| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Case Report
Volume 12, Number 1, February 2023, pages 42-48
Mature Type T-Lymphoblastic Leukemia/Lymphoma Presenting With Isolated Central Nervous System Symptomatology in a Patient With Giant Cell Arteritis on Long-Term Steroid Treatment
John Kolton Smitha, f, Xinmin Zhangb, Stephen C. Machnickic, Salman Azhard, Morana Vojnica, e
aDepartment of Medicine, Lenox Hill Hospital, Northwell Health, New York, NY, USA
bDepartment of Pathology, Northwell Health, Greenvale, NY, USA
cDepartment of Radiology, Lenox Hill Hospital, Northwell Health, New York, NY, USA
dDepartment of Neurology, Lenox Hill Hospital, Northwell Health, New York, NY, USA
eDivision of Hematology and Oncology, Lenox Hill Hospital, Northwell Health, New York, NY, USA
fCorresponding Author: John Kolton Smith, Department of Medicine, Lenox Hill Hospital, Northwell Health, New York, NY 10028, USA
Manuscript submitted December 13, 2022, accepted January 12, 2023, published online February 25, 2023
Short title: Mature Type T-ALL With Isolated CNS Symptom
doi: https://doi.org/10.14740/jh1037
| Abstract | ▴Top |
T-lymphoblastic leukemia/lymphoma (T-ALL/T-LBL) is a malignancy comprised of T-lymphoblasts that can present as one of four clinical subtypes (pro-T, pre-T, cortical T, and mature T). Clinical presentation is typically characterized by leukocytosis with diffuse lymphadenopathy and/or hepatosplenomegaly. Beyond clinical presentation, specific immunophenotypic and cytogenetic classifications are utilized to diagnose mature T-ALL. In later disease stages it can spread to the central nervous system (CNS); however, presentation of mature T-ALL by way of CNS pathology and clinical symptomatology alone is rare. Even more rare is the presence of poor prognostic factors without correlating significant clinical presentation. We present a case of mature T-ALL in an elderly female with isolated CNS symptoms in combination with poor prognostic factors including terminal deoxynucleotidyl transferase (TdT) negativity and a complex karyotype. Our patient lacked the classical symptomatology and laboratory findings of mature T-ALL but deteriorated quickly upon diagnosis due to the aggressive genetic profile of her cancer.
Keywords: T-lymphoblastic leukemia/lymphoma; Central nervous system; Disease; Cells
| Introduction | ▴Top |
T-lymphoblastic leukemia/lymphoma (T-ALL/T-LBL) is a neoplasm of lymphoblasts committed to T-cell lineage involving bone marrow (BM), blood, or presenting as a tissue-based mass involving the thymus, lymph nodes, or extranodal sites [1]. The designation of T-ALL is best used when there is extensive blood and BM involvement. In the United States, the incidence of T-ALL is 0.3 to 1 case per 100,000 population [2]. The diagnosis of T-ALL is based on clinical, morphological, immunophenotypic, and cytogenetic features [1]. The clinical presentation of T-ALL is characterized by some combination of high leukocyte count, mediastinal or other tissue mass, hepatosplenomegaly, hypercalcemia, and lymphadenopathy [1, 3]. Based on maturation stage, T-ALL is classified into four subgroups: pro-T, pre-T, cortical T, and mature T (medullary). Mature T-ALL is typically positive for CD3, CD7, CD4+/-, CD8+/- and negative for CD1a and CD34 [4, 5]. Terminal deoxynucleotidyl transferase (TdT) is typically positive in T-ALL, but TdT-negative cases have been rarely reported [6]. Central nervous system (CNS) involvement can be present at initial diagnosis in a small subset of T-ALL cases [7]. However, initial presentation by way of CNS symptomatology alone is exceedingly rare. In this case we present an elderly female with the diagnosis of a TdT-negative, mature T-ALL who presented as the result of persistent neurological complaints in the absence of the other typical findings of this disease.
| Case Report | ▴Top |
A 67-year-old female with a past medical history of giant cell arteritis (GCA), coronary artery disease, non-ST elevation myocardial infarction, cerebrovascular stenosis, Pneumocystis carinii pneumonia (PCP, diagnosed 1 year prior, with a lactate dehydrogenase (LDH) level of 421 U/L on diagnosis) and thyroid cancer status post thyroidectomy and thyrotropin suppression presented to the hospital with headaches accompanied by extremity numbness and fatigue of a few weeks’ duration. During her hospitalization, investigatory measures led physicians to diagnose her with a flare of vasculitis and she was discharged on a steroid taper after receiving pulse dose steroids inpatient. After initially improving upon discharge, the patient returned to the hospital about a month later and reported that she was experiencing worsening confusion, weakness, and dizziness. A caretaker reported that the patient had become practically bed-bound and had intermittently been unable to recognize familiar faces over the past couple of weeks.
Initial physical exam was limited due to severe lethargy of the patient but did not demonstrate any lymphadenopathy, hepatosplenomegaly, bony tenderness, or dermatologic findings. Upon admission, the patient’s complete blood count (CBC) showed white blood cell count of 12,000 × 103/µL, with hemoglobin and platelet counts in normal range. Her slight leukocytosis was not out of the realm of possibility in the setting of recent steroid use. The most notable laboratory finding was a sodium of 119 mmol/L, which became the working rationale as to the etiology of her neurological symptomatology. However, following correction of her serum sodium, her neurological symptoms persisted. Magnetic resonance imaging (MRI) of the head revealed ependymal enhancement in the bilateral frontal horns consistent with either chronic small vessel disease versus an infectious process such as meningitis or ventriculitis (Fig. 1). A lumbar puncture was inconsistent with infection but did show increased cerebrospinal fluid (CSF) protein at 110 mg/dL, glucose of 117 mg/dL, a total nucleated cell count of 12 cells/µL, with “atypical cells” that could not be further classified. Flow cytometry of the CSF showed no diagnostic abnormalities. The common infectious or metabolic causes of encephalopathy were negative upon further workup including negative autoimmune and infectious testing of the CSF, negative blood and urine cultures, and normal electrolyte values.
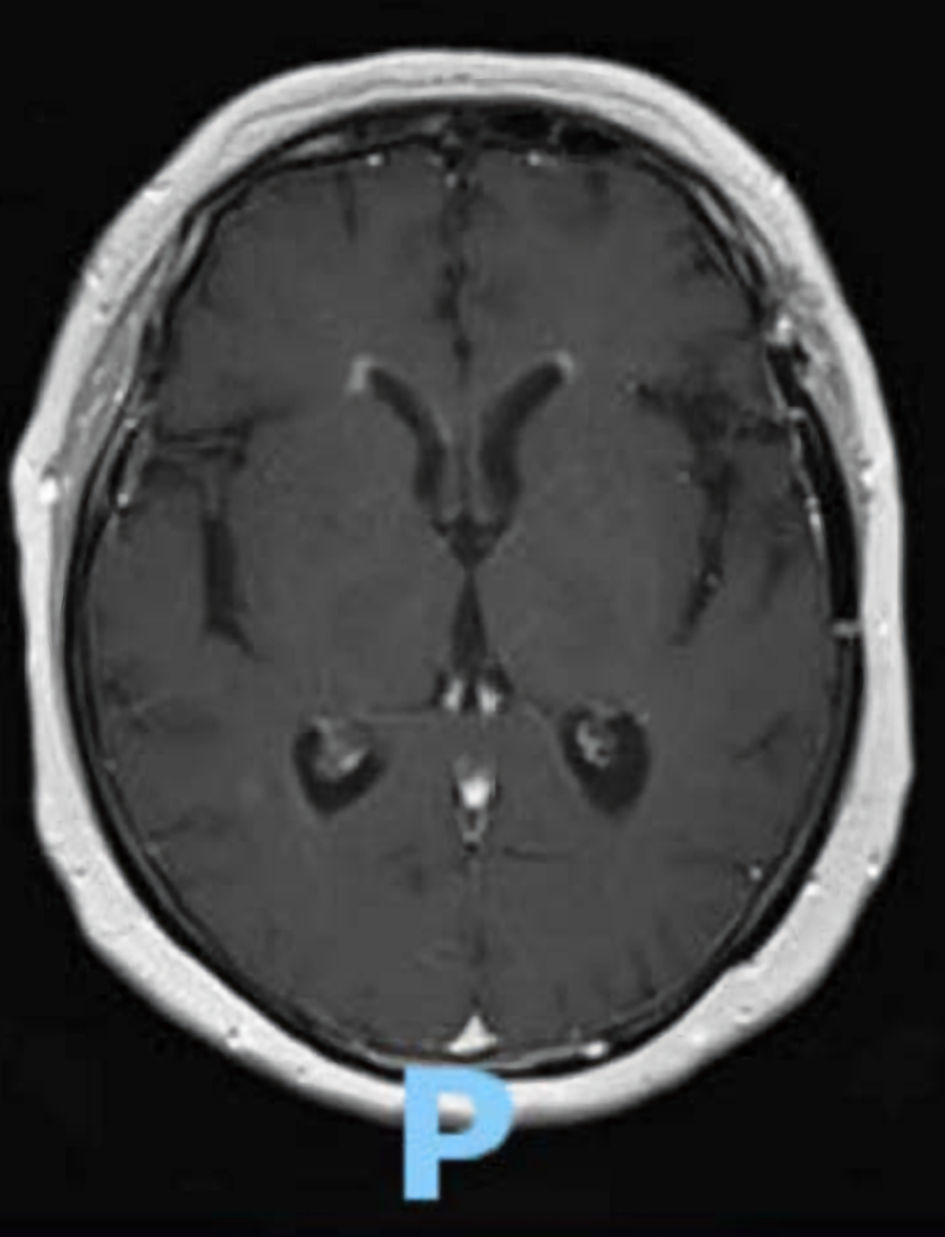 Click for large image | Figure 1. T1 weighted MRI of the head, axial view. This scan exhibits bilateral ependymal brain enhancement of the frontal horns, suggestive of ventriculitis, an uncommon imaging finding in mature T-lymphoblastic lymphoma/leukemia. MRI: magnetic resonance imaging. |
Ten days into her hospitalization, hematology/oncology was consulted for leukoerythroblastosis and rouleaux formation on peripheral smear. Immunofixation and serum protein electrophoresis (SPEP) were both unremarkable at initial consultation and LDH was 409 U/L. Given the continued encephalopathy, a repeat lumbar puncture was performed that demonstrated increased CSF protein at 75 mg/dL, glucose of 80 mg/dL and more of the “atypical cells” that could not be further classified. Flow cytometric analysis of peripheral blood did not show significant numbers of circulating blasts or an abnormal lymphoid or myeloid population, in contrast to the findings on automated peripheral smear. Still the patient had a physical exam that did not signal any suspicion for malignancy with the absence of lymphadenopathy, hepatosplenomegaly, bone pain or dermatologic lesions. Over the next 10 to 14 days the patient developed a normocytic anemia and thrombocytopenia that progressed with a new finding of schistocytes and atypical lymphocytes on peripheral smear (Fig. 2) as well as an LDH of 1,360 U/L (Fig. 3). She also developed diffuse petechiae and purpura. A repeat head computed tomography (CT) scan showed a new hypodense focus of the right lentiform nucleus suggestive of a stroke vs. encephalitic process (Fig. 4). The patient also got a positron emission tomography (PET)/CT scan that showed a few areas of increased uptake in the lungs and periesophageal area that were deemed to be nonspecific. The patient then developed a low reticulocyte count, prompting a bone marrow biopsy.
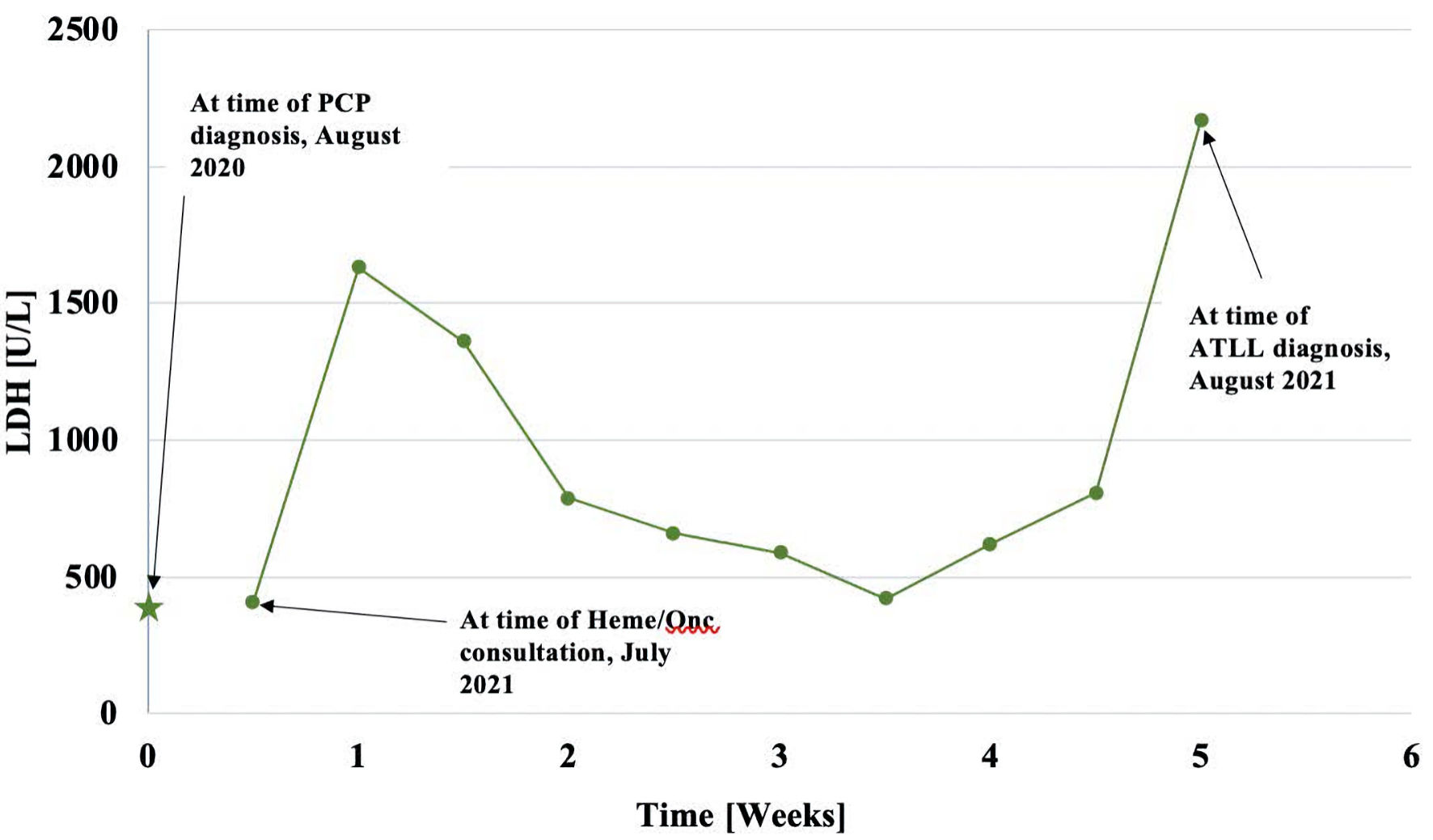 Click for large image | Figure 2. This graph represents the patient’s LDH values over time. The star represents the initial LDH (1 year prior) while point 1 represents LDH upon initial hematology consultation. The rest of the points represent the progression of LDH through the disease course. LDH: lactate dehydrogenase; PCP: Pneumocystis carinii pneumonia; ATLL: adult T-cell leukemia/lymphoma; Heme/Onc: hematology and oncology. |
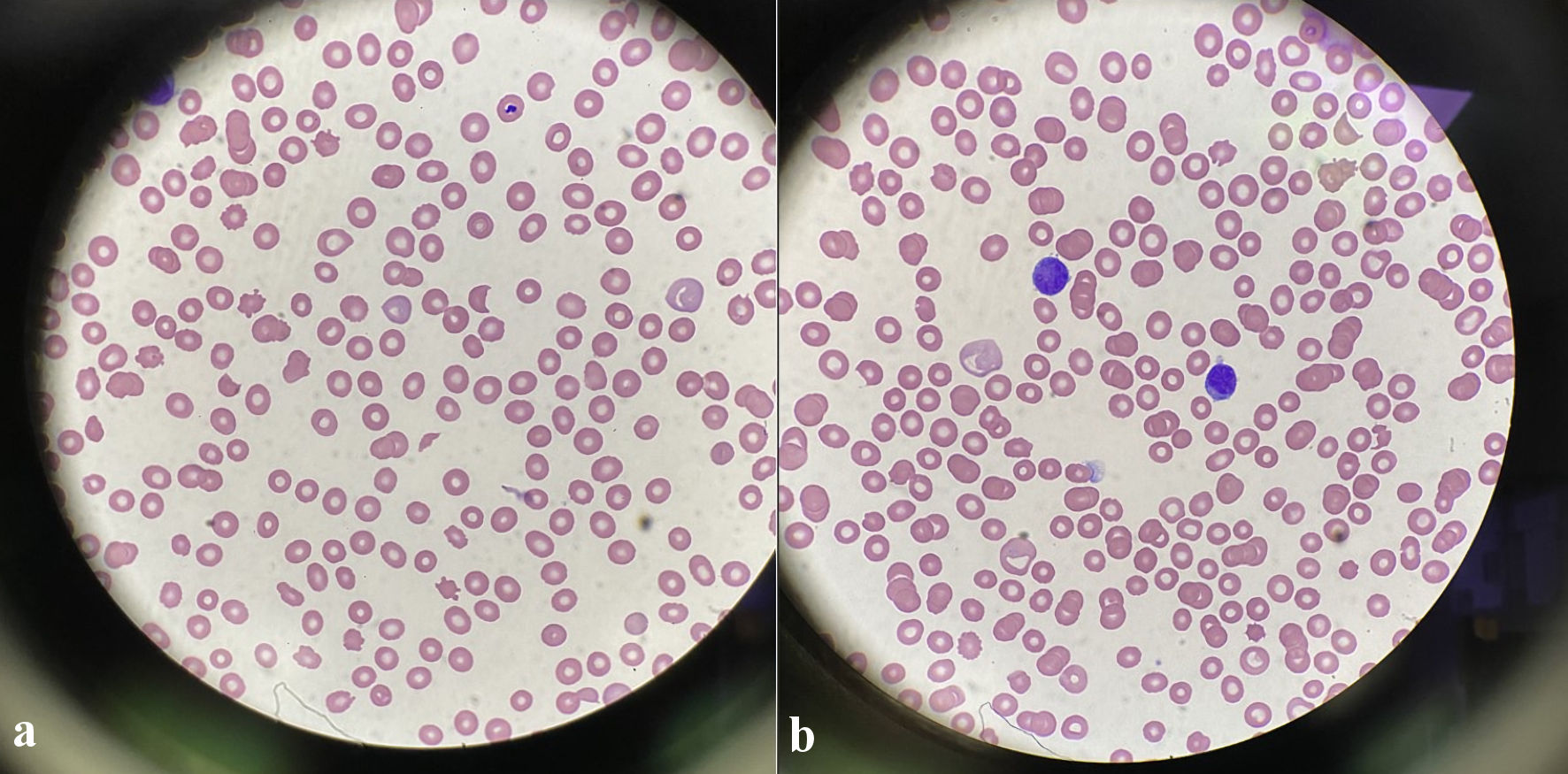 Click for large image | Figure 3. (a) Peripheral blood smear shows the presence of schistocytes, helmet cells (× 100, magnification). (b) Peripheral blood smear shows lymphocytic appearing cells with large irregular nuclei, similar to the appearance of the atypical cells in the CSF sample. CSF: cerebrospinal fluid. |
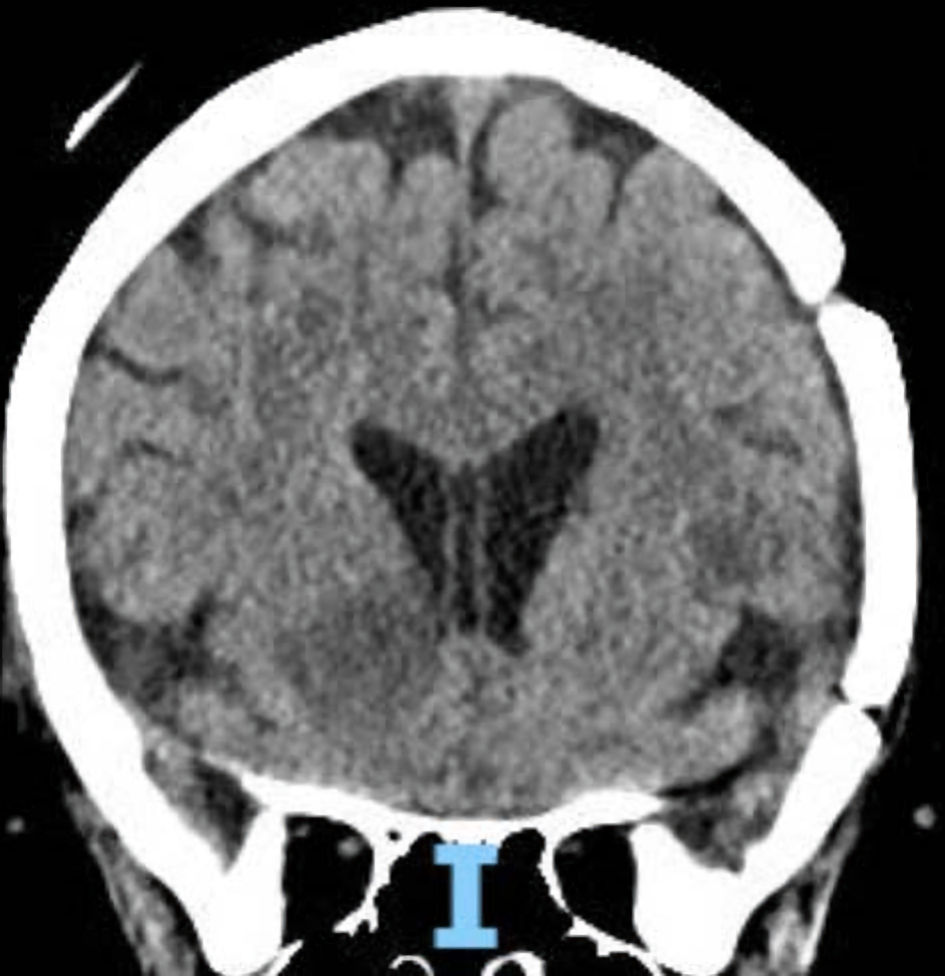 Click for large image | Figure 4. CT scan of the head, sagittal view. There is hypodensity of the right lentiform nucleus, most consistent with acute vascular pathology vs. inflammatory changes, an uncommon imaging finding in mature T-ALL. Solid masses are more characteristic of mature-T-ALL. CT: computed tomography; T-ALL: T-lymphoblastic leukemia. |
By the time bone marrow biopsy was performed, the patient’s hemoglobin had dropped from over 13 g/dL on admission to 7.1 g/dL and her platelet count had dropped from over 300 × 103/µL to 11 × 103/µL. At no point during either hospitalization did the patient become hypercalcemic. Bone marrow pathology report indicated neoplastic cells comprising up to 40% of the bone marrow aspirate that showed an immature morphology, including some with “hand mirror” appearance (Fig. 5). Flow cytometry analysis of bone marrow revealed that the blasts had a higher side scatter (SSC) and were positive for CD2, CD3, CD4, CD5, CD7, CD8 (partial), CD56, CD45 and negative for TdT, CD34, T-cell receptor (TCR)-gamma/delta. TCR-alpha/beta was negative to very dim compared to the residual T lymphocytes (Fig. 6). Immunohistochemical studies performed on bone marrow biopsy showed that the blasts were positive for CD2, CD3, CD4, CD5, CD7, CD8 (partial), p53 and negative for TdT (Fig. 7). Cytogenetics showed deletion 16 and t(1;9) with complete karyotype/cytogenetics. The clinicopathological findings supported a diagnosis of mature T-ALL. The patient was informed of the grim prognosis and her poor candidacy for chemotherapy. The patient quickly deteriorated, developing profound transaminitis and hypotension with subsequent transfer to the intensive care unit for intubation and blood pressure support. The patient expired within weeks of her development of cytopenias due to multi-organ failure in the setting of system wide inflammation presumed to be secondary to progression of the T-ALL.
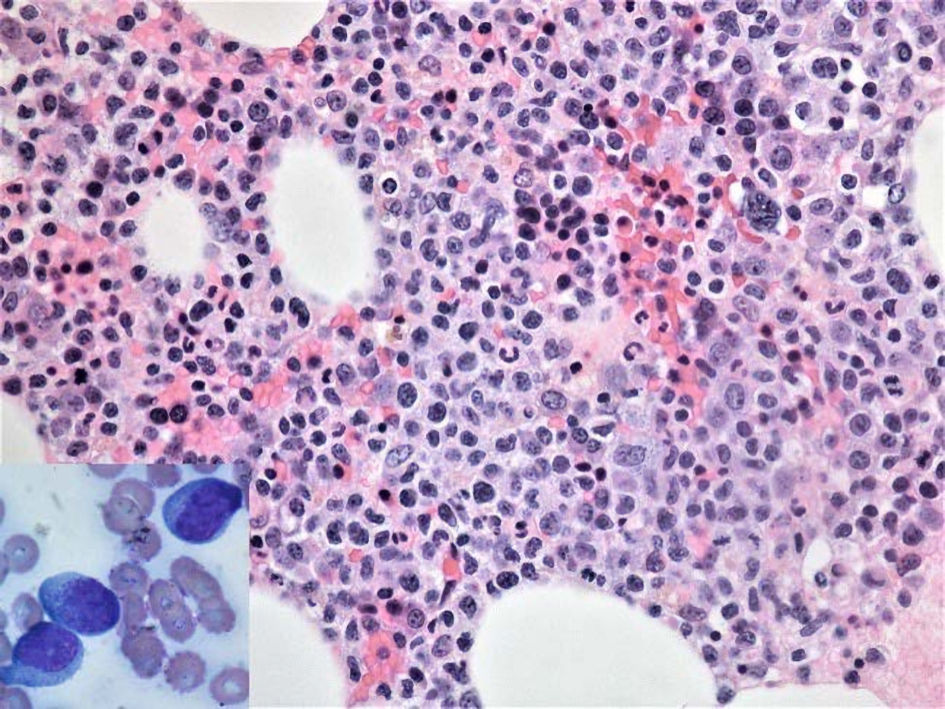 Click for large image | Figure 5. Hematoxylin and eosin stain reveals hypercellular bone marrow with extensive immature infiltrate in a background of decreased trilineage hematopoiesis (× 400, magnification). Bone marrow aspirate smear (inset) shows presence of lymphoblasts (medium-sized to large cells with higher nuclear to cytoplasm ratio, round to oval-shaped nucleus with fine chromatin and small nucleoli (× 1,000, magnification). |
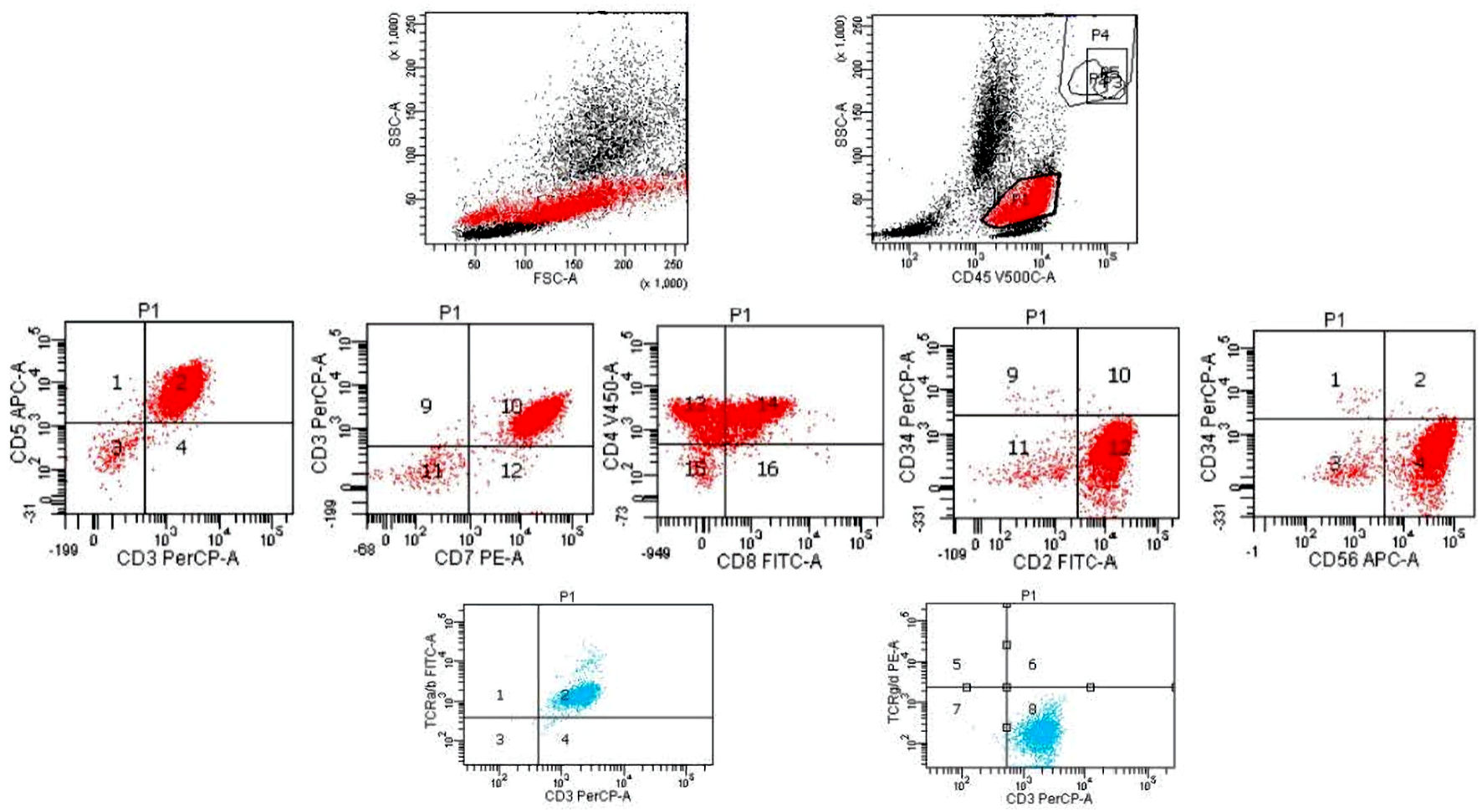 Click for large image | Figure 6. Flow cytometry histograms of the patient’s bone marrow sample. The blasts have a higher sight scatter (SSC) and forward scatter (FSC) and express CD2, CD3, CD4, CD5, CD7, CD8 (partial), CD56, negative to very dim TCR-alpha/beta (much weaker than the residual normal T cells), and negative for CD34, TCR-gamma/delta. TCR: T-cell receptor. |
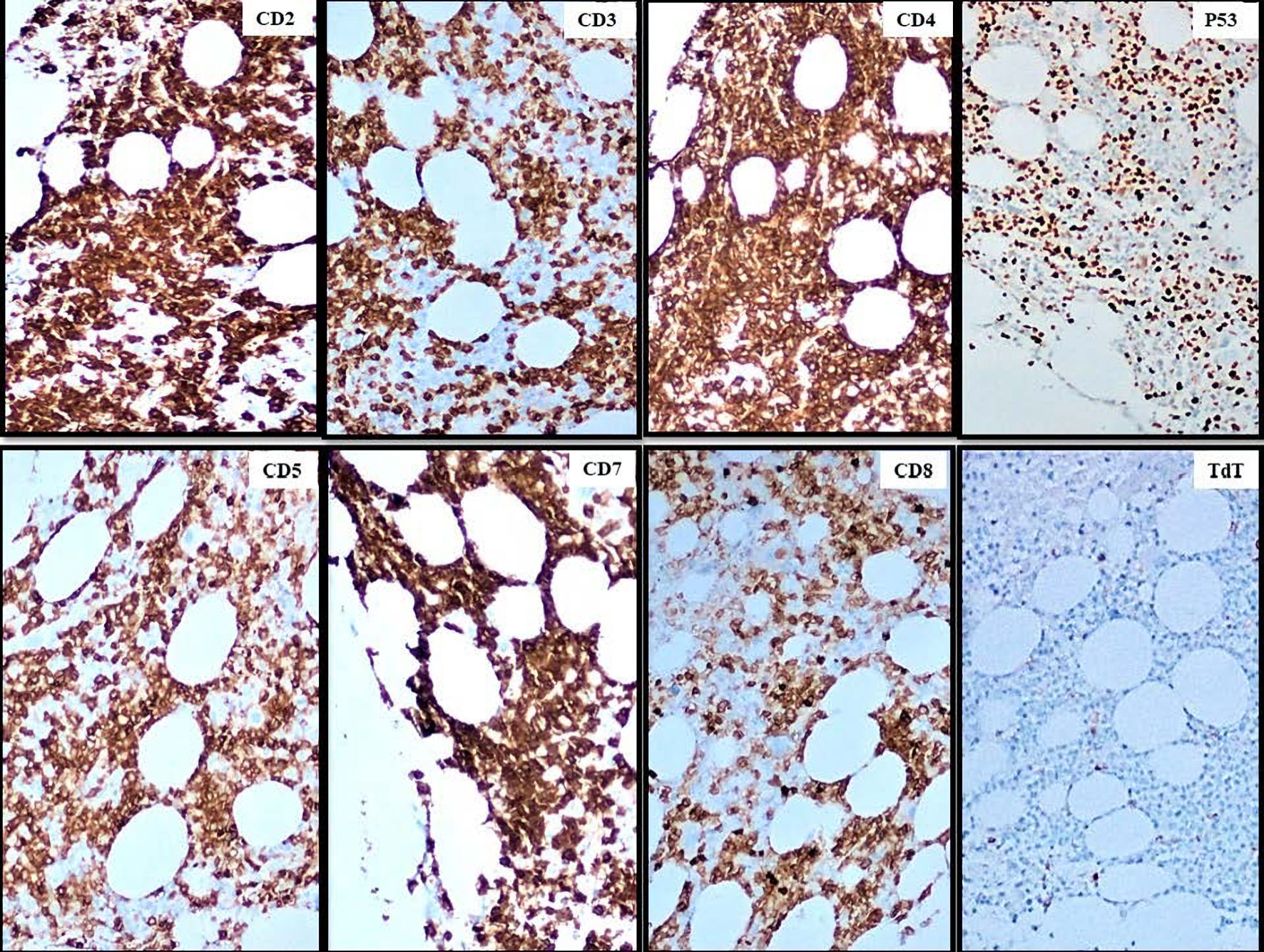 Click for large image | Figure 7. Immunohistochemistry of the patient’s bone marrow sample, displaying the staining patterns of each of the cell markers denoted in the top right corner. The blasts are positive for CD2, CD3, CD4, CD5, CD7, CD8 (partial), p53 and negative for TdT (× 100, magnification). TdT: terminal deoxynucleotidyl transferase. |
| Discussion | ▴Top |
This patient is unique and notable due to the fact that she presented with isolated CNS involvement and poor prognostic factors although the remainder of the clinical picture did not suggest mature T-ALL. It is more likely, given the lack of cytopenias and circulating blasts, that the disease originated in the CNS or at the least spread to the CNS prior to invading the bone marrow. In this case, the main differential diagnoses were T-ALL, T-prolymphocytic leukemia (T-PLL) and peripheral T-cell lymphoma (PTCL) including adult T-cell leukemia/lymphoma (ATLL). The clinical findings and negative T-cell leukemia 1 (TCL1) by immunohistochemistry did not support T-PLL. Given a negative TdT result, PTCL could not be completely excluded. The diagnosis of ATLL requires positive Human T-cell leukemia virus type 1 (HTLV-1) testing, which could not be obtained in our patient due to her expiration. However, the neoplastic cells demonstrated an immature morphology, thymic origin (CD4+, CD8+) and a complex karyotype. With the lack of extramedullary involvement, these findings supported a diagnosis of mature T-ALL. Unusual features in this case include TdT negativity, CD56 positivity, and p53 expression. Though rare, TdT-negative cases of T-ALL have been reported in the literature [6]. Negativity of TdT in T-ALL has been associated with poorer prognosis [6]. CD56 and p53 are also associated with an unfavorable prognosis in T-ALL [8, 9].
At no point during the progression of our patient’s disease did she develop the systemic signs and symptoms of T-ALL. She never developed hepatosplenomegaly, lymphadenopathy, skin lesions or hypercalcemia. Though CNS involvement is present in a small subset of T-ALL cases, CNS disease as the only clinical manifestation is rare [7]. Commonly observed CNS clinical manifestations in these cases include altered mental status, seizures, syndrome of inappropriate antidiuretic hormone and the potential for leukemic meningitis [10]. According to the literature, CNS involvement will typically present on imaging as a single or multiple discrete masses that exhibit a pathological enhancement on CT scan [11, 12]. Furthermore, cases in which the disease has progressed to the CNS typically exhibit diffuse uptake in the liver, spleen, and mediastinal lymph nodes on PET/CT scan. Our patient presented with isolated CNS symptomatology, had no characteristic radiological findings of disease, and was subsequently diagnosed with mature T-ALL.
Previous studies have indicated that long-term steroid use can be linked to the development of hematologic malignancies [13-20]. Our patient was on long-term steroid therapy for GCA as well as PCP pneumonia, therefore it is not out of the question for her steroid use to have been a contributing factor in her development of T-ALL. It is important to point out, though, that review articles have not found a particular association between GCA-associated steroid use and the development of malignancy [21]. Questions remain regarding the link between steroids and the development of cancer, and it will be important for future studies to tease out if steroids themselves or the underlying pathology they are treating contribute to the development of malignancies. Overall, we do not believe that our patients steroid use led to the development of her T-ALL.
Furthermore, our patient had atypical CSF findings, which raised suspicion for vasculitis, but cytology found atypical cells that could not be further classified but that were similar in morphology to atypical cells found in the peripheral blood. Flow cytometry sample showed cell degeneration, which is a common challenge that laboratories face. It can take as little as 30 min for cells in a CSF sample to degenerate, likely due to poor viability of malignant cells combined with low cell counts in the samples [22]. Recent advances in the technology of stabilizing collection media have greatly improved the reliability of flow cytometry in the diagnosis of CNS malignancies, though degeneration is still a consideration [23]. Though our institution has quality assurance guidelines that include proper stabilization of the CSF sample prior to flow cytometry, the sample showed cell degeneration which may represent an error or time lapse in the protocol. Flow cytometry is a valuable asset in the investigation of cancer, but newer technology exists that can increase the sensitivity of diagnosing hematologic diseases. One such tool that could have proven useful is next generation sequencing, a process which has shown great promise in the detection of infection and circulating tumor DNA in the CSF [24].
Treatment for T-ALL depends on the clinical subtype, severity of disease, as well as other patient specific factors. In adults with T-ALL, survival rates have hovered between 40% and 50% over the past decade [25]. Management of T-ALL in adults has progressed from the use of historical lymphoma regimens to the use of leukemia regimens, including induction, consolidation, delayed intensification, and maintenance phases with CNS prophylaxis. One regimen termed hyper-CVAD employs classical chemotherapeutics including cyclophosphamide, vincristine, adriamycin, and dexamethasone alternating with high-dose methotrexate and cytarabine. Studies have also demonstrated improved survival in patients treated with hyper-CVAD when combined with the purine nucleoside analog nelarabine [26]. Considering the poor survival of adults with T-ALL, recent consideration has been given to the use of stem cell transplantation in these patients. Trials have demonstrated survival benefits of allotransplant in patients who have either achieved their second complete remission or high-risk patients who have achieved their first complete remission [27]. Ongoing investigations will demonstrate the viability of stem cell transplantation in the treatment of this disease [10].
In conclusion, we have described a case in which mature T-ALL presented in a unique fashion. Even though the patient lacked systemic signs of disease, CNS involvement in combination with a unique immunophenotype proved to be negative prognostic indicators. It is quite possible that this patient had the disease for an extended period of time, even more than a year, without detection.
Acknowledgments
The authors have no acknowledgements to make.
Financial Disclosure
The authors received no funding from any source.
Conflict of Interest
The authors have no conflict of interest to declare.
Informed Consent
Informed consent was not obtained, as the patient has expired and did not participate in any experimental treatments or interventions, the patient had no known next of kin. The patient’s identity was concealed throughout the entirety of the case report.
Author Contributions
John Kolton Smith contributed to the collection of data, analysis, wrote with input from all authors. Xinmin Zhang and Stephen C. Machnicki: contribution of data and analysis, revision. Salman Azhar: analysis and revision. Morana Vojnic contributed to the conception, planning and design, wrote with input from all authors, revision.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Swerdlow SH, Campo E, Harris NL, et al. World Health Organization Classification of Tumors of Haematopoietic and Lymphoid Tissues. 4th ed. Lyon, France: IARC. 2008:176-178.
- Yamamoto JF, Goodman MT. Patterns of leukemia incidence in the United States by subtype and demographic characteristics, 1997-2002. Cancer Causes Control. 2008;19(4):379-390.
doi pubmed - Marks DI, Paietta EM, Moorman AV, Richards SM, Buck G, DeWald G, Ferrando A, et al. T-cell acute lymphoblastic leukemia in adults: clinical features, immunophenotype, cytogenetics, and outcome from the large randomized prospective trial (UKALL XII/ECOG 2993). Blood. 2009;114(25):5136-5145.
doi pubmed - Marks DI, Rowntree C. Management of adults with T-cell lymphoblastic leukemia. Blood. 2017;129(9):1134-1142.
doi pubmed - You MJ, Medeiros LJ, Hsi ED. T-lymphoblastic leukemia/lymphoma. Am J Clin Pathol. 2015;144(3):411-422.
doi pubmed - Zhou Y, Fan X, Routbort M, Cameron Yin C, Singh R, Bueso-Ramos C, Thomas DA, et al. Absence of terminal deoxynucleotidyl transferase expression identifies a subset of high-risk adult T-lymphoblastic leukemia/lymphoma. Mod Pathol. 2013;26(10):1338-1345.
doi pubmed - Lazarus HM, Richards SM, Chopra R, Litzow MR, Burnett AK, Wiernik PH, Franklin IM, et al. Central nervous system involvement in adult acute lymphoblastic leukemia at diagnosis: results from the international ALL trial MRC UKALL XII/ECOG E2993. Blood. 2006;108(2):465-472.
doi pubmed - Fischer L, Gokbuget N, Schwartz S, Burmeister T, Rieder H, Bruggemann M, Hoelzer D, et al. CD56 expression in T-cell acute lymphoblastic leukemia is associated with non-thymic phenotype and resistance to induction therapy but no inferior survival after risk-adapted therapy. Haematologica. 2009;94(2):224-229.
doi pubmed - Gao L, Harbaugh B, Parr K, Patel P, Golem S, Zhang D, Woodroof J, et al. MYC Expression Is Associated With p53 Expression and TP53 Aberration and Predicts Poor Overall Survival in Acute Lymphoblastic Leukemia/Lymphoma. Am J Clin Pathol. 2022;157(1):119-129.
doi pubmed - Jabbour E, Thomas D, Cortes J, Kantarjian HM, O'Brien S. Central nervous system prophylaxis in adults with acute lymphoblastic leukemia: current and emerging therapies. Cancer. 2010;116(10):2290-2300.
doi pubmed - Otero HJ, Jagannathan JP, Prevedello LM, Johnston CJ, Ramaiya NH, Van den Abbeele AD, DiPiro PJ. CT and PET/CT findings of T-cell lymphoma. AJR Am J Roentgenol. 2009;193(2):349-358.
doi pubmed - Munch V, Trentin L, Herzig J, Demir S, Seyfried F, Kraus JM, Kestler HA, et al. Central nervous system involvement in acute lymphoblastic leukemia is mediated by vascular endothelial growth factor. Blood. 2017;130(5):643-654.
doi pubmed - Teshima T, Akashi K, Shibuya T, Taniguchi S, Okamura T, Harada M, Sumida I, et al. Central nervous system involvement in adult T-cell leukemia/lymphoma. Cancer. 1990;65(2):327-332.
doi pubmed - Cartwright RA, McKinney PA, O'Brien C, Richards ID, Roberts B, Lauder I, Darwin CM, et al. Non-Hodgkin's lymphoma: case control epidemiological study in Yorkshire. Leuk Res. 1988;12(1):81-88.
doi pubmed - Bernstein L, Ross RK. Prior medication use and health history as risk factors for non-Hodgkin's lymphoma: preliminary results from a case-control study in Los Angeles County. Cancer Res. 1992;52(19 Suppl):5510s-5515s.
- Zhang Y, Holford TR, Leaderer B, Zahm SH, Boyle P, Morton LM, Zhang B, et al. Prior medical conditions and medication use and risk of non-Hodgkin lymphoma in Connecticut United States women. Cancer Causes Control. 2004;15(4):419-428.
doi pubmed - Ye X, Casaclang N, Mahmud SM. Use of non-steroidal anti-inflammatory drugs and risk of non-Hodgkin lymphoma: a systematic review and meta-analysis. Hematol Oncol. 2015;33(4):176-186.
doi pubmed - Holly EA, Bracci PM. Population-based study of non-Hodgkin lymphoma, histology, and medical history among human immunodeficiency virus-negative participants in San Francisco. Am J Epidemiol. 2003;158(4):316-327.
doi pubmed - Beiderbeck AB, Holly EA, Sturkenboom MC, Coebergh JW, Stricker BH, Leufkens HG. No increased risk of non-Hodgkin's lymphoma with steroids, estrogens and psychotropics (Netherlands). Cancer Causes Control. 2003;14(7):639-644.
doi pubmed - Sorensen HT, Mellemkjaer L, Nielsen GL, Baron JA, Olsen JH, Karagas MR. Skin cancers and non-hodgkin lymphoma among users of systemic glucocorticoids: a population-based cohort study. J Natl Cancer Inst. 2004;96(9):709-711.
doi pubmed - Askling J, Klareskog L, Hjalgrim H, Baecklund E, Bjorkholm M, Ekbom A. Do steroids increase lymphoma risk? A case-control study of lymphoma risk in polymyalgia rheumatica/giant cell arteritis. Ann Rheum Dis. 2005;64(12):1765-1768.
doi pubmed - de Jongste AH, de Graaf MT, van den Broek PD, Kraan J, Sillevis Smitt PA, Gratama JW. Effector memory and late memory T cells accumulate in the blood of CMV-carrying individuals but not in their cerebrospinal fluid. Cytometry B Clin Cytom. 2013;84(4):218-221.
doi pubmed - Bento LC, Correia RP, Alexandre AM, Nosawa ST, Pedro EC, Vaz ADC, Schimidell D, et al. Detection of central nervous system infiltration by myeloid and lymphoid hematologic neoplasms using flow cytometry analysis: diagnostic accuracy study. Front Med (Lausanne). 2018;5:70.
doi pubmed - Zhao Y, He JY, Cui JZ, Meng ZQ, Zou YL, Guo XS, Chen X, et al. Detection of genes mutations in cerebrospinal fluid circulating tumor DNA from neoplastic meningitis patients using next generation sequencing. BMC Cancer. 2020;20(1):690.
doi pubmed - Litzow MR, Ferrando AA. How I treat T-cell acute lymphoblastic leukemia in adults. Blood. 2015;126(7):833-841.
doi pubmed - Abaza Y, H MK, Faderl S, Jabbour E, Jain N, Thomas D, Kadia T, et al. Hyper-CVAD plus nelarabine in newly diagnosed adult T-cell acute lymphoblastic leukemia and T-lymphoblastic lymphoma. Am J Hematol. 2018;93(1):91-99.
doi pubmed - Hamilton BK, Rybicki L, Abounader D, Adekola K, Advani A, Aldoss I, Bachanova V, et al. Allogeneic hematopoietic cell transplantation for adult T cell acute lymphoblastic leukemia. Biol Blood Marrow Transplant. 2017;23(7):1117-1121.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


