| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Original Article
Volume 10, Number 5, October 2021, pages 207-211
Validation of a Modified Version of the Myeloproliferative Neoplasm Symptom Assessment Form Total Symptom Score
Blake T. Langlaisa, Gina L. Mazzaa, Heidi E. Kosioreka, Jeanne Palmerb, Ruben Mesac, Amylou C. Duecka, d
aDepartment of Quantitative Health Sciences, Mayo Clinic, Scottsdale, AZ, USA
bDepartment of Hematology and Oncology, Mayo Clinic, Phoenix, AZ, USA
cMays Cancer Center at UT Health San Antonio MD Anderson, San Antonio, TX, USA
dCorresponding Author: Amylou C. Dueck, Department of Quantitative Health Sciences, Mayo Clinic, 13400 E. Shea Blvd., Scottsdale, AZ 85259, USA
Manuscript submitted August 26, 2021, accepted September 24, 2021, published online October 31, 2021
Short title: A Modified Version of the MPN-SAF TSS
doi: https://doi.org/10.14740/jh914
| Abstract | ▴Top |
Background: Patients with myeloproliferative neoplasms (MPNs) suffer from chronic and progressive symptom burden. MPN trials capturing patient-reported symptoms routinely administer the MPN Symptom Assessment Form (SAF). The MPN-10 assesses 10 of the most clinically relevant symptoms, including fatigue and generates a Total Symptom Score (TSS). The original MPN-10 included a fatigue item from the Brief Fatigue Inventory (BFI). The myelofibrosis-specific symptom assessment tool called the MFSAF v4 utilizes a fatigue item developed to be consistent with other items within the SAF. This study sought to validate a modified version of the MPN-10 TSS using the SAF fatigue item for harmonization with MFSAF v4.
Methods: Survey data from two cohorts of patients with essential thrombocythemia, polycythemia vera, or myelofibrosis assessing MPN characteristics and symptom burden were used.
Results and conclusion: BFI and SAF fatigue items were highly correlated in raw score (Pearson r = 0.88), comparable in their severity categorizations (89% agreement for severe versus non-severe) and respective contributions to the TSS (both Cronbach’s alpha = 0.89). Reliability of SAF fatigue was acceptable and independently associated with known disease-related characteristics (splenomegaly, low quality-of-life, and distress). Fatigue in patients with MPNs is measured with high similarity using the SAF fatigue item within the MPN-10 in harmonization with the MFSAF v4.
Keywords: Myeloproliferative neoplasm; Symptom Assessment Form; Total Symptom Score; Brief Fatigue Inventory; MPN-10; MFSAF v4
| Introduction | ▴Top |
The myeloproliferative neoplasms (MPNs), including essential thrombocythemia (ET), polycythemia vera (PV), and myelofibrosis (MF), are progressive bone marrow diseases marked by chronic and often debilitating symptom burden. Hematopoietic stem cell transplantations are potentially curative measures but are generally reserved for only a small fraction of younger patients with good performance status [1]. For the remaining majority, alleviating symptom suffering, reducing clinical complications, and slowing progression are typical treatment goals in the wake of increasingly aggressive disease. Evaluation of experimental therapeutics in trials relies on patient-reported symptoms using the MPN Symptom Assessment Form (SAF) in conjunction with evaluation of treatment response [2]. The SAF assesses 18 symptoms related to MPNs scored from 0 (absent) to 10 (worst imaginable). A later rendition of the SAF, called the MPN-10, condensed the 18 items to 10 of the most clinically meaningful symptoms [3]. The Total Symptom Score (TSS) is computed by summing the MPN-10 symptom scores. Chief among the most prevalent and severe symptoms in patients with MPNs is fatigue. Though the SAF includes a fatigue item, initial deployments included an item from the Brief Fatigue Inventory (BFI), which asks, “Please rate your fatigue (weariness, tiredness) by choosing the one number that best describes your WORST level of fatigue during the past 24 hours.” [4]. Subsequently, the MFSAF v4 was developed for MF trials which included a fatigue item developed to be consistent with other items within the SAF [5]. This item asks, “During the past 24 hours, how severe was your worst fatigue (weariness, tiredness)?”
This study aimed to validate a modified version of the MPN-10 which includes the SAF fatigue item to harmonize with the MFSAF v4.
| Materials and Methods | ▴Top |
Study data
Data from two prior independent surveys [6, 7] were used for this secondary analysis. Approval for each study was given by the Mayo Clinic Institutional Review Board and surveys were executed in compliance with applicable ethical standards of the Health Insurance Portability and Accountability Act and the Helsinki Declaration. For both surveys, participant consent was provided and survey responses were anonymous. Links to the respective surveys were promoted via multiple MPN-related websites and their affiliated Facebook pages.
Study 1: BFI and SAF fatigue comparison sample
To validate the harmonized SAF fatigue item within the MPN-10, data were used from a randomized survey deployed in 2017 designed to test measure equivalence, validity, and reliability for the MFSAF v4. Patients completed surveys in one of eight possible, randomly assigned, ordering sequences. Sequences in which patients completed both the BFI and SAF fatigue items in addition to the remainder of the MPN-10 items are included in this current study for comparison of BFI and SAF fatigue. Sequences in which patients completed the SAF fatigue item twice are included here as a comparator for test/retest reliability. The recall period throughout this survey was the last 24 h.
Study 2: SAF fatigue implementation sample
To evaluate the relationship between known MPN-disease corelates with this modified version of the MPN-10, data were used from a survey assessing the impact of the coronavirus disease 2019 (COVID-19) pandemic on patient care during June of 2020. SAF fatigue was used in place of BFI fatigue to construct the MPN-10. The recall period throughout this survey was the past week.
Statistical analysis
Within study 1, agreement between BFI and SAF fatigue scores, and twice administered SAF fatigue scores, were assessed using Pearson correlations (r) and Bland-Altman methods. Fatigue was categorized as severe (≥ 7) versus non-severe (< 7); and absent (0), mild (1 - 3), moderate (4 - 6), and severe (7 - 10). Absolute agreement between categories was computed. MPN-10 TSS was computed using BFI and SAF fatigue items, separately, and Pearson correlation was computed between the TSS. Internal consistency of the TSS was assessed using Cronbach’s alpha. Order effects were evaluated using paired t-tests. Within study 2, known-groups validity was evaluated by comparing SAF fatigue between patients with and without reported splenomegaly using a t-test [8]. Convergent validity was assessed using Pearson correlation between SAF fatigue and overall quality-of-life measured by a numeric rating scale (0 - 10) [9] and distress measured by the National Comprehensive Cancer Network Distress Thermometer [10]. Means and 95% confidence intervals (CIs) were reported. P values less than 0.05 were considered statistically significant.
| Results and Discussion | ▴Top |
Study 1
There were 464 consenting adults with ET (n = 165), PV (n = 196), or MF (n = 103). Mean age was 58 years (range 27 - 90 years), 371 (80%) were female, and 493 (96%) were White. After randomization, 224 (48%) completed the BFI and SAF items and 240 (52%) completed the SAF items twice. Among those completing BFI and SAF items, 52% (116/224) received the BFI item first and 48% (108/224) received the SAF item first. There was no difference between first and second fatigue scores (mean difference (first score minus second) = 0.01; CI: -0.16, 0.19). The BFI and SAF fatigue scores were highly correlated (Fig. 1a; r = 0.88, P < 0.001) and severity categorization agreement was high (89% (199/224) for severe versus non-severe; 74% (165/224) for overall categories). Bland-Altman methods indicate the BFI and SAF fatigue items have high agreement with no evidence of directional bias (regression slope = -0.03; P = 0.33) and only clinically insignificant overall bias (mean difference = 0.20; CI: 0.03, 0.37; Fig. 1b). Computing the scale score with BFI and SAF separately resulted in nearly identical TSS (r = 0.997, P < 0.001) and equivalent internal consistency (both Cronbach’s alpha = 0.89). Among those completing the SAF item twice, SAF fatigue scores were highly correlated (Fig. 1c; r = 0.89, P < 0.001) and severity categorization agreement was high (87% (208/240) for severe versus non-severe; 75% (181/240) for overall categories), metrics which mirrored the BFI/SAF comparisons. Bland-Altman methods also showed strong indication of test/retest reliability with no evidence of directional bias (regression slope = -0.01, P = 0.77) and only clinically insignificant overall bias (mean difference (first score minus second) = 0.27; CI: 0.10, 0.43; Fig. 1d).
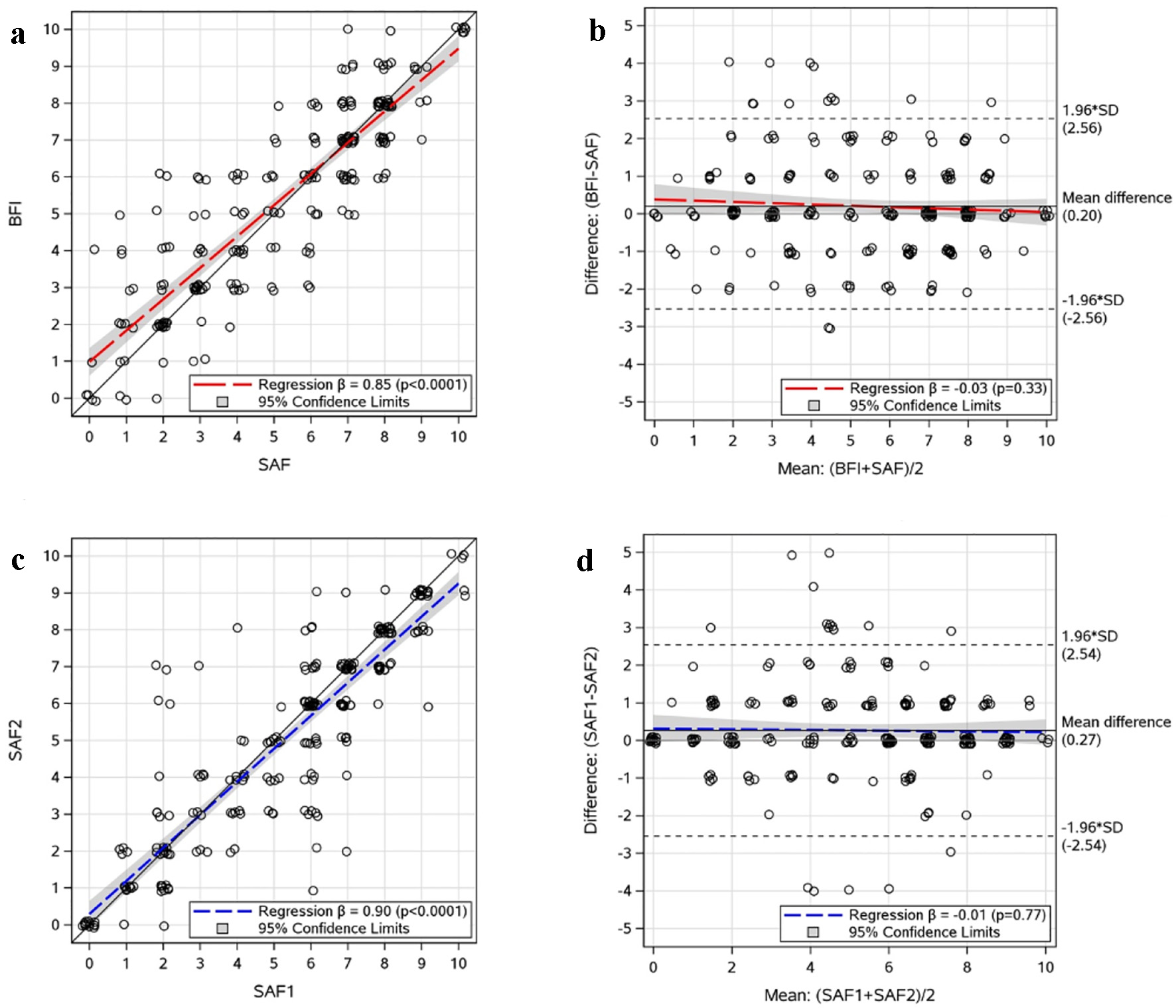 Click for large image | Figure 1. Scatter and Bland-Altman plots of the BFI/SAF fatigue comparison and SAF fatigue test/retest data. (a) BFI/SAF comparison: Jittered scatter plot of paired scores. (b) BFI/SAF comparison: Jittered Bland-Altman plot of paired scores. (c) Test/retest: Jittered scatter plot of paired SAF1 and SAF2. (d) Test/retest: Jittered Bland-Altman plot of paired SAF1 and SAF2. SAF: Symptom Assessment Form; BFI: Brief Fatigue Inventory; SAF1: first administration of SAF fatigue question; SAF2: second administration of SAF fatigue question; SD: standard deviation. |
Study 2
There were 1,129 consenting adults with ET (n = 522), PV (n = 387), or MF (n = 220). Mean age was 61 years (range 21 - 93 years), 848 (75%) were female, and 1,074 (95%) were White. Known-groups analysis showed those who reported splenomegaly reported higher MPN-10 TSS (computed with SAF fatigue) than those without (n = 291 and 602, respectively; mean difference = 7.7; CI: 5.3, 10.0). Those with higher MPN-10 TSS reported lower quality-of-life scores (r = -0.50, P < 0.001) and higher levels of distress (r = 0.41, P < 0.001).
Fatigue is a key disease-related symptom among patients with MPNs [11]. Measuring fatigue within the MPN-10 is essential for characterizing symptomatic burden [8, 12]. As noted by Gwaltney et al, the SAF fatigue item was modeled after the BFI fatigue item. The BFI and SAF versions evaluating fatigue are very similar in wording. As such, it should be unsurprising that the results presented here support BFI and SAF fatigue items being highly comparable in raw score, severity categorizations, and in their respective contributions to the MPN-10 TSS. Further, the test/retest results of the SAF fatigue item support acceptable reliability. When implementing the SAF fatigue item in an independent, subsequent survey of MPN patients, the modified MPN-10 TSS was associated with known disease-related characteristics and other patient-reported outcomes demonstrating construct validity. Notwithstanding, there are limitations to data from anonymous internet-based surveys such as volunteer bias. For example, both surveys asked about MPN diagnosis and splenomegaly without confirmatory clinical data. However, it is generally accepted that MPN patients have good comprehension of their diagnosis and MPN-related health status. Also, participants from both surveys were disproportionally female (80% and 75% for studies 1 and 2, respectively). Though the general MPN patient population is roughly 53% female [13], a bias towards female survey participation is common among other independent surveys of patients with MPNs [1, 14-18]. This may be a function of the survey dissemination method using internet platforms with disproportionally female users, an unmeasured gender survey-participation bias phenomenon, or some other uncontrolled mechanism. Future internet-based MPN patient surveys should aim to reduce this participation bias during the survey design phase. Ultimately, we believe these limitations do not jeopardize these findings. Disease-related fatigue is measured equivalently using the SAF fatigue item within the MPN-10 in harmonization with the MFSAF v4.
Acknowledgments
None to declare.
Financial Disclosure
This work was supported in part by National Institute of Health grants P01CA108671, P30CA015083, and UL1TR002377, as well as internal funding by Mayo Clinic.
Conflict of Interest
RM received research funding from Celgene, Incyte, Abbvie, Samus, Genotech, Promedior, CTI, and Constellation Pharmaceuticals, as well as received consultancy fees from Novartis, Sierra Oncology, LA Jolla Pharmaceuticals, Pharma, and Constellation Pharmaceuticals. AD received consultancy fees from Constellation Pharmaceuticals, as well as royalties from licensing for commercial purposes of the MPN-SAF.
Informed Consent
Data for this analysis originated from anonymous internet-based surveys. Survey participants provided informed consent in order to initiate the survey.
Author Contributions
BTL performed the statistical analysis. BTL and ACD wrote the manuscript. JP, RM, and ACD collected and provided data. GLM, HEK, JP, and RM contributed interpretation and edits to the work.
Data Availability
Any inquiries regarding supporting data availability of this study should be directed to the corresponding author.
Abbreviations
BFI: Brief Fatigue Inventory; CI: confidence interval; ET: thrombocythemia; MF: myelofibrosis; MPN: myeloproliferative neoplasm; PV: polycythemia vera; r: Pearson correlations; TSS: Total Symptom Score
| References | ▴Top |
- Palmer J, Scherber R, Girardo M, Geyer H, Kosiorek H, Dueck A, Jain T, et al. Patient perspectives regarding allogeneic bone marrow transplantation in myelofibrosis. Biol Blood Marrow Transplant. 2019;25(2):398-402.
doi pubmed - Scherber R, Dueck AC, Johansson P, Barbui T, Barosi G, Vannucchi AM, Passamonti F, et al. The Myeloproliferative Neoplasm Symptom Assessment Form (MPN-SAF): international prospective validation and reliability trial in 402 patients. Blood. 2011;118(2):401-408.
doi pubmed - Emanuel RM, Dueck AC, Geyer HL, Kiladjian JJ, Slot S, Zweegman S, te Boekhorst PA, et al. Myeloproliferative neoplasm (MPN) symptom assessment form total symptom score: prospective international assessment of an abbreviated symptom burden scoring system among patients with MPNs. J Clin Oncol. 2012;30(33):4098-4103.
doi pubmed - Mendoza TR, Wang XS, Cleeland CS, Morrissey M, Johnson BA, Wendt JK, Huber SL. The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer. 1999;85(5):1186-1196.
doi - Gwaltney C, Paty J, Kwitkowski VE, Mesa RA, Dueck AC, Papadopoulos EJ, Wang L, et al. Development of a harmonized patient-reported outcome questionnaire to assess myelofibrosis symptoms in clinical trials. Leuk Res. 2017;59:26-31.
doi pubmed - Dueck AC, Gwaltney C, Chen W, Wang L, Pierson R, Trudeau J, Eremenco S, et al. Quantitative testing of the myelofibrosis symptom assessment form version 4.0, a harmonized patient-reported outcome measure for collecting key secondary endpoint data in myelofibrosis clinical trials. Blood. 2017;130(Supplement 1):2168.
doi - Palmer JM, Mead-Harvey C, Harrison C, Dueck AC, Eckert R, Kosiorek HE, Muxi P, et al. Impact of COVID19 pandemic on an international MPN patient population: survey results from 1560 MPN patients. Blood. 2020;136(Supplement 1):1-3.
doi - Mesa RA, Niblack J, Wadleigh M, Verstovsek S, Camoriano J, Barnes S, Tan AD, et al. The burden of fatigue and quality of life in myeloproliferative disorders (MPDs): an international Internet-based survey of 1179 MPD patients. Cancer. 2007;109(1):68-76.
doi pubmed - Langlais BT, Geyer H, Scherber R, Mesa RA, Dueck AC. Quality of life and symptom burden among myeloproliferative neoplasm patients: do symptoms impact quality of life? Leuk Lymphoma. 2019;60(2):402-408.
doi pubmed - Holland JC, Bultz BD, National comprehensive Cancer N. The NCCN guideline for distress management: a case for making distress the sixth vital sign. J Natl Compr Canc Netw. 2007;5(1):3-7.
doi pubmed - Geyer H, Mesa RA. Approach to MPN symptom assessment. Curr Hematol Malig Rep. 2017;12(5):381-388.
doi pubmed - Scherber RM, Kosiorek HE, Senyak Z, Dueck AC, Clark MM, Boxer MA, Geyer HL, et al. Comprehensively understanding fatigue in patients with myeloproliferative neoplasms. Cancer. 2016;122(3):477-485.
doi pubmed - Price GL, Davis KL, Karve S, Pohl G, Walgren RA. Survival patterns in United States (US) medicare enrollees with non-CML myeloproliferative neoplasms (MPN). PLoS One. 2014;9(3):e90299.
doi pubmed - Geyer HL, Andreasson B, Kosiorek HE, Dueck AC, Scherber RM, Martin KA, Butler KA, et al. The role of sexuality symptoms in myeloproliferative neoplasm symptom burden and quality of life: An analysis by the MPN QOL International Study Group. Cancer. 2016;122(12):1888-1896.
doi pubmed - Huberty J, Eckert R, Dueck A, Kosiorek H, Larkey L, Gowin K, Mesa R. Online yoga in myeloproliferative neoplasm patients: results of a randomized pilot trial to inform future research. BMC Complement Altern Med. 2019;19(1):121.
doi pubmed - Padrnos L, Scherber R, Geyer H, Langlais BT, Dueck AC, Kosiorek HE, Senyak Z, et al. Depressive symptoms and myeloproliferative neoplasms: Understanding the confounding factor in a complex condition. Cancer Med. 2020;9(22):8301-8309.
doi pubmed - Gowin K, Langlais BT, Kosiorek HE, Dueck A, Millstine D, Huberty J, Eckert R, et al. The SIMM study: Survey of integrative medicine in myeloproliferative neoplasms. Cancer Med. 2020;9(24):9445-9453.
doi pubmed - Christensen SF, Scherber RM, Mazza GL, Dueck AC, Brochmann N, Andersen CL, Hasselbalch HC, et al. Tobacco use in the Myeloproliferative neoplasms: symptom burden, patient opinions, and care. BMC Cancer. 2021;21(1):691.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


