| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Case Report
Volume 11, Number 1, February 2022, pages 40-44
Ischemic Stroke and Bilateral Pulmonary Embolism in COVID-19: COVID-Associated Coagulopathy or Heparin-Induced Thrombocytopenia
Sara Solimana, b, c , Medhat Ghalya, b
aDepartment of Internal Medicine, Waterbury Hospital, Waterbury, CT, USA
bDepartment of Internal Medicine, Yale School of Medicine, CT, USA
cCorresponding Author: Sara Soliman, Department of Internal Medicine, Waterbury Hospital, Waterbury, CT, USA
Manuscript submitted December 7, 2021, accepted January 17, 2022, published online February 26, 2022
Short title: HIT in COVID-19
doi: https://doi.org/10.14740/jh956
| Abstract | ▴Top |
A main feature of coronavirus disease 2019 (COVID-19) pathogenesis is the high frequency of thrombosis, predominantly pulmonary embolism (PE). Anticoagulation therapy is a crucial part of the management. Heparin use for anticoagulation could increase the risk of heparin-induced thrombocytopenia (HIT), a potentially fatal complication that presents with thrombocytopenia with or without thrombosis. We present a 69-year-old unvaccinated female patient with severe COVID-19 pneumonia. Initial laboratory investigation was significant for thrombocytopenia and low D-dimer levels. She was initially started on enoxaparin followed by unfractionated heparin. On hospital day 8, she developed left facial droop and dysarthria and was found to have non-occlusive thrombus in proximal middle cerebral artery as well as bilateral pulmonary emboli. She received intravenous thrombolysis followed by heparin infusion. On day 13 of hospitalization, platelet count dropped from 120,000/mm3 to 43,000/mm3, raising suspicion of HIT. Heparin was stopped and fondaparinux was started. After 3 days, HIT antibody testing returned positive, then a positive serotonin release assay confirmed the diagnosis. On discharge, she was transitioned to apixaban to complete 3 months of anticoagulation for provoked PE. This case represents the diagnostic challenge of HIT in COVID-19 patients. Thrombocytopenia after heparin infusion should raise clinical suspicion of HIT, which allows appropriate discontinuation of heparin products and initiation of alternative anticoagulants to limit devastating complications. To our knowledge, this is the first case report of a COVID-19 patient presenting with venous thrombosis as well as arterial thrombotic event in the context of underlying HIT.
Keywords: COVID-19; HIT; Hypercoagulability; Thrombosis; Thrombocytopenia
| Introduction | ▴Top |
At the end of 2019, a novel coronavirus was identified as the cause of a cluster of pneumonia cases in Wuhan, China. It rapidly spread, resulting in an epidemic throughout China, followed by an increasing number of cases worldwide [1]. Although coronavirus disease 2019 (COVID-19) usually presents with severe acute respiratory syndrome, a striking feature of the disease is high frequency of thrombosis. The available literature to date has documented high rates of venous thromboembolism (VTE), predominantly pulmonary embolism (PE) in critically ill patients with COVID-19 pneumonia [2, 3]. Multiple reports have also shown substantial micro thrombosis in postmortem autopsies [4]. Recommendations regarding the rate and type of VTE prophylaxis in those patients vary widely among the studies.
Heparin is frequently used to prevent thrombosis in those patients, sometimes in higher dosage and for prolonged periods. Heparin use increases the risk of heparin-induced thrombocytopenia (HIT), a rare but potentially fatal complication of heparin exposure that usually presents with thrombocytopenia with or without thrombosis. Thrombocytopenia is the most common presenting sign of HIT but it is also frequently seen in severe COVID-19 infections. Overlapping symptoms between HIT and COVID-associated thrombosis or thrombocytopenia make diagnosis extremely challenging.
Here we report a case of a 69-year-old female patient with severe COVID-19 pneumonia who developed acute ischemic stroke and bilateral pulmonary emboli and hospital course was complicated by a sudden drop of platelet count leading to diagnosis of HIT. We discuss the diagnostic challenge of HIT in those patients and the importance of early diagnosis to limit morbidity and mortality.
| Case Report | ▴Top |
A 69-year-old female patient with past medical history of hypertension, hyperlipidemia and obstructive sleep apnea presented with 3 days of cough, shortness of breath and diarrhea. The patient was unvaccinated against COVID-19. On examination, she was febrile (100.6 °F) and oxygen saturation was 88% on room air. She was in mild respiratory distress and lung auscultation revealed diffuse bilateral crackles. On admission, laboratory investigations revealed white blood cell count of 3.2 × 103/mm3, platelet count of 128 × 103/mm3, lymphocytes of 0.3 × 103/mm3, sodium of 131 mmol/L, potassium of 2.7 mmol/L, prothrombin time of 13.7 s, international normalized ratio (INR) of 1.2 and D-dimer of < 150 ng/mL. Chest X-ray did show bilateral interstitial opacities. She tested positive for COVID-19 by nasopharyngeal reverse-transcriptase-polymerase chain reaction.
She received supplemental oxygen and was started on dexamethasone, remdesivir and low molecular weight heparin (enoxaparin) 40 mg daily for thrombosis prophylaxis. Leukopenia and thrombocytopenia were believed to be secondary to viral infection.
On hospital day 3, she developed acute worsening of respiratory status with increased work of breathing requiring urgent mechanical ventilation. Chest imaging showed marked bilateral infiltrates consistent with progression of underlying viral pneumonia and acute respiratory distress syndrome. Repeat laboratory testing showed white blood cell count of 6.3 × 103/mm3 and platelet count of 150 × 103/mm3. Inflammatory markers were markedly elevated including C-reactive protein (CRP) > 270 mg/L, lactate dehydrogenase (LDH) 1,553 U/L and D-dimer 606 ng/mL. Due to worsening kidney function, enoxaparin was changed to unfractionated heparin (UFH) 5,000 IU/mL three times daily.
Inflammatory markers continued to rise and D-dimer peaked at 21,143 ng/mL on hospital day 5 for which she received one dose of 400 mg of tocilizumab to reduce the consequences of impending cytokine storm (Fig. 1). On hospital day 6, she was successfully extubated.
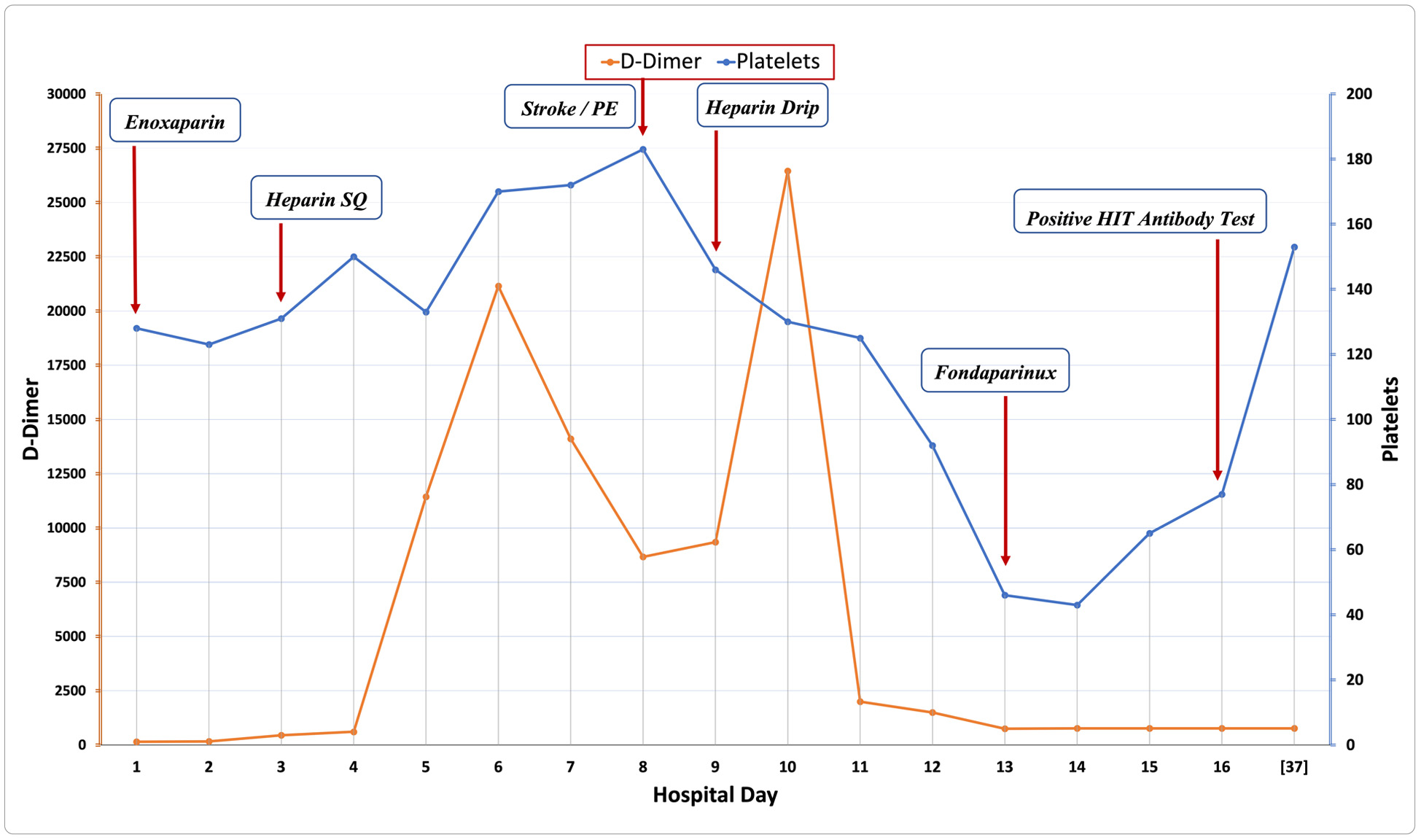 Click for large image | Figure 1. Platelets and D-dimer levels in relation to day of hospitalization. Blue line represents the trend of platelet count (× 103/µL) during inpatient hospitalization. Note that the patient presented with mild thrombocytopenia but levels remained consistently above 120 × 103/µL. The graph highlights the remarkable decrease in platelet count around hospital day 13 that led to diagnosis of HIT and appropriate management. Red line shows the trend of D-dimer levels (ng/mL) during hospitalization. Note that levels have peaked on hospital day 5 as well as other inflammatory markers consistent with impending cytokine storm and respiratory failure. Levels have markedly increased again around hospital days 10-11 in the setting of bilateral PE. HIT: heparin-induced thrombocytopenia; PE: pulmonary embolism. |
On hospital day 8, she acutely developed new left-sided facial droop, mild left-sided upper and lower extremity weakness with mild dysarthria. National Institutes of Health (NIH) stroke scale score was 8 and immediate computed tomography (CT) scan of the head showed no acute intracranial process, but CT angiography (CTA) of the head and neck showed non-occlusive thrombus in the proximal right M2 branch as well as emboli in the bilateral distal main and upper lobe pulmonary arteries. She received intravenous thrombolysis with tissue plasminogen activator (t-PA) 0.9 mg/kg. A repeat CT of head after 24 h did not show any evidence of intracranial hemorrhage or evolving infarct. Transthoracic echocardiogram with bubble study did not show any evidence of intra-cardiac shunt making paradoxical embolism unlikely. Heparin infusion was subsequently started for management of bilateral pulmonary emboli. Later, a magnetic resonance imaging (MRI) of head confirmed an acute 5 mm left cerebellar infarct.
On hospital day 13, platelet count has dropped from 120 × 103/mm3 to 43,000/mm3. The steady drop and recent thrombosis raised suspicion of possible underlying HIT. The 4T score was calculated at 6 corresponding to high pretest probability of HIT. Heparin was immediately stopped and fondaparinux was started. After 3 days, HIT antibody testing (anti-platelet factor 4 (PF4)/heparin antibody, by enzyme-linked immunosorbent assay) returned positive with an optical density (OD) of 1.345 OD units, subsequently diagnosis of HIT was confirmed by positive functional assay (serotonin release assay). Platelet count has gradually improved (Fig. 1). The patient was transitioned to apixaban and was discharged home on anticoagulation with a close outpatient follow-up with hematology. On outpatient follow-up, her symptoms have resolved, and serial D-dimer levels were normal. She has completed 3 months of oral anticoagulation for provoked PE in the setting of HIT with thrombosis.
| Discussion | ▴Top |
Severe COVID-19 infection is associated with high risk of thrombosis since patients exhibit all three components of Virchow’s triad [2]. Endotheliitis and hyperinflammation predispose to diffuse thrombosis which is a major characteristic of severe COVID-19 infection [5, 6]. Hyperfibrinogenemia and markedly elevated D-dimers reflect underlying pro-coagulant state. Factor VIII and Von Willebrand factors were found to be also increased in COVID-19 infection reflecting a severe inflammatory and hypercoagulable state [7].
The high incidence of thrombotic events even with prophylactic dose anticoagulation led physicians to use intermediate or even therapeutic doses of anticoagulants [7]. Enoxaparin, low molecular weight heparin (LMWH) is usually used as it can be administered once daily; when contraindicated as in cases of renal failure, UFH is preferred [2].
HIT is a rare but potentially life-threatening complication of heparin exposure seen in about 1 in 5,000 hospitalized patients. It is characterized by platelet count drop of 50% or greater usually within 5 - 10 days of exposure to heparin. It results from autoantibody directed against endogenous PF4 in complex with heparin. The IgG-PF4-heparin immune complexes cross-link FCy (γ) receptors on platelets and monocytes resulting in its activation. Activated platelets subsequently induce thrombin release and predispose patients to thrombotic complications. Untreated HIT has a mortality rate as high as 20% [8].
Worsening thrombocytopenia after exposure to heparin is usually the initial sign to suspect HIT; however, thrombotic manifestations can precede thrombocytopenia. In a series of 408 patients of HIT with thrombosis, around 33% of patients developed thrombosis before the decrease in platelet counts was noted, so thrombotic manifestation with recent heparin exposure should raise the clinical suspicion of HIT even in absence of thrombocytopenia [9].
When HIT is suspected, pre-test probability is calculated using the 4T score which evaluates four indicators: the relative platelet count fall, the timing of the onset of the platelet count fall, the presence of thrombosis, and the likelihood of other causes of thrombocytopenia. A total score of less than 4 points has a very high negative predictive value (97-99%) and should preclude testing for HIT. Magnitude of anti-PF4-heparin reactivity on enzyme immunoassay should be considered, for example, an OD < 1.0 on enzyme immunoassay is rarely associated with clinically relevant anti-PF4-heparin antibodies [8, 9].
HIT reports in severe COVID-19 infection are limited but initial reports have shown higher frequency of HIT in COVID-19 patients. In a retrospective review analysis of HIT cases, seven cases of HIT were described among 86 patients with severe COVID-19 representing an incidence of HIT of 8% in patients with severe COVID-19 infection. Although the results of the study are limited by retrospective nature and small sample size, it has reported a nearly 10-fold higher occurrence of HIT during severe COVID-19 [9]. Rushed et al have also analyzed 88 COVID-19 patients who received at least 5 days of UFH, and among eight patients where there was suspicion for HIT, five patients tested positive for HIT antibodies [10].
Diagnosis of HIT in patients with positive PF4 antibody should be followed by confirmatory serotonin release assay (SRA) to limit overdiagnosis.
Prasanth et al reported a case of a 63-year-old male patient with COVID-19 pneumonia who developed a right femoral deep venous thrombosis and was started on therapeutic anticoagulation. Then he subsequently had significant platelet drop raising suspicion for HIT. Heparin was switched to argatroban and diagnosis was confirmed by positive SRA. The case series also reported four additional patients who had thrombotic complications and positive PF4 antibodies, but SRA was negative in those cases [11].
Richard et al also reported three cases of thrombocytopenia with PF4 antibodies among 16 intubated patients with severe COVID-19 infection. All three patients had evidence of thrombosis. Though all patients had positive PF4 antibodies, only one patient was confirmed by SRA. This highlights the importance of confirmatory testing of HIT in those patients [12]. One problem with HIT is overdiagnosis. Only half of patients with positive immunoassays have positive confirmatory testing, and the latter usually needs specialized laboratory centers and may not be readily available initially. Results should be carefully interpreted to avoid overdiagnosis and use of alternate anticoagulants that are expensive, can cause major bleeding and their reversal agents are not readily available [13].
Severe COVID-19 infection can induce anti-heparin-PF4 antibodies causing thrombocytopenia without heparin exposure. In a study of 61 critically ill intensive care unit (ICU) patients, Xuan et al surprisingly found that HIT occurred not only in patients with heparin exposure but also in heparin-naive patients, suggesting that a spontaneous HIT might occur in COVID-19 patients, probably related to the formation of PF4 tetrameric complexes during viral or secondary bacterial infection [14].
Antiphospholipid syndrome has also been reported in critically ill COVID-19 patients, and those patients can falsely test positive for HIT serology. For those reasons, results should be carefully interpreted in the context of clinical pictures and other relevant test results [15, 16].
When HIT is suspected, the first step is to discontinue all heparin products and start a non-heparin anticoagulant. Argatroban, a short-lived direct thrombin inhibitor, is FDA approved for use in HIT. In vitro data suggest that direct oral anticoagulants such as rivaroxaban and apixaban might be used in patients with acute HIT [17]. However, limited data are currently available to support its use. Xuan et al reported a case of a 43-year-old male patient with severe COVID-19 pneumonia necessitating the use of veno-venous extracorporeal membrane oxygenation (ECMO), and platelet count has dropped to 44 × 109/L 1 day after ECMO. HIT was suspected and PF4 antibodies returned positive with high antibody titer. Due to unavailability of recommended non-heparin anticoagulants, he was started on rivaroxaban and was able to make full recovery. Here we report a good response in our patient treated with fondaparinux before she was transitioned to oral apixaban [18].
Finally, an extremely rare but fatal complication called vaccine-induced thrombotic thrombocytopenia (VITT) was found to occur in patients who received adenoviral COVID-19 vaccines (AstraZeneca and Johnson & Johnson), within 4 - 30 days of vaccine administration, and those patients developed thrombosis and thrombocytopenia. Surprisingly, those patients were all found to have positive HIT antibodies without known recent heparin exposure. VITT was thought to have the same underlying pathophysiology of HIT; however, the exact trigger is unknown, suggesting possible antigenic component in viral genome that induces formation of HIT antibodies in those patients. Heparin is also contraindicated and treatment should be initiated with non-heparin anticoagulation [19].
Our case initially represented a common clinical scenario with severe COVID-19 infection complicated by thrombotic manifestations that were appropriately managed by anticoagulation with intravenous heparin; however, worsening thrombocytopenia after heparin exposure raised the question of HIT leading to appropriate testing and management with non-heparin anticoagulant. Since our patient did not receive any kind of COVID vaccine, VITT was unlikely and symptoms were likely secondary to HIT, a relatively common consequence in COVID-19 infection.
Learning points
Use of heparin for anticoagulation prophylaxis in hospitalized COVID-19 patients places the patient at risk of developing HIT, a potentially fatal complication. Diagnosis of HIT in the context of COVID-19 is extremely challenging since both conditions are prothrombotic and can lead to thrombocytopenia, so high index of suspicion is needed for timely diagnosis and appropriate intervention. Physicians should always consider HIT as an underlying cause of otherwise unexplained drop in platelet count or suspicion of thrombotic events in those patients. Careful interpretation of the immunoassay results and pretest test probability limits overdiagnosis of HIT.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Informed consent was obtained.
Author Contributions
Sara Soliman developed the original idea, reviewed literature data, prepared the manuscript, and provided additional review. Medhat Ghaly provided revision of the manuscript for important intellectual content.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
HIT: heparin-induced thrombocytopenia; PE: pulmonary embolism; VTE: venous thromboembolism; INR: international normalized ratio; CRP: C-reactive protein; LDH: lactate dehydrogenase; NIH: National Institutes of Health; CTA: computed tomography angiography; t-PA: tissue plasminogen activator; MRI: magnetic resonance imaging; OD: optical density; PF4: platelet factor 4; SRA: serotonin release assay; VITT: vaccine-induced thrombotic thrombocytopenia
| References | ▴Top |
- Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708-1720.
doi pubmed - Chowdhury JF, Moores LK, Connors JM. Anticoagulation in hospitalized patients with COVID-19. N Engl J Med. 2020;383(17):1675-1678.
doi pubmed - Moores LK, Tritschler T, Brosnahan S, Carrier M, Collen JF, Doerschug K, Holley AB, et al. Prevention, diagnosis, and treatment of VTE in patients with coronavirus disease 2019: CHEST guideline and expert panel report. Chest. 2020;158(3):1143-1163.
doi pubmed - Wichmann D, Sperhake JP, Lutgehetmann M, Steurer S, Edler C, Heinemann A, Heinrich F, et al. Autopsy Findings and Venous Thromboembolism in Patients With COVID-19: A Prospective Cohort Study. Ann Intern Med. 2020;173(4):268-277.
doi pubmed - Teuwen LA, Geldhof V, Pasut A, Carmeliet P. COVID-19: the vasculature unleashed. Nat Rev Immunol. 2020;20(7):389-391.
doi pubmed - Lowenstein CJ, Solomon SD. Severe COVID-19 is a microvascular disease. Circulation. 2020;142(17):1609-1611.
doi pubmed - Panigada M, Bottino N, Tagliabue P, Grasselli G, Novembrino C, Chantarangkul V, Pesenti A, et al. Hypercoagulability of COVID-19 patients in intensive care unit: A report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost. 2020;18(7):1738-1742.
doi pubmed - Greinacher A. Clinical Practice. Heparin-induced thrombocytopenia. N Engl J Med. 2015;373(3):252-261.
doi pubmed - Daviet F, Guervilly C, Baldesi O, Bernard-Guervilly F, Pilarczyk E, Genin A, Lefebvre L, et al. Heparin-induced thrombocytopenia in severe COVID-19. Circulation. 2020;142(19):1875-1877.
doi pubmed - Patell R, Khan AM, Bogue T, Merrill M, Koshy A, Bindal P, Joyce R, et al. Heparin induced thrombocytopenia antibodies in Covid-19. Am J Hematol. 2020;95(10):E295-E296.
doi pubmed - Lingamaneni P, Gonakoti S, Moturi K, Vohra I, Zia M. Heparin-induced thrombocytopenia in COVID-19. J Investig Med High Impact Case Rep. 2020;8:2324709620944091.
doi pubmed - Riker RR, May TL, Fraser GL, Gagnon DJ, Bandara M, Zemrak WR, Seder DB. Heparin-induced thrombocytopenia with thrombosis in COVID-19 adult respiratory distress syndrome. Res Pract Thromb Haemost. 2020;4(5):936-941.
doi pubmed - Cuker A, Gimotty PA, Crowther MA, Warkentin TE. Predictive value of the 4Ts scoring system for heparin-induced thrombocytopenia: a systematic review and meta-analysis. Blood. 2012;120(20):4160-4167.
doi pubmed - Liu X, Zhang X, Xiao Y, Gao T, Wang G, Wang Z, et al. Heparin-induced thrombocytopenia is associated with a high risk of mortality in critical COVID-19 patients receiving heparin-involved treatment. medRxiv. 2020:2020.04.23.20076851.
doi - Zhang Y, Xiao M, Zhang S, Xia P, Cao W, Jiang W, Chen H, et al. Coagulopathy and Antiphospholipid Antibodies in Patients with Covid-19. N Engl J Med. 2020;382(17):e38.
doi pubmed - Smythe MA, Warkentin TE, Woodhouse AL, Zakalik D. Venous limb gangrene and fatal hemorrhage: adverse consequences of HIT "overdiagnosis" in a patient with antiphospholipid syndrome. Am J Hematol. 2011;86(2):188-191.
doi pubmed - Krauel K, Hackbarth C, Furll B, Greinacher A. Heparin-induced thrombocytopenia: in vitro studies on the interaction of dabigatran, rivaroxaban, and low-sulfated heparin, with platelet factor 4 and anti-PF4/heparin antibodies. Blood. 2012;119(5):1248-1255.
doi pubmed - Phan XT, Nguyen TH, Tran TT, Huynh TT, Hoang TT, Nguyen VV, Pham TNT. Suspected heparin-induced thrombocytopenia in a COVID-19 patient on extracorporeal membrane oxygenation support: a case report. Thromb J. 2020;18(1):37.
doi pubmed - Pavord S, Scully M, Hunt BJ, Lester W, Bagot C, Craven B, Rampotas A, et al. Clinical features of vaccine-induced immune thrombocytopenia and thrombosis. N Engl J Med. 2021;385(18):1680-1689.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


