| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Case Report
Volume 10, Number 5, October 2021, pages 212-216
Progressive Multifocal Leukoencephalopathy After Chimeric Antigen Receptor T-Cell Therapy for Recurrent Non-Hodgkin Lymphoma
Jared T. Ahrendsena , Kartik Sehgalb, Sasmit Sarangic, Erik J. Uhlmannc, Hemant Varmaa, Jon Arnasonb, David Aviganb, d
aDepartment of Pathology, Beth Israel Deaconess Medical Center, Boston, MA 02215, USA
bDivision of Hematology and Medical Oncology, Department of Medicine, Beth Israel Deaconess Medical Center, Boston, MA 02215, USA
cDepartment of Neurology, Beth Israel Deaconess Medical Center, Boston, MA 02215, USA
dCorresponding Author: David Avigan, Department of Medicine, Harvard Medical School, 330 Brookline Avenue, Boston, MA 02215, USA
Manuscript submitted July 26, 2021, accepted September 1, 2021, published online October 5, 2021
Short title: PML After CAR T-Cell Therapy
doi: https://doi.org/10.14740/jh903
| Abstract | ▴Top |
Chimeric antigen receptor (CAR) T-cell therapy targeting cluster of differentiation (CD)19 has had a transformative impact on patient outcomes in a subset of patients with relapsed/refractory non-Hodgkin lymphoma. We present a patient with refractory large B-cell lymphoma in complete remission for 2 years following treatment with CD19-targeted CAR T-cell therapy, who presented with 2 weeks of progressive aphasia. Imaging revealed a left occipital brain lesion and biopsy demonstrated features diagnostic of progressive multifocal leukoencephalopathy. Further evaluation revealed severe hypogammaglobulinemia and a low CD4 count. She was treated with pembrolizumab and intravenous immunoglobulin resulting in decreased cerebrospinal fluid viral load without clinical improvement and died 8 weeks after presentation. This case highlights that there is potential for severe opportunistic infections after CAR T-cell therapy, including fatal progressive multifocal leukoencephalopathy. Strategies to enhance post-treatment immune reconstitution are essential to further harness the unique potency of CAR T-cell therapy.
Keywords: Chimeric antigen receptor; CAR T-cell therapy; Progressive multifocal leukoencephalopathy; Opportunistic infection
| Introduction | ▴Top |
Progressive multifocal leukoencephalopathy (PML) is a devastating opportunistic infection caused by reactivation of John Cunningham virus (JC virus, also known as human polyomavirus 2) within the central nervous system that is characteristically observed in settings of profound immunosuppression [1]. There is increasing evidence that iatrogenic immunosuppression in the setting of immunomodulatory therapies may have an impact on the rising incidence of PML [2]. Specifically, chimeric antigen receptor (CAR) T-cell therapy targeting cluster of differentiation (CD)19 induces durable complete remissions in a subset of patients with relapsed/refractory B-cell lymphomas, dramatically impacting outcomes in this patient population. Unfortunately, the administration of CAR T-cells following lymphodepleting chemotherapy is associated with significant toxicity [3]. The impact of therapy on long-term immune dysregulation has not been well delineated although there is increasing awareness of the incidence of opportunistic infection [4, 5].
We present a patient treated with CAR T-cells for refractory non-Hodgkin lymphoma who was diagnosed with PML 2 years after infusion. This case highlights the potential for severe opportunistic infections in this population, possibly related to persistent lymphopenia, hypogammaglobulinemia, and immune dysregulation after CAR T-cell infusion.
| Case Report | ▴Top |
Investigations
A 68-year-old woman with history of recurrent/refractory non-Hodgkin lymphoma in complete remission since CAR T-cell therapy 2 years prior presented with 2 weeks of progressive word finding difficulty. Her oncologic history dated back to 2007 (56-years-old at that time) when a mass was discovered in her left breast. Biopsy revealed involvement by a B-cell non-Hodgkin lymphoma with follicular derivation. Her treatment course after diagnosis was notable for multiply recurrent disease following standard induction chemoimmunotherapy, high-dose chemotherapy with autologous stem cell rescue, several subsequent salvage chemotherapies, and targeted therapies (Fig. 1). Despite these efforts, transformation into diffuse large B-cell lymphoma (DLBCL) occurred in August 2016. Her treatment course was also notable for persistent lymphopenia and hypogammaglobulinemia (Fig. 1). In April 2017, she underwent lymphodepletion with fludarabine and cyclophosphamide followed by CD19-targeted CAR T-cell therapy (lisocabtagene maraleucel) as part of a clinical trial (see reference [6] for additional details), and was in complete remission since May 2017. Following CAR T-cell infusion, her clinical course was uneventful and only notable for persistent hypogammaglobulinemia. Notably, a bone marrow biopsy done prior to CAR T-cell administration was free of any significant pathology. Similarly, her hemoglobin and platelet levels normalized after CAR T-cell administration, with only residual lymphopenia and continued hypogammaglobulinemia (Fig. 1).
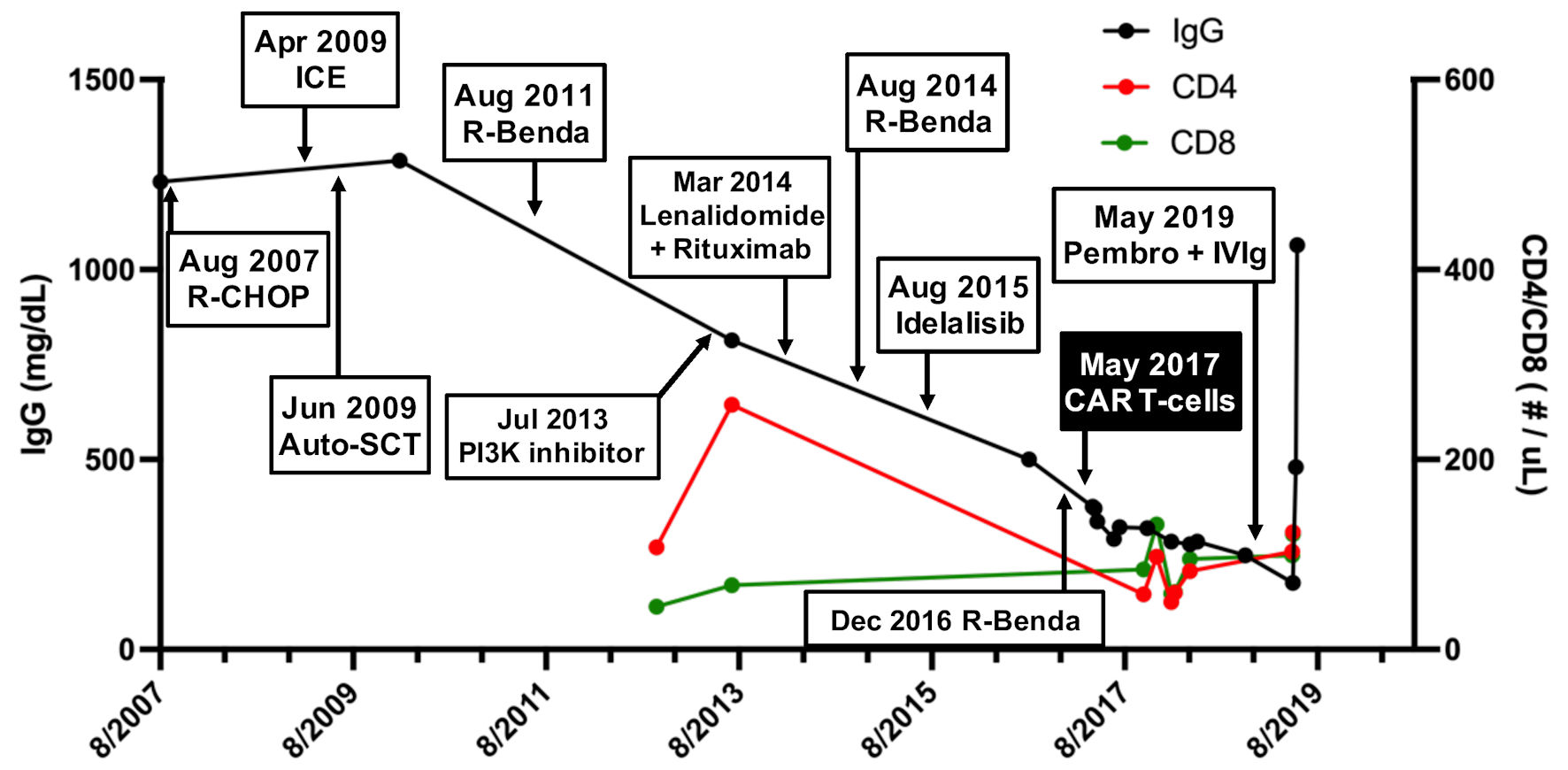 Click for large image | Figure 1. Serum immunoglobulin (IgG) levels and CD4 T-cell counts in response to various lymphoma treatments. A steady decline in serum IgG levels (black) and absolute CD4 counts (red), reaching a nadir after infusion of CD19-targeted CAR T-cells in May 2017. Absolute CD8 counts (green) were relatively preserved. R-CHOP: rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone; ICE: ifosfamide, carboplatin, etoposide; Auto-SCT: autologous stem cell transplant; R-benda: rituximab, bendamustine; CAR: chimeric antigen receptor; Pembro + IVIG: pembrolizumab, intravenous immunoglobulin; CD: cluster of differentiation. |
In April 2018, approximately 1 year after CAR T-cell therapy, she noticed symptoms of simple visual hallucinations. Neurological exam revealed right inferior quadrantanopia and brain magnetic resonance imaging (MRI) demonstrated signal abnormality on fluid-attenuated inversion recovery (FLAIR) images in the left occipital pole (Fig. 2b), which was not present at the time of CAR T-cell infusion (Fig. 2a). Follow-up imaging in October 2018 again revealed FLAIR signal abnormality in the left occipital lobe, with extension into adjacent areas compared to prior scans (not shown). This was thought to represent evolving sequelae of infarction.
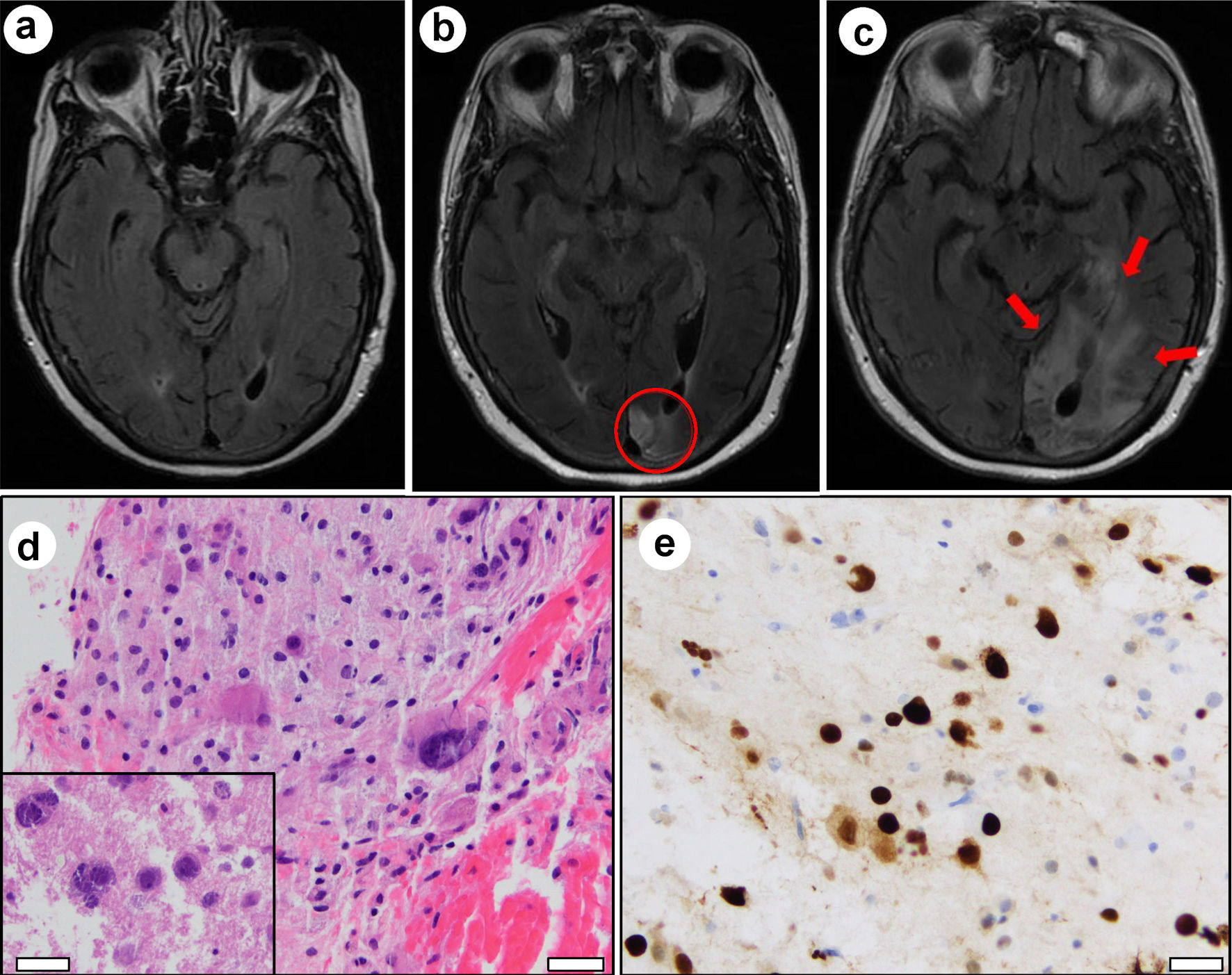 Click for large image | Figure 2. Magnetic resonance imaging evolution and brain biopsy. (a) FLAIR signal abnormality was absent in May 2017 at the time of CAR T-cell infusion and prior to onset of neurological symptoms. (b) FLAIR signal abnormality in the left occipital lobe at the time of initial presentation for visual disturbance in April 2018 (red circle). (c) Expansion of FLAIR signal (red arrows) involving the left occipital lobe and left posterior temporal lobe in April 2019 after presentation with progressive word finding difficulty. Hematoxylin and eosin (H&E) stained sections from left occipital brain biopsy show (d) bizarre-appearing astrocytes and violaceous oligodendroglial nuclear inclusions (inset). Immunohistochemistry demonstrates strong nuclear positivity for SV-40 (e). White scale bars = 100 µm. |
The patient was enrolled and cared for on a clinical trial approved by the Dana Farber/Harvard Cancer Center (DF/HCC) Institutional Review Board (IRB). As per IRB policies, all study treatments and procedures were performed after informed consent was obtained.
Diagnosis
In April 2019, 2 years after CAR T-cell therapy, she presented with 2 weeks of progressive word finding difficulty. Physical exam revealed alexia without agraphia, non-fluent aphasia, and right homonymous hemianopia. Brain MRI revealed further expansion of FLAIR hyperintense signal in the left occipital lobe and an area of new enhancement (Fig. 2c). Due to worsening symptoms and evolving imaging findings, a malignant process was suspected. Stereotactic brain biopsy was performed to obtain a tissue diagnosis.
Hematoxylin and eosin (H&E) stained sections from biopsy tissue revealed hypercellular gray matter with bizarre astrocytes (Fig. 2d). Enlarged violaceous nuclei were also observed, suggestive of viral inclusions (Fig. 2d, inset). SV-40 immunostain was strongly positive in large, atypical lesional cells, confirming infection by JC virus (Fig. 2e). Additional immunohistochemical and cytogenetic analyses confirmed the absence of a neoplastic process. Cerebrospinal fluid analysis subsequently tested positive for JC virus DNA by polymerase chain reaction (PCR, > 500,000 viral copies/mL) and serum was positive for anti-JC virus antibodies by enzyme-linked immunosorbent assay (ELISA). Together, these results were diagnostic of PML.
Treatment
During her hospitalization, she was initially treated with dexamethasone, which was discontinued following the diagnosis of PML. Her clinical symptoms gradually worsened with increasing word finding difficulty and worsening mental status. She was subsequently administered three doses of intravenous immunoglobulins (IVIGs), and mirtazapine was administered in an attempt to inhibit viral entry into cells [7, 8]. Pembrolizumab was initiated in light of evidence demonstrating efficacy of immune checkpoint inhibitors for PML [9].
Follow-up and outcomes
Despite these efforts, she became increasingly somnolent and passed away after 8 weeks of hospitalization.
| Discussion | ▴Top |
CAR T-cells targeting CD19 have demonstrated striking efficacy in B-cell malignancies including acute lymphocytic leukemia and non-Hodgkin lymphoma [3]. A majority of patients with relapsed/refractory disease achieve complete remission and a subset of patients may achieve durable response. CAR T-cell therapy is associated with significant toxicity in the early post-infusion period including cytopenias, infections resulting from lymphodepleting chemotherapy, and cytokine release syndrome (CRS) [10]. Neurologic toxicities following CAR T-cell infusion may follow CRS and are characterized by symptoms ranging in severity, including aphasia, confusion, altered mental status, and seizure [3].
PML has been described in settings of severe immune compromise resulting in JC viral reactivation and expansion with devastating consequences. It has been previously described in patients with advanced human immunodeficiency virus (HIV) disease with profound CD4 lymphopenia, following allogeneic transplantation in the setting of chronic immune suppression, and in rheumatologic diseases requiring intensive chronic immune suppression [1]. More recently, PML has also been reported in patients with hematologic malignancies on chronic B-cell depleting therapy [2].
Immune dysregulation following CAR T-cell therapy is multifactorial and may result in the emergence of opportunistic pathogens. Patients with lymphoid malignancies undergoing CAR T-cell therapy often have received multiple prior chemotherapy regimens in conjunction with antibody-mediated B-cell depletion, and as a consequence may be immunosuppressed prior to CAR T-cell therapy. Additionally, prolonged B-cell aplasia due to CAR T-cell-mediated depletion of the normal B-cell repertoire with consequent hypogammaglobulinemia is increasingly recognized as a serious complication of CAR T-cell therapy, and may result in an increased risk of opportunistic infection [11]. Hypogammaglobulinemia has been described as soon as 9 weeks and as late as 4 years after infusion [11]. One prospective study reported bacterial, viral, and fungal infections following CD19-targeted CAR T-cell therapy up to 90 days after infusion in 30 of 133 treated patients [4]. Similarly, a retrospective study found that late infections (> 90 days after infusion) were caused by bacteria, respiratory viruses, and (rarely) fungi. Interestingly, 61% of patients in this study had at least one late infection [5].
The impact of CAR T-cell therapy on cell-based immunity may also play a pivotal role for opportunistic infections. For example, lymphodepleting chemotherapy with fludarabine may be associated with prolonged cytopenias via targeting of hematopoietic stem cells as well as functional and quantitative suppression of helper and cytotoxic T-cell populations. In addition, immune dysregulation and cytopenias may be associated with CAR T-cell-induced inflammatory states such as hemophagocytic or macrophage activating syndromes. Finally, hyperstimulation of the CAR T-cell population in the setting of constitutive expression of costimulatory molecules may ultimately result in compensatory activation of tolerance pathways that suppress T-cell immunity.
Our case highlights a devastating late consequence potentially related to prolonged and profound hypogammaglobulinemia, raising an important question on how to optimally monitor patients for long-term effects of CAR T-cell therapy. Similar to other recent reports (Table 1 [12, 13]), CAR T-cells are unlikely the sole contributor to PML development in this patient. Indeed, the patient described here had several other PML risk factors, including history of hematological malignancy, prior rituximab treatment, chronic lymphopenia/hypogammaglobulinemia, and possibly immunosenescence [1] . However, her lymphoma had been in complete remission for 2 years following CAR T-cell infusions. Furthermore, first dose of rituximab was 10 years (and her last dose 2 years) prior to PML diagnosis (Fig. 1). Supporting this notion, one study found that the median time to PML diagnosis following first rituximab dose was 16 months, with almost two-thirds of cases occurring within 2 years of first exposure to rituximab [2]. Thus, it is likely that CAR T-cell therapy significantly contributed to the development of PML in this patient.
 Click to view | Table 1. Review of Prior Reports of Progressive Multifocal Leukoencephalopathy (PML) After CAR T-Cell Therapy |
Learning points
This case supports the accumulating literature highlighting the importance of vigilance for PML in patients with late neurologic findings, and emphasizes the critical importance of establishing PML as a differential diagnosis by clinicians caring for patients treated with CAR T-cells. Furthermore, there is a critical need for the further interrogation of the late immunologic implications of this highly promising therapy informing surveillance of opportunistic infection and associated complications. Strategies to enhance post-treatment immune reconstitution are essential as we seek to further harness the unique potency of CAR T-cell therapy.
Acknowledgments
None to declare.
Financial Disclosure
There are no financial disclosures or funding sources related to this manuscript.
Conflict of Interest
The authors have no conflict of interest to disclose.
Informed Consent
The patient was enrolled and cared for on a clinical trial approved by the Dana Farber/Harvard Cancer Center Institutional Review Board (IRB). As per IRB policies, all study treatments and procedures were performed after informed consent was obtained.
Author Contributions
JTA, JA, and DA performed the research, analyzed the data, and wrote the manuscript. SS and KS analyzed the data and wrote the manuscript. EJU and HV analyzed the data.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
Auto-SCT: autologous stem cell transplant; CAR: chimeric antigen receptor; CRS: cytokine release syndrome; PML: progressive multifocal leukoencephalopathy; DLBCL: diffuse large B-cell lymphoma; FLAIR: fluid-attenuated recovery inversion; H&E: hematoxylin and eosin; ICE: ifosfamide, carboplatin, etoposide; JC virus: John Cunningham virus; IRB: Institutional Review Board; MRI: magnetic resonance imaging; Pembro + IVIG: pembrolizumab, intravenous immunoglobulin; R-CHOP: rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone; R-benda: rituximab, bendamustine
| References | ▴Top |
- Tan CS, Koralnik IJ. Progressive multifocal leukoencephalopathy and other disorders caused by JC virus: clinical features and pathogenesis. Lancet Neurol. 2010;9(4):425-437.
doi - Focosi D, Tuccori M, Maggi F. Progressive multifocal leukoencephalopathy and anti-CD20 monoclonal antibodies: What do we know after 20 years of rituximab. Rev Med Virol. 2019;29(6):e2077.
doi pubmed - June CH, O'Connor RS, Kawalekar OU, Ghassemi S, Milone MC. CAR T cell immunotherapy for human cancer. Science. 2018;359(6382):1361-1365.
doi pubmed - Hill JA, Li D, Hay KA, Green ML, Cherian S, Chen X, Riddell SR, et al. Infectious complications of CD19-targeted chimeric antigen receptor-modified T-cell immunotherapy. Blood. 2018;131(1):121-130.
doi pubmed - Cordeiro A, Bezerra ED, Hirayama AV, Hill JA, Wu QV, Voutsinas J, Sorror ML, et al. Late events after treatment with CD19-targeted chimeric antigen receptor modified T cells. Biol Blood Marrow Transplant. 2020;26(1):26-33.
doi pubmed - Abramson JS, Palomba ML, Gordon LI, Lunning MA, Wang M, Arnason J, Mehta A, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396(10254):839-852.
doi - Trentalange A, Calcagno A, Ghisetti V, Atzori C, Busolli P, Bonora S, Imperiale D. Clearance of cerebrospinal fluid JCV DNA with mirtazapine in a patient with progressive multifocal leukoencephalopathy and sarcoidosis. Antivir Ther. 2016;21(7):633-635.
doi pubmed - Mullins C, Miranda J, Sandoval H, Ramos-Duran L, Tonarelli SB. The benefit of mirtazapine in the treatment of progressive multifocal leukoencephalopathy in a young HIV-positive patient: A Case Report. Innov Clin Neurosci. 2018;15(1-2):33-35.
- Cortese I, Muranski P, Enose-Akahata Y, Ha SK, Smith B, Monaco M, Ryschkewitsch C, et al. Pembrolizumab treatment for progressive multifocal leukoencephalopathy. N Engl J Med. 2019;380(17):1597-1605.
doi pubmed - Brudno JN, Kochenderfer JN. Toxicities of chimeric antigen receptor T cells: recognition and management. Blood. 2016;127(26):3321-3330.
doi pubmed - Doan A, Pulsipher MA. Hypogammaglobulinemia due to CAR T-cell therapy. Pediatr Blood Cancer. 2018;65:e26914.
doi pubmed - Sdrimas K, Diaz-Paez M, Camargo JF, Lekakis LJ. Progressive multifocal leukoencephalopathy after CAR T therapy. Int J Hematol. 2020;112(1):118-121.
doi pubmed - Mian A, Andrapalliyal N, Weathers AL, Pohlman B, Hill BT. Late occurrence of progressive multifocal leukoencephalopathy after anti-CD19 chimeric antigen receptor T-cell therapy. Eur J Haematol. 2021;106(4):584-588.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


