| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Case Report
Volume 10, Number 5, October 2021, pages 228-231
Non-Invasive Prenatal Testing Leading to Detection of Asymptomatic Acute Myeloid Leukemia in a 30-Year-Old Patient: A Case Report
Sterre P.E. Willemsa, Marianne Stenstrab, Mojca Jongen-Lavrencicc, Michelle E.M.H. Westerhuisd, H. Berna Beverlooe, Gerard Vreugdenhilf, g
aDepartment of Internal Medicine, Radboudumc, Nijmegen, The Netherlands
bVelthuis Kliniek, Eindhoven/Rotterdam, The Netherlands
cDepartment of Haematology, Erasmus Medisch Centrum, Rotterdam, The Netherlands
dDepartment of Gynaecology, Catharina Hospital, Eindhoven, The Netherlands
eDepartment of Clinical Genetics, Erasmus Medisch Centrum, Rotterdam, The Netherlands
fDepartment of Internal Medicine, Maxima Medisch Centrum, Eindhoven/Veldhoven, The Netherlands
gCorresponding Author: Gerard Vreugdenhil, Department of Internal Medicine, Maxima Medisch Centrum, Eindhoven/Veldhoven, The Netherlands
Manuscript submitted September 18, 2021, accepted October 20, 2021, published online October 31, 2021
Short title: NIPT Leads to Detction of Asymptomatic AML
doi: https://doi.org/10.14740/jh908
| Abstract | ▴Top |
The widely use of non-invasive prenatal testing (NIPT) may lead to accidental findings and the discovery of malignancy in pregnancy, often in asymptomatic patients. Diagnosis of such subclinical malignancy during pregnancy in the asymptomatic patient poses a predicament for both doctor and patient. The risks and benefits of possible treatment for both mother and child have to be weighted, and there is often limited scientific evidence available. We present a case of an abnormal NIPT result, leading to the diagnosis of acute myeloid leukemia (AML) in an asymptomatic pregnant patient. After multiple multidisciplinary meetings and an elaborate shared decision making (SDM) process, a watch and wait strategy was implemented, in contradiction with general treatment recommendations. Following this approach, it was possible to achieve a near term pregnancy before delivery of a healthy baby girl. The patient could subsequently commence treatment of her AML and is still in complete remission after a follow-up of 25 months. Our case report highlights the possibility of watch and wait strategy in selected cases and the importance of multidisciplinary collaboration and SDM, when faced with the accidental finding of AML through NIPT.
Keywords: Acute myeloid leukemia; Non-invasive prenatal testing; Pregnancy; Shared decision making; Watch and wait strategy
| Introduction | ▴Top |
Non-invasive prenatal testing (NIPT) is based on the measurement of circulating cell-free DNA released by apoptotic cells in the human body. In pregnant women, the cell-free fraction is predominantly (roughly 90%) maternal and for a lesser fraction originating from the placenta and therefore fetal [1]. It can be used for detection of trisomy 13, 18 and 21 only or it can be used as a screening test for anomalies other than these trisomies, resulting in additional findings (other trisomies, structural chromosomal aberrations and abnormal profiles indicative of maternal malignancies). Within a developing fetus, some chromosomal aberrations are not compatible with life. Complex patterns of multiple (sub)chromosomal aberrations are therefore indicative of maternal malignancy. In the Netherlands, pregnant women who are at risk for carrying a child with said trisomies, qualify for NIPT as an alternative for invasive prenatal testing. Since 2017 every pregnant woman can opt for NIPT in exchange for a small fee. Results are monitored in a nationwide study. The widely usage of NIPT leads to accidental findings and is indicative of maternal malignancy in 0.02%, most likely in asymptomatic patients [2]. Suspicion of malignancy during pregnancy leads to various diagnostic investigations, some more invasive than others, posing a high psychological burden on the mother and her environment. Whether the early detection of subclinical malignancy leads to improved outcomes in these patients remains to be seen [3].
In general, patients present with symptoms at the time they are diagnosed with acute myeloid leukemia (AML). The moment of disease onset is difficult to determine. It is reasonable to suspect that patients have had bone marrow involvement for weeks, maybe even months, prior to initial presentation. Since patients principally present in symptomatic phase of AML, there is little known regarding the natural course of AML in this “silent” phase. There are no case reports concerning the diagnosis of asymptomatic acute leukemia in pregnancy as a consequence of abnormal NIPT results.
| Case Report | ▴Top |
An asymptomatic 30-year-old pregnant woman with no relevant medical history decided to perform an NIPT at 12 weeks of gestation. She appeared to be in good health and experienced no restraint while cycling endurance tours of 150 km or longer. NIPT screening revealed an abnormal NIPT result consistent with a small deletion in chromosome band 3q26 and the presence of trisomy 8. Blood tests showed slight anemia (hemoglobin 11.8 g/dL or g%) and leukopenia (white blood cell count 2.8 × 109/L) with blasts (3.5%) in a peripheral blood smear. Chorion villi sampling did not demonstrate these abnormalities within the fetus. Additional karyotyping and fluorescent in situ hybridization (FISH) of the maternal blood showed an abnormal karyotype with an inversion of chromosome 16 (inv(16)(p13q22)) and an additional copy of chromosome 8. In addition, single nucleotide polymorphism (SNP) array investigation showed a subclonal deletion in chromosome region 3q26.31q26.32 (Fig. 1). However, the MECOM (EVI1) gene was not located in this region and FISH did not show a MECOM (EVI1) rearrangement. The bone marrow aspirate demonstrated a diagnosis of AML with 40% blasts with WHO 2016 classification, AML with inv(16)(p13q22), gene fusion of CBFB and MYH11. This AML is classified as favorable within the European LeukemiaNet (ELN) risk stratification and is associated with a high rate of complete remission (CR) and a favorable overall survival [4].
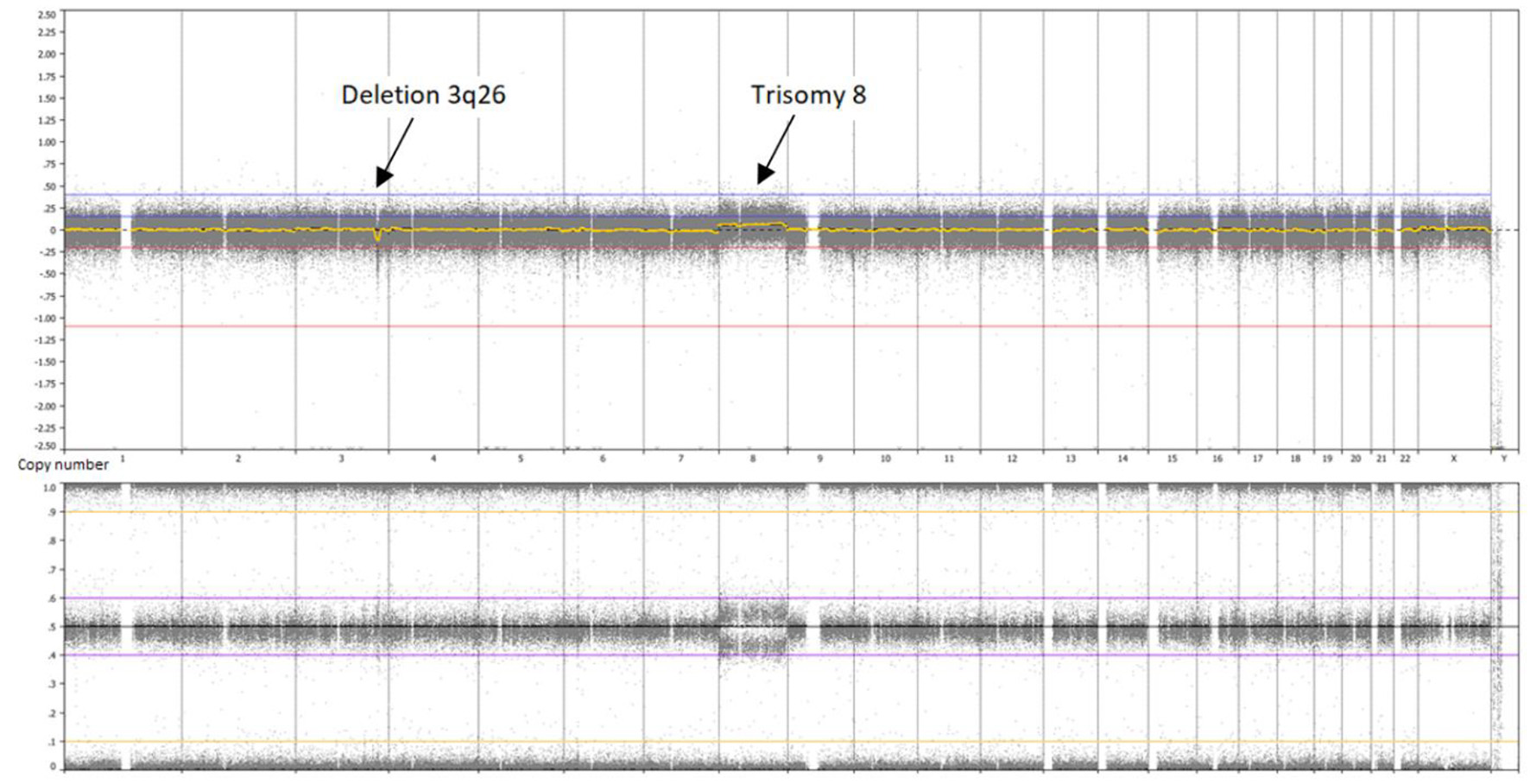 Click for large image | Figure 1. Whole genome overview, single nucleotide polymorphism (SNP) array of the patient’s bone marrow. A deletion 3q26 is visible in approximately 15% of cells and a trisomy 8 in approximately 40% of cells. |
Initial advice from her treating hemato-oncologist was instantaneous start of intensive chemotherapy treatment, at the expense of fetal preservation. Her case was submitted to a national advisory council (Adviesgroep Kanker in de Zwangerschap (AKZ)), specialized in cancer during pregnancy. The AKZ recommended the immediate initiation of chemotherapy during pregnancy and advised that the pregnancy would be terminated at 28 weeks to allow a chance for the fetus to survive. The patient, a cosmetic doctor herself, wished to maintain pregnancy. At that time, she was at 18 weeks of gestation without experiencing any symptoms and stable blood counts. During a multidisciplinary meeting between her treating hemato-oncologist, obstetrician and neonatologist, abovementioned options were discussed and a third option was added: to maintain pregnancy and postpone treatment as long as patient’s symptoms and blood count remained stable. The patient was present during these meetings. A careful watch and wait strategy was approved and implemented, consisting of weekly blood analysis and consultation with her hematologist and obstetrician. Fetal growth was monitored every 2 weeks.
During follow-up, the patient remained asymptomatic, despite slight fatigue and could continue her cycling endurance tours. Blood count was stable with minimal increase of blast percentage, ranging from 5% to 13% blasts. At 32 weeks of gestation, a delivery date was scheduled considering possible growth restriction of the fetus seen on prenatal ultrasound and increasing blast percentage (19-24%) with a stable leukocyte count of 3.1 × 109/L. The patient was admitted to the hospital at 32 + 5 weeks and labor was induced after completing fetal lung ripening by one course of maternal corticosteroids. However, due to acute dyspnea caused by rhinovirus-induced pneumonia, an emergency cesarean section was performed at 33 + 1 weeks. A healthy baby girl was born with a normal birth weight of 2,010 g.
The patient was temporarily admitted to the intensive care unit (ICU) for high flow oxygen therapy and discharged a few days later. Re-admission for induction chemotherapy was planned within a week. A repeated bone marrow aspirate now revealed 80% blasts and karyotyping showed trisomy of chromosome 8 and a fusion transcript of CBFB/MYH11. Two cycles of induction chemotherapy therapy (3 + 7) were scheduled according to Dutch guidelines (www.hovon.nl) and resulted in CR at bone marrow examination. Consolidation with autologous hematopoietic stem cell transplantation (HSCT) could not be accomplished, as a consequence of failure in harvesting autologous stem cells. Therefore, the patient received one cycle of consolidation chemotherapy with mitoxantrone and etoposide, which is the standard treatment according to Dutch HOVON guidelines for patients with good risk AML. International guidelines differ on the recommended treatment for patients with low or intermediate risk AML in which a planned autologous HSCT can not be accomplished. For example, consolidation with high dose cytarabine is considered standard care for inv(16) AML in the USA. Due to amenorrhea and a low estradiol suggestive of menopause accompanying post-menopausal complaints, she started with hormonal suppletion therapy (HST). Estradiol levels recovered spontaneously after 10 months, therefore HST was discontinued. At the moment of writing this paper, after a follow-up of 25 months, patient is still alive and in good health, together with a healthy daughter.
| Discussion | ▴Top |
AML is considered a hematological emergency and therefore the general recommendation is immediate initiation of therapy to minimize disease-related mortality and morbidity. Untreated AML has a poor prognosis and retrospective analyses demonstrated that delayed treatment is associated with worse CR rates and overall survival in young patients [5]. However, other studies regarding the effect of time from diagnosis to treatment (TDT) are contradictory. A single-center retrospective analysis found no significant association of TDT with response rate or overall survival after adjustments for other risk factors [6]. A more recent study suggested no relation between TDT and survival or overall response in both older and younger individuals [7]. All of the aforementioned studies concern delayed treatment with a median of 4, 8 and 3 days. Hence, the risks of delayed treatment for more than 8 days are unclear.
When a diagnosis of AML is made in the first trimester of pregnancy, elective termination of pregnancy is considered as the safest option. A successful outcome of pregnancy is unlikely in this situation and spontaneous miscarriage poses a high risk for the mother [8]. In addition, exposure to chemotherapy in the first trimester leads to a 10-20% chance of major malformations [9]. Chemotherapy can be safely administered during second and third trimester, although no data regarding long-term effects on offspring are available. After a gestational period of 24 weeks, the risks of fetal exposure to chemotherapy should be weighed against the risks of extreme premature delivery. Elective delivery should be planned as soon as fetal maturity allows, although it has to be timed carefully and avoided during the maternal nadir period. It seems justifiable to deliver the fetus when the presentation is beyond 32 weeks of gestation [8]. Standard AML induction chemotherapy consists of combination of cytarabine and daunorubicin. Treatment with daunorubicin is preferred over idarubicin, which is more lipophilic and therefore has a higher transplacental transfer. Dose-adjustment for weight gain during pregnancy should be made, because pregnancy-related physiological changes can lead to decreased blood drug levels [10]. Transplacental transmission of AML in the fetus is extremely rare due to the functioning of the placental barrier and the fetal immune system. However, vertical transmission of maternal acute lymphocytic leukemia cells has been described, with spontaneous elimination of leukemic cells in the neonate after 6 weeks [11]. Current guidelines advise no delay in AML treatment, as this carries a significant risk to the health of both mother and child [8, 12]. However, these guidelines are based on newly diagnosed AML patients with symptomatic disease and therefore not directly applicable to our asymptomatic patient, resulting in the necessity of a personalized approach. Although final outcome in our patient was favorable, the choice of prolonged treatment delay should be weighted very carefully against the risk of severe infection, fetal growth restriction and even death. A watch and wait strategy can be implemented with extreme precaution.
Shared decision making (SDM)
Diagnosis of AML in pregnancy poses a predicament for both doctor and patient, and achievement of a favorable balance in risk and benefit ratio for both baby and mother can be a challenge. In this particular case, this was even a greater dilemma, because of the scarcity of scientific evidence of accidental discovery of AML through NIPT. Under conditions of uncertainty, SDM is important. Patients who participate in SDM may have greater satisfaction and less decisional conflict [13]. Given the major psychological impact of the disease, especially during pregnancy, this can be of added value. Before SDM can take place, three essential elements must be present. First, the acknowledgement of both patient and health care provider that a decision is required. Secondly, knowledge and understanding of the best available evidence, risks and benefits is needed. Specific advisory councils should be consulted. Lastly, patients’ preferences, values and circumstances have to be taken into consideration, as well as the guidance the clinician provides [14]. During the decision making process, it is valuable to consider the limits of SDM. Impaired arithmetic skills in patients can form an obstacle in the understanding of available evidence. Clinicians can also lack numeracy skill and therefore may fail to communicate data in a comprehensible manner to their patients. Furthermore, a human being, whether he/she is a doctor or a patient, is not always capable of rational decision making. Choices are, more often than not, based on various irrational biases. A person may overestimate small probabilities and the idea of a potential loss could be of greater influence in decision making than the gain of something of equal value [15]. Multidisciplinary collaboration is indispensable for correct assessment of the risks and provision of information to the patient. In this case, the background of the patient as a medical professional ensured she could partake in the multidisciplinary meetings regarding her treatment plan. We believe that her attendance had a positive impact on the decision making progress.
Conclusion
Here we report a case where we were confronted with the potential diagnosis of AML through NIPT in an asymptomatic pregnant patient. An individualized treatment plan was conducted through multidisciplinary collaboration, with due consideration of patient’s wishes and the help of SDM. A watch and wait strategy in the asymptomatic favorable risk AML patient and a (near) normal and stable blood count during the waiting period seems feasible. Further experience in the accidental discovery and treatment of AML through NIPT is needed.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Informed consent was obtained from the patient.
Author Contributions
All authors contributed to drafting and editing the manuscript. SW prepared the manuscript. BB provided the figure. All authors approve the final version of the manuscript.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
NIPT: non-invasive prenatal testing; AML: acute myeloid leukemia; SDM: shared decision making; ELN: European LeukemiaNet; SNP: single nucleotide polymorphism; HST: hormonal suppletion therapy; AKZ: Adviesgroep Kanker in de Zwangerschap (National Advisory Council for Cancer During Pregnancy); HSCT: hematopoietic stem cell transplantation; CR: complete remission; TDT: time from diagnosis to treatment
| References | ▴Top |
- Janssens K, Deiteren K, Verlinden A, Rooms L, Beckers S, Holmgren P, Vermeulen K, et al. Detection of a case of chronic myeloid leukaemia with deletions at the t(9;22) translocation breakpoints by a genome-wide non-invasive prenatal test. Prenat Diagn. 2016;36(8):760-765.
doi pubmed - van der Meij KRM, Sistermans EA, Macville MVE, Stevens SJC, Bax CJ, Bekker MN, Bilardo CM, et al. TRIDENT-2: national implementation of genome-wide non-invasive prenatal testing as a first-tier screening test in the Netherlands. Am J Hum Genet. 2019;105(6):1091-1101.
doi pubmed - Prasad V. Non-invasive, serum DNA pregnancy testing leading to incidental discovery of cancer: a good thing? Eur J Cancer. 2015;51(16):2272-2274.
doi pubmed - Grimwade D, Hills RK, Moorman AV, Walker H, Chatters S, Goldstone AH, Wheatley K, et al. Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood. 2010;116(3):354-365.
doi pubmed - Sekeres MA, Elson P, Kalaycio ME, Advani AS, Copelan EA, Faderl S, Kantarjian HM, et al. Time from diagnosis to treatment initiation predicts survival in younger, but not older, acute myeloid leukemia patients. Blood. 2009;113(1):28-36.
doi pubmed - Bertoli S, Berard E, Huguet F, Huynh A, Tavitian S, Vergez F, Dobbelstein S, et al. Time from diagnosis to intensive chemotherapy initiation does not adversely impact the outcome of patients with acute myeloid leukemia. Blood. 2013;121(14):2618-2626.
doi pubmed - Rollig C, Kramer M, Schliemann C, Mikesch JH, Steffen B, Kramer A, Noppeney R, et al. Does time from diagnosis to treatment affect the prognosis of patients with newly diagnosed acute myeloid leukemia? Blood. 2020;136(7):823-830.
doi pubmed - Ali S, Jones GL, Culligan DJ, Marsden PJ, Russell N, Embleton ND, Craddock C, et al. Guidelines for the diagnosis and management of acute myeloid leukaemia in pregnancy. Br J Haematol. 2015;170(4):487-495.
doi pubmed - Weisz B, Meirow D, Schiff E, Lishner M. Impact and treatment of cancer during pregnancy. Expert Rev Anticancer Ther. 2004;4(5):889-902.
doi pubmed - Horowitz NA, Henig I, Henig O, Benyamini N, Vidal L, Avivi I. Acute myeloid leukemia during pregnancy: a systematic review and meta-analysis. Leuk Lymphoma. 2018;59(3):610-616.
doi pubmed - van der Velden VH, Willemse MJ, Mulder MF, Szczepanski T, Langerak AW, Wijkhuijs JM, van Dongen JJ. Clearance of maternal leukaemic cells in a neonate. Br J Haematol. 2001;114(1):104-106.
doi pubmed - Greenlund LJ, Letendre L, Tefferi A. Acute leukemia during pregnancy: a single institutional experience with 17 cases. Leuk Lymphoma. 2001;41(5-6):571-577.
doi pubmed - Shay LA, Lafata JE. Where is the evidence? A systematic review of shared decision making and patient outcomes. Med Decis Making. 2015;35(1):114-131.
doi pubmed - Legare F, Witteman HO. Shared decision making: examining key elements and barriers to adoption into routine clinical practice. Health Aff (Millwood). 2013;32(2):276-284.
doi pubmed - Avorn J. The psychology of clinical decision making - implications for medication use. N Engl J Med. 2018;378(8):689-691.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


