraneoplastic hematologic abnormalities observed include AIHA, hypogammaglobulinemia (Good syndrome), pure white cell aplasia, immune thrombocytopenia, aplastic anemia, lymphoproliferative disorders, and others [3, 4].
PRCA occurs in an estimated 2-5% of patients with thymoma and is characterized by the absence of erythropoiesis in the bone marrow with reticulocytopenia (commonly ≤ 1%) [5, 6]. Treatment approaches for thymoma-associated PRCA, used alone or in combination, include thymectomy, corticosteroids, cyclosporine, cyclophosphamide, octreotide, IVIG, ATG, plasma exchange, splenectomy, and others [5, 7]. In a recently published systematic review of the literature, rates of complete remission (CR), defined as normalization of hemoglobin levels, were reported for various therapies: cyclosporine alone (CR 74%, n = 23), thymectomy with adjuvant immunosuppressive therapy (IST) (CR 56%, n = 12), corticosteroids alone (CR 41%, n = 25), and thymectomy alone (CR 29%, n = 23) [8]. Though these findings suggest a superior efficacy of IST alone or in combination with thymectomy, the reported results must be tempered with the risk of morbidity and mortality secondary to IST. The review found that of 58 reported deaths, < 15% were attributed to thymoma progression while > 60% were due to complications of PRCA treatments, most notably infection in the setting of IST [8]. Additionally, IST and thymectomy are not appropriate/available to all patients, thus highlighting the need for further treatment options. Although limited to case reports, octreotide alone or in combination with corticosteroids has demonstrated promise for thymoma with PRCA [9-11]. The mechanism of action is complex and incompletely understood but based on the immunomodulatory and anti-tumor effects of somatostatin analogues [9, 12-15]. Normal, healthy thymic tissue expresses high-affinity somatostatin receptors and certain thymic neoplasms have demonstrated high levels of uptake of indium-labeled octreotide (111In-DTPA-D-Phe1-octreotide) [16-18]. Corticosteroids are often added based off a proposed synergistic effect and their ability to induce tumor regression [9, 13]. Palmieri et al utilized this approach for a patient with thymoma and PRCA and reported complete resolution of PRCA with shrinkage of the thymic neoplasm [9]. Since this publication in 1997, two additional case reports have trialed octreotide +/- corticosteroids for treatment of thymoma-associated PRCA with mixed results (Table 2) [9-11].
 Click to view | Table 2. Cases of Thymoma-Associated PRCA Treated With Octreotide +/- Corticosteroids |
Another paraneoplastic hematologic abnormality seen with thymoma is AIHA, albeit significantly less common than thymoma-associated PRCA [19]. AIHA is a hemolytic anemia characterized by autoantibodies targeting self-antigens expressed on red blood cells [20]. The etiology of AIHA can be classified as primary (idiopathic) or secondary to underlying medical conditions, medications, etc. In addition, AIHA can be further classified based on characteristics of the autoantibodies including warm, cold agglutinin disease, or mixed-type. Determining the AIHA subtype is imperative as such classifications guide therapeutic approach [21]. Thymoma-associated AIHA is therefore classified as secondary AIHA. In the present case, warm autoantibodies were also identified and thus the comprehensive diagnostic designation was secondary, warm AIHA. Other cases of AIHA secondary to thymoma can be found in the literature with reported resolution of AIHA after treatment with corticosteroids, thymectomy, or both [22-25].
Thymoma with concurrent (defined as diagnoses ≤ 1 month apart) PRCA and AIHA is exceedingly rare. Four such cases have been described in the literature with key findings presented in Table 3 [26]. Response rate to thymectomy in all four cases was 100% [26]. In contrast to the above cases, upfront surgical excision of thymoma was not possible in the current case. Alternatively, the patient was medically managed with methylprednisolone and octreotide. This therapeutic regimen was selected based off the aforementioned success of such therapy in treating a patient with thymoma-associated PRCA, though octreotide scintigraphy was not performed in the current case [9]. The patient responded very well with attainment of transfusion-independence and improvement in hemoglobin from 6.1 g/dL to 11.3 g/dL within three months of treatment. To the best of our knowledge, this is the first reported case of thymoma with concurrent PRCA and AIHA successfully treated with corticosteroids and octreotide.
 Click to view | Table 3. Cases of Thymoma With PRCA and AIHA |
In addition to concurrent paraneoplastic anemias, the patient also developed neutropenia and thrombocytopenia. Hypothesized etiologies for the neutropenia and thrombocytopenia included an occult presentation of T-LGLL causing cytopenias versus thymoma-associated neutropenia and thrombocytopenia, though the latter is extremely rare [4, 27, 28]. In contrast, the relationship between T-LGLL and immune-mediated cytopenias is well described in the literature [29]. For the current patient, it is possible that underlying T-LGLL was present from the initial presentation of hematologic abnormalities and could be speculated as the etiology for the neutropenia and thrombocytopenia. This assessment is complicated by several suboptimal bone marrow specimens making exclusion of a prior T-LGLL clone difficult.
T-LGLL is yet another hematologic abnormality observed in patients with thymoma [30, 31]. T-LGLL is characterized by a clonal proliferation of a distinct subtype of lymphocytes: large granular lymphocytes (LGLs). There are two subtypes of LGLL: T-cell (T-LGLL), which is the most common with immunophenotype CD3+, CD57+, CD56-, and natural killer cell (NK-LGLL) with immunophenotype CD3-, CD56+ [32, 33]. The etiology of LGL clonal proliferation remains unknown; hypotheses include chronic antigen activation, JAK/STAT mutations inducing overexpression of anti-apoptotic pathways, cytokine stimulation secondary to immune dysfunction, and others [31, 34, 35]. The concurrence of T-LGLL and thymoma is considered rare, reported as occurring in < 5% of patients with T-LGLL [30]. In contrast, patients with T-LGLL frequently develop cytopenias. Neutropenia, anemia, and thrombocytopenia occur in up to 80%, 48%, and 20% of patients with T-LGLL, respectively [29]. More specifically, an association between T-LGLL and PRCA has been described. In one report by Gurnari et al, 7.4% (n = 19) of patients with T-LGLL also had PRCA [36]. Notably, in another study by Go et al that included 15 patients with T-LGLL and PRCA, all of whom received concurrent diagnoses. There were no reported cases of patients who initially presented with PRCA, achieved remission, then were subsequently diagnosed with T-LGLL [37]. This discrepancy between the present case and the 15 cases in this study could be supportive of separate and distinct pathologies in the current case and argue against the theory that an occult T-LGLL was present from the initial hematologic abnormalities. Furthermore, cases of T-LGLL, PRCA, and thymoma have also been reported [37]. Previous studies have identified a STAT3 mutation in many of these patients and hypothesized that this mutation may serve as the common link between diagnoses [36]. However, in the current case and others, STAT3 mutational testing was negative, indicating a need for further exploration into alternative theories of pathophysiology [36]. Regardless of concurrent diagnoses, first-line treatment of T-LGLL generally consists of methotrexate alone or in combination with corticosteroids. Other options include cyclophosphamide and cyclosporine, with the latter generally reserved for cases refractory to methotrexate and cyclophosphamide [32, 33].
In summary, numerous paraneoplastic syndromes are associated with thymoma and though the pathogenesis is incompletely understood, thymic tissue disruption causing a lapse in regulation of autoreactive T cells has been proposed as a theory [1]. A normally functioning thymus will challenge immature T cells with processes of positive and negative selection, with the ultimate survival of T cells that are able to recognize foreign antigens without demonstrating strong reactivity toward self-antigens. Thus, an invading neoplasm, such as thymoma, can exert a detrimental impact on the maturation and proper functioning of T cells [1]. Regardless of exact mechanism, it is hypothesized that autoreactive T cells are then able to escape into peripheral circulation, where consequent reaction and destruction of self-antigens ensues. For hematologic paraneoplastic syndromes specifically, dysregulated T cells may be targeting stem cells, other early precursor cells in the bone marrow, and/or mature cells circulating in the periphery [1, 38]. An overview of paraneoplastic syndromes seen in this patient are summarized in Table 4.
 Click to view | Table 4. Overview of Diagnoses, Treatments, and Outcomes |
Learning points
This case report details an unusual presentation of an advanced stage thymoma occurring in combination with several rare hematologic abnormalities in a single patient. To the best of our knowledge, this is the first report of a single patient diagnosed with thymoma, PRCA, AIHA, and T-LGLL.
In addition to the diagnostic rarity, this case is also novel in regards to therapeutic approach. We describe the first case of thymoma-associated PRCA and AIHA successfully treated with corticosteroids and octreotide. This alternative treatment option may be useful for clinicians caring for similar patients with unresectable thymomas or perhaps in the setting of paraneoplastic anemia persistent or refractory to thymectomy. More research is needed to determine the efficacy of this treatment option.
The development of neutropenia and subsequent T-LGLL while on therapy with octreotide and corticosteroids highlights the complex immune environment in those with thymomas and the need to better understand the underlying drivers of various paraneoplastic processes. Hemoglobin was continuing to improve at the time of development of neutropenia suggesting the underlying pathophysiologies of these entities are distinct.
Additional areas for future research include discerning the interrelationship, if any, between multiple paraneoplastic syndromes in patients with thymomas and to determine the best therapeutic course of action for these complex patients. Careful monitoring with surveillance imaging and routine blood work is warranted in patients with thymomas with a high index of suspicion for new disorders even years into the treatment course.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Written informed consent was obtained from the patient.
Author Contributions
All authors reviewed the patient case. TS performed a literature review, created the tables and graph, and authored the manuscript. AO and PL edited the manuscript and supervised the interpretation and presentation of information, contributing expertise in the areas of hematology and oncology, respectively.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
AIHA: autoimmune hemolytic anemia; AML: acute myeloid leukemia; ANC: absolute neutrophil count; ATG: anti-thymocyte globulin; CR: complete remission; FISH: fluorescence in situ hybridization; G-CSF: granulocyte colony-stimulating factor; IM: intramuscular; IST: immunosuppressive therapy; IVIG: intravenous immunoglobulin; LDH: lactate dehydrogenase; LGL: large granular lymphocytes; MCV: mean corpuscular volume; MDS: myelodysplastic syndrome; PCR: polymerase chain reaction; PO: per os; PRCA: pure red cell aplasia; RBC: red blood cell; SVC: superior vena cava; TCR-gamma: T-cell receptor gamma; TLGLL: T-cell large granular lymphocytic leukemia; WBC: white blood cell; WHO: World Health Organization
| References | ▴Top |
- Weksler B, Lu B. Alterations of the immune system in thymic malignancies. J Thorac Oncol. 2014;9(9 Suppl 2):S137-142.
doi pubmed - Padda SK, Yao X, Antonicelli A, Riess JW, Shang Y, Shrager JB, Korst R, et al. Paraneoplastic syndromes and thymic malignancies: an examination of the international thymic malignancy interest group retrospective database. J Thorac Oncol. 2018;13(3):436-446.
doi pubmed - Blum TG, Misch D, Kollmeier J, Thiel S, Bauer TT. Autoimmune disorders and paraneoplastic syndromes in thymoma. J Thorac Dis. 2020;12(12):7571-7590.
doi pubmed - Qin J, Liu L. Thymoma with idiopathic thrombocytopenic purpura: report of a case. J Thorac Cardiovasc Surg. 2005;129(2):453.
doi pubmed - Yen CC, Huang WL, Li SS, Chen YP, Tseng YL, Yen YT, Chu CY, et al. Pure red cell aplasia and other haematological diseases associated with thymoma: a case series and systematic review. Front Med (Lausanne). 2021;8:759914.
doi pubmed - Means RT Jr. Pure red cell aplasia. Hematology Am Soc Hematol Educ Program. 2016;2016(1):51-56.
doi pubmed - Sawada K, Fujishima N, Hirokawa M. Acquired pure red cell aplasia: updated review of treatment. Br J Haematol. 2008;142(4):505-514.
doi pubmed - Lesire B, Durieux V, Grigoriu B, Girard N, Berghmans T. Management of thymoma associated autoimmune pure red cell aplasia: Case report and systematic review of the literature. Lung Cancer. 2021;157:131-146.
doi pubmed - Palmieri G, Lastoria S, Colao A, Vergara E, Varrella P, Biondi E, Selleri C, et al. Successful treatment of a patient with a thymoma and pure red-cell aplasia with octreotide and prednisone. N Engl J Med. 1997;336(4):263-265.
doi pubmed - Zaucha R, Zaucha JM, Jassem J. Resolution of thymoma-related pure red cell aplasia after octreotide treatment. Acta Oncol. 2007;46(6):864-865.
doi pubmed - Larroche C, Mouthon L, Casadevall N, Le Roux G, Casassus P, Guillevin L. Successful treatment of thymoma-associated pure red cell aplasia with intravenous immunoglobulins. Eur J Haematol. 2000;65(1):74-76.
doi pubmed - Ferone D, van Hagen MP, Kwekkeboom DJ, van Koetsveld PM, Mooy DM, Lichtenauer-Kaligis E, Schonbrunn A, et al. Somatostatin receptor subtypes in human thymoma and inhibition of cell proliferation by octreotide in vitro. J Clin Endocrinol Metab. 2000;85(4):1719-1726.
doi pubmed - Pettit L, El-Modir A. The role of somatostatin analogues in the treatment of advanced malignant thymomas: case report and review of the literature. Br J Radiol. 2011;84(997):e7-e10.
doi pubmed - von Arx C, Rea G, Napolitano M, Ottaiano A, Tatangelo F, Izzo F, Petrillo A, et al. Effect of octreotide long-acting release on Tregs and MDSC cells in neuroendocrine tumour patients: a pivotal prospective study. Cancers (Basel). 2020;12(9):2422.
doi pubmed - Loehrer PJ, Sr., Wang W, Johnson DH, Aisner SC, Ettinger DS, Eastern Cooperative Oncology Group Phase IIT. Octreotide alone or with prednisone in patients with advanced thymoma and thymic carcinoma: an Eastern Cooperative Oncology Group Phase II Trial. J Clin Oncol. 2004;22(2):293-299.
doi pubmed - Reubi JC, Waser B, Horisberger U, Krenning E, Lamberts SW, Gebbers JO, Gersbach P, et al. In vitro autoradiographic and in vivo scintigraphic localization of somatostatin receptors in human lymphatic tissue. Blood. 1993;82(7):2143-2151.
doi pubmed - Lastoria S, Vergara E, Palmieri G, Acampa W, Varrella P, Caraco C, Bianco RA, et al. In vivo detection of malignant thymic masses by indium-111-DTPA-D-Phe1-octreotide scintigraphy. J Nucl Med. 1998;39(4):634-639.
- Palmieri G, Montella L, Martignetti A, Muto P, Di Vizio D, De Chiara A, Lastoria S. Somatostatin analogs and prednisone in advanced refractory thymic tumors. Cancer. 2002;94(5):1414-1420.
doi pubmed - De Keyzer K, Peeters P, Verhelst C, Dendooven A, Vonck A, Vanholder R. Autoimmune haemolytic anaemia associated with a thymoma: case report and review of the literature. Acta Clin Belg. 2009;64(5):447-451.
doi pubmed - Barcellini W. Immune hemolysis: diagnosis and treatment recommendations. Semin Hematol. 2015;52(4):304-312.
doi pubmed - Berentsen S, Barcellini W. Autoimmune hemolytic anemias. N Engl J Med. 2021;385(15):1407-1419.
doi pubmed - Suzuki K, Inomata M, Shiraishi S, Hayashi R, Tobe K. Thymoma with autoimmune hemolytic anemia. Case Rep Oncol. 2014;7(3):764-768.
doi pubmed - Rubinstein I, Langevitz P, Hirsch R, Berkowicz M, Lieberman Y, Shibi G. Autoimmune hemolytic anemia as the presenting manifestation of malignant thymoma. Acta Haematol. 1985;74(1):40-42.
doi pubmed - Rennenberg RJ, Pauwels P, Vlasveld LT. A case of thymoma-associated autoimmune haemolytic anaemia. Neth J Med. 1997;50(3):110-114.
doi pubmed - Arntzenius AB, Bieger R. Disappearance of autoantibody-induced haemolysis after excision of a malignant thymoma. Neth J Med. 1991;38(3-4):117-121.
- Wang W, Chen LY, Zhao W, Ren Y, Wang L, Li X, Jiang Y, et al. Coexistence of pure red cell aplasia and autoimmune haemolytic anaemia associated with thymoma. Acta Haematol. 2020;143(5):491-495.
doi pubmed - Oyenuga M, Shaikh S, Harris B, Sinha J, Lacasse A. Recurrent neutropenia and chronic diarrhea following thymectomy: the good, the bad, and the ugly. J Community Hosp Intern Med Perspect. 2021;11(2):263-265.
doi pubmed - Youssef M, Stratton TW, Gallant RC, Young C, Li DY, Piran S. Pure white cell aplasia and immune thrombocytopenia after thymoma resection: a case report and review of the literature. Case Rep Hematol. 2022;2022:8271069.
doi pubmed - Lamy T, Loughran TP Jr. Current concepts: large granular lymphocyte leukemia. Blood Rev. 1999;13(4):230-240.
doi pubmed - Zambello R, Semenzato G. Large granular lymphocytosis. Haematologica. 1998;83(10):936-942.
- Nearman ZP, Wlodarski M, Jankowska AM, Howe E, Narvaez Y, Ball E, Maciejewski JP. Immunogenetic factors determining the evolution of T-cell large granular lymphocyte leukaemia and associated cytopenias. Br J Haematol. 2007;136(2):237-248.
doi pubmed - Cheon H, Dziewulska KH, Moosic KB, Olson KC, Gru AA, Feith DJ, Loughran TP, Jr. Advances in the diagnosis and treatment of large granular lymphocytic leukemia. Curr Hematol Malig Rep. 2020;15(2):103-112.
doi pubmed - Zawit M, Bahaj W, Gurnari C, Maciejewski J. Large granular lymphocytic leukemia: from immunopathogenesis to treatment of refractory disease. Cancers (Basel). 2021;13(17):4418.
doi pubmed - Teramo A, Gattazzo C, Passeri F, Lico A, Tasca G, Cabrelle A, Martini V, et al. Intrinsic and extrinsic mechanisms contribute to maintain the JAK/STAT pathway aberrantly activated in T-type large granular lymphocyte leukemia. Blood. 2013;121(19):3843-3854.S3841.
doi pubmed - Yang J, Epling-Burnette PK, Painter JS, Zou J, Bai F, Wei S, Loughran TP, Jr. Antigen activation and impaired Fas-induced death-inducing signaling complex formation in T-large-granular lymphocyte leukemia. Blood. 2008;111(3):1610-1616.
doi pubmed - Gurnari C, Durrani J, Pagliuca S, Kishtagari A, Awada H, Kerr CM, Adema V, et al. Novel invariant features of Good syndrome. Leukemia. 2021;35(6):1792-1796.
doi pubmed - Go RS, Li CY, Tefferi A, Phyliky RL. Acquired pure red cell aplasia associated with lymphoproliferative disease of granular T lymphocytes. Blood. 2001;98(2):483-485.
doi pubmed - Scarpino S, Di Napoli A, Stoppacciaro A, Antonelli M, Pilozzi E, Chiarle R, Palestro G, et al. Expression of autoimmune regulator gene (AIRE) and T regulatory cells in human thymomas. Clin Exp Immunol. 2007;149(3):504-512.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


