| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Original Article
Volume 10, Number 2, April 2021, pages 53-63
Acute Promyelocytic Leukemia: A Long-Term Retrospective Study in Mexico
Nidia Zapata-Cantoa, v, Manuel Aguilara, Luara Aranab, Efren Montanob, Cristian Ramos-Penafielc, Jose Antonio De la Penab, Jose Luis Alvarez-Verab, Eugenia Espitia-Riosb, Juan Manuel Perez Zunigab, Eleazar Hernandez-Ruizb, Eduardo Cerveraa, Ramiro Espinoza-Zamoraa, Alejandro Sosa-Espinozaa, Juan Carlos Solis-Poblanod, Roberta Demichelise, David Gomez-Almaguerf, Esperanza Barrerag, Javier Mijangosh, Ruben Solis-Armentai, Oscar de Jesus Perezi, Miguel Herreraj, Guillermo Diaz-Vargask, Alvaro Cabrera-Garcial, Juan Antonio Flores-Jimenezm, Javier Morales-Adriann, Eva Fabiola Ramirez-Romeroo, Adrian Ceballos-Lopezp, Victor Antonio Guillermoq, Manuel Solano Manuelr, Esthela Juan Lien-Chang Lourdess, Juan Ojeda-Tovart, Gladys Gomez-Perdomou, Martha Alvarado-Ibarrab
aInstituto Nacional de Cancerologia, Ciudad de Mexico, Mexico
bHospital 20 de Noviembre ISSSTE, Ciudad de Mexico, Mexico
cHospital General de Mexico, Ciudad de Mexico, Mexico
dUnidad Medica Onco-Hematologica Puebla, Puebla, Mexico
eInstituto Nacional de la Nutricion Salvador Zubiran, Ciudad de Mexico, Mexico
fHospital Universitario, Dr. Jose E. Gonzalez, Monterrey, Nuevo Leon, Mexico
gHospital Civil de Guadalajara “Fray Antonio Alcande”, Guadalajara, Jalisco, Mexico
hHospital PEMEX Norte, Ciudad de Mexico, Mexico
iHospital Central Dr. Ignacio Morones Prieto, San Luis Potosi, Mexico
jHospital Lomas de San Luis Potosi, San Luis Potosi, Mexico
kCentro Oncologico Estatal ISSEMyM, Estado de Mexico, Mexico
lHospital Regional de Alta Especialidad Iztapaluca, Estado de Mexico, Mexico
mCentro Medico Puerta de Hierro Sur, Guadalajara, Jalisco, Mexico
nHospital ISSSTE, Merida, Yucatan, Mexico
oHospital ISSSTE, Oaxaca, Oaxaca, Mexico
pClinica de Merida, Merida Yucatan, Mexico
qHospital Regional de Alta Especialidad, Merida, Yucatan, Mexico
rHospital San Javier, Guadalajara Jalisco, Mexico
sUnidad de Oncologia de los Servicios de Salud del Estado de Puebla, Puebla, Mexico
tHospital Regional de Alta Especialidad del Bajio, Guanajuato, Mexico
uCentro Estatal de Cancerologia Dr. Miguel Dorantes Mesa, Xalapa Veracruz, Mexico
vCorresponding Author: Nidia Zapata-Canto, Instituto Nacional de Cancerologia Mexico (INCan), San Fernando Avenue 22, Col. Seccion XVI Tlalpan, 14080 Mexico City, Mexico
Manuscript submitted November 9, 2020, accepted April 20, 2021, published online April 27, 2021
Short title: Acute Promyelocytic Leukemia in Mexico
doi: https://doi.org/10.14740/jh773
| Abstract | ▴Top |
Background: The present retrospective study reviewed acute promyelocytic leukemia (APL) cases recorded in Mexico between January 2007 and January 2017. The primary objective of the study was to evaluate overall survival (OS) in Mexican patients with APL. Secondary objective was to evaluate the impact of induction treatment with different anthracyclines on OS, event-free survival (EFS) and complications in this patient population.
Methods: The medical charts of patients referred to medical institutions in Mexico from January 2007 through January 2017 for the treatment of suspected APL were reviewed retrospectively. Patients aged 15 - 75 years, in whom the diagnosis of APL was confirmed, who had an Eastern Cooperative Group performance status of 0 - 2, and who were eligible for combined treatment with intensive chemotherapy and all-trans retinoic acid (ATRA), were included in the study. Study participants received induction and consolidation treatment with ATRA plus either daunorubicin or idarubicin, followed by 2 years of single-agent ATRA as maintenance therapy. Patients who were unable to pay for ATRA treatment received anthracycline-based induction and consolidation, with methotrexate plus mercaptopurine as maintenance therapy.
Results: A total of 360 patients from 21 public and private hospitals were included in the study. The median age of the population was 37 years, and 51% were male. Of the 360 patients, 205 (57%) vs. 155 (43%) received daunorubicin vs. idarubicin as induction treatment for APL. ATRA was administered to 201 (98%) patients in the daunorubicin group vs. 138 (89%) in the idarubicin group (P = 0.001), and was initiated at diagnosis in 92% vs. 73% of recipients, respectively (P = 0.0001). At 150 months, OS and EFS for the entire population were 84% and 79%, respectively. Both OS (90% vs. 76%, P = 0.003) and EFS (85% vs. 72%, P = 0.001) were significantly prolonged in daunorubicin vs. idarubicin recipients. Rates of complications were similar in the two groups.
Conclusions: As arsenic trioxide (ATO) is not currently available in Mexico, anthracycline plus ATRA is the mainstay of treatment for APL here. Our results confirm the efficacy of this strategy, with high OS and EFS rates being observed 12.5 years after diagnosis.
Keywords: Acute promyelocytic leukemia; Mexico; Retrospective study; ATRA; Daunorubicin; Idarubicin; Anthracycline
| Introduction | ▴Top |
Acute promyelocytic leukemia (APL) is a distinctive subtype of acute myeloid leukemia (AML) that is characterized by specific biological and clinical findings. These include: bone marrow infiltration by atypical promyelocytes [1-3]; a balanced reciprocal translocation between chromosomes 15 and 17 (t(15;17)), which fuses the promyelocyte (PML) gene on chromosome 15 to the retinoic acid receptor alfa (RAR-alfa) on chromosome 17 [2, 4, 5]; and coagulopathy associated with a severe hemorrhagic diathesis [5, 6]. The coagulopathy associated with APL is complex and incorporates several processes, including disseminated intravascular coagulation (DIC), fibrinolysis and proteolysis [7]. It is potentially life-threatening and, while typically present at diagnosis, is worsened by the initiation of chemotherapy [1, 5, 8].
There are two main morphological variants of APL: a hypergranular variant, with cells that typically contain bundles of Auer rods and have a reniform or bilobed nucleus [5, 9]; and a microgranular form, in which Auer rods are less common and no granules are visible on light microscopy [5, 10]. The hypergranular variant is the more common, representing around 75-79% of cases [10, 11]. In a typical APL case, abnormal promyelocytes constitute at least 30% of the myeloid cells in a bone marrow sample [2], and staining with myeloperoxidase is strongly positive [12].
In the United States [13] and in Europe [14], arsenic trioxide (ATO) is approved in combination with all-trans retinoic acid (ATRA) for the treatment of newly diagnosed patients with low-to-intermediate risk APL (white blood cell (WBC) count ≤ 10 × 109/L). In cases where ATO is either contraindicated or unavailable, the National Comprehensive Cancer Network (NCCN) recommends induction treatment with an anthracycline plus ATRA [15]. As ATO is not currently reimbursed in Mexico as a front-line treatment for APL, anthracyclines are the cornerstone of induction and consolidation regimens here, with ATRA being added when possible. The present study aimed to evaluate survival in Mexican patients with APL, and the impact of different anthracyclines on both survival rates and complications in this population. The study is registered with ClinicalTrials.gov identifier NCT04562818.
| Materials and Methods | ▴Top |
Patients
Patients aged 15 - 75 years, who were treated for APL at one of 21 hospitals in Mexico between January 2007 and January 2017, were eligible for inclusion. The diagnosis of APL was confirmed by the detection of abnormal promyelocytes in bone marrow samples and verification of the t(15;17) chromosomal translocation either by karyotyping or by reverse transcriptase polymerase chain reaction (RT-PCR) identification of the APL-specific genetic lesion. Additional study inclusion criteria were: Eastern Cooperative Oncology Group (ECOG) performance status of 0 - 2; and eligibility for combination treatment with intensive chemotherapy plus ATRA.
Study design and treatments
This was a retrospective, multicenter, longitudinal study. Consistent with standard medical practice in Mexico, patients received: one cycle of anthracycline and ATRA-based induction treatment; three cycles of anthracycline and ATRA-based consolidation; and single-agent ATRA as maintenance therapy for 2 years. In patients with WBC ≤ 10 × 109/L, induction treatment comprised either idarubicin 12 mg/m2 or daunorubicin 60 mg/m2 on days 2, 4, 6, and 8 of a 28-day cycle, plus ATRA 45 mg/m2 daily in divided doses. Patients with WBC > 10 × 109/L received the same induction regimen, but with cytarabine 100 mg/m2 being added on seven consecutive days in each 28-day cycle. Patients who were unable to pay for ATRA treatment received anthracycline-based induction and consolidation without ATRA, and methotrexate plus mercaptopurine as maintenance therapy. In addition to the above treatments, all patients received intrathecal methotrexate 12 mg as central nervous system prophylaxis. Patients with WBC ≤ 10 × 109/L received three cycles of methotrexate, while those with WBC > 10 × 109/L received six cycles.
Ethical issues
In accordance with the Declaration of Helsinki, all patients provided written informed consent prior to receiving chemotherapy. The study was approved by the investigational committee of the National Institute of Oncology, Mexico.
Study objectives
The primary objective of the study was to evaluate OS in Mexican patients with APL. Secondary objective was to evaluate OS, event-free survival (EFS) and complications in this population following daunorubicin- vs. idarubicin-based induction treatment plus 2 years of maintenance therapy.
Study assessments and definitions
OS was defined as the time from diagnosis of APL to death from any cause. EFS was defined as the time from diagnosis of APL to the first occurrence of an event, where an event was defined as: failure to achieve a complete response (CR), as demonstrated by continued detection of the PML-RAR-alfa fusion gene on PCR analysis; disease relapse after prior achievement of a CR; or death. Treatment response was assessed by complete blood count (CBC) after the first month of induction, and subsequently by PCR monthly during consolidation and every 3 months during the maintenance phase. CR was defined as the achievement of a normalized CBC and PML-RAR-alfa transcript level below the PCR detection limit. Disease relapse was defined as CBC evidence of pancytopenia in combination with PCR detection of the PML-RAR-alfa fusion gene in a patient with a previously confirmed CR. High-risk APL was defined as WBC count >10 × 109/L; intermediate-risk APL as WBC count ≤ 10 × 109/L and platelet count ≤ 40 × 109/L; and low-risk APL as WBC count ≤ 10 × 109/L and platelet count > 40 × 109/L. Early death was defined as death within 30 days of APL diagnosis.
Statistical methods
Nominal variables were expressed as percentages and compared using a Chi-squared test. Numerical variables were presented as mean and median values, with minima, maxima and standard deviations as appropriate for handling the described variable. Numerical variables were compared using Student’s t-test or analysis of variance (ANOVA). Non-parametric tests (Kruskal-Wallis or Mann-Whitney) were used for multivariate analyses. Survival analyses were conducted using Kaplan-Meier methodology. P was considered statistically significant at a value of < 0.05.
| Results | ▴Top |
| Patients and treatments | ▴Top |
A total of 360 patients were eligible for inclusion in the study. The median age of the population was 37 years. Seventy-six (21.1%) of the 360 patients presented with hemorrhage, and 112 (31.1%) had DIC at diagnosis. Diabetes was the most common comorbidity, being reported in 35 (9.7%) patients. A total of 136 (37.8%) patients were categorized as low risk, 81 (22.5%) as intermediate risk and 143 (39.7%) as high risk. Patient demographics and disease characteristics at diagnosis are summarized in Table 1.
 Click to view | Table 1. Patient Demographics and Disease Characteristics at Diagnosis |
Two hundred five (56.9%) patients received daunorubicin as induction treatment, while 155 (43.1%) patients received idarubicin. The choice of anthracycline was determined by availability at the treating institution. A total of 339 (94.2%) patients received ATRA in addition to an anthracycline as induction therapy: 201 (98.0%) in the daunorubicin group and 138 (89.0%) in the idarubicin group (P = 0.001) (Table 2). Among patients who received ATRA, initiation of ATRA treatment at diagnosis was significantly more common in the daunorubicin group vs. the idarubicin group (91.5% vs. 72.5%; P = 0.0001). The median duration of induction and consolidation therapy with ATRA was 75 days for the entire population, and did not differ significantly by anthracycline (73 days in the daunorubicin group vs. 77 days in the idarubicin group; P = 0.41) (Table 2).
 Click to view | Table 2. ATRA Treatment and Response to Induction Therapy |
Efficacy
Treatment response
Overall, 310 patients achieved a CR following induction treatment: 187 (91.2%) patients in the daunorubicin group vs. 123 (79.4%) patients in the idarubicin group (P = 0.04) (Table 2). Disease relapse occurred in similar numbers of patients in the two anthracycline groups: 12 (6%) daunorubicin patients vs. 11 (7%) idarubicin patients (P = 0.32).
Survival
After a median follow-up of 150 months, OS was 83.6% in the total population (Fig. 1a), and was significantly prolonged in the daunorubicin group vs. the idarubicin group (89.8% vs 75.5%, P = 0.003; Fig. 1b). EFS for the total population was 79.2% (Fig. 2a). Similar to OS, EFS was significantly prolonged in the daunorubicin group vs. the idarubicin group (84.9% vs. 71.6%, P = 0.001; Fig. 2b).
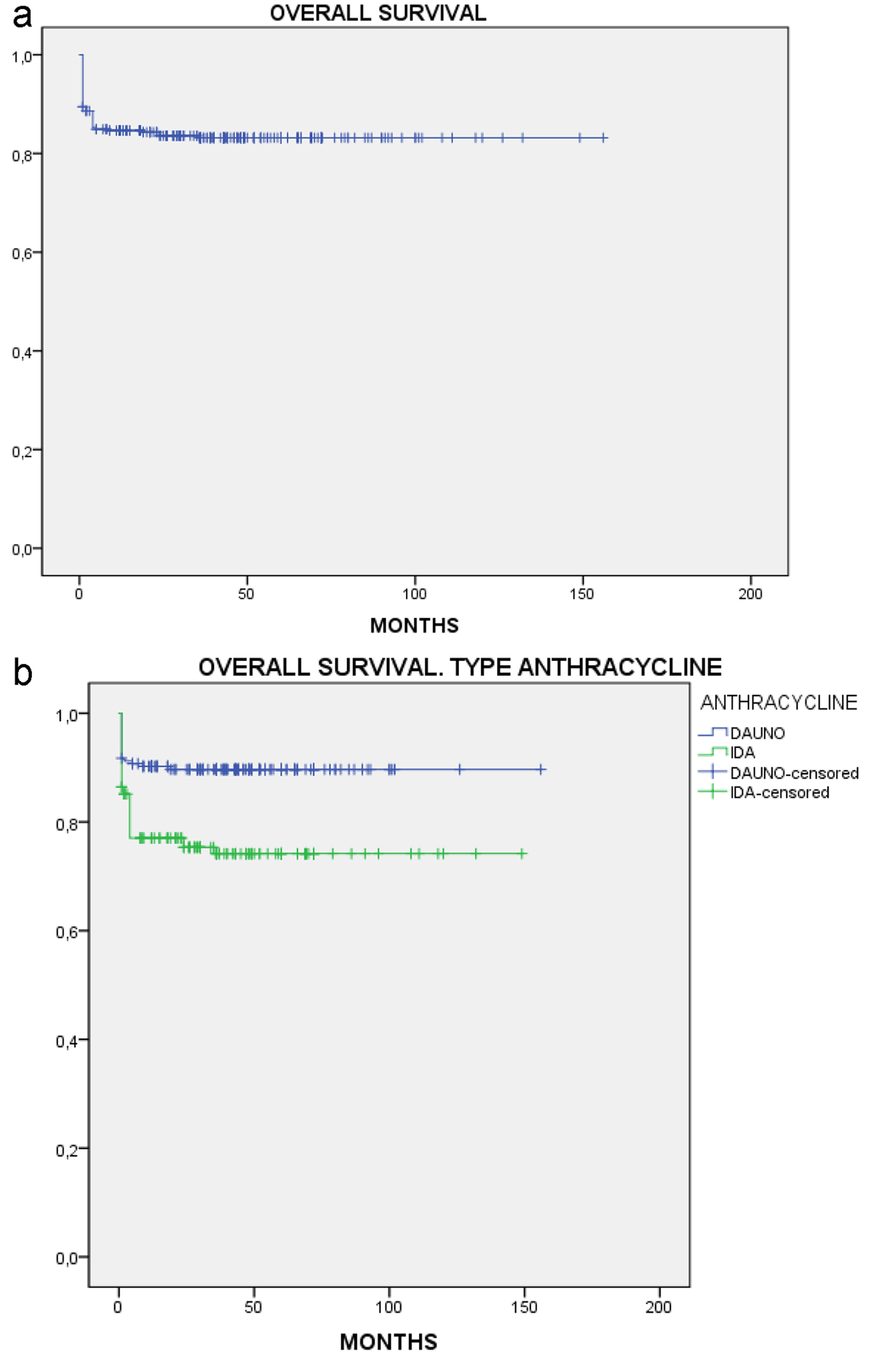 Click for large image | Figure 1. Overall survival. (a) All patients. (b) By anthracycline. |
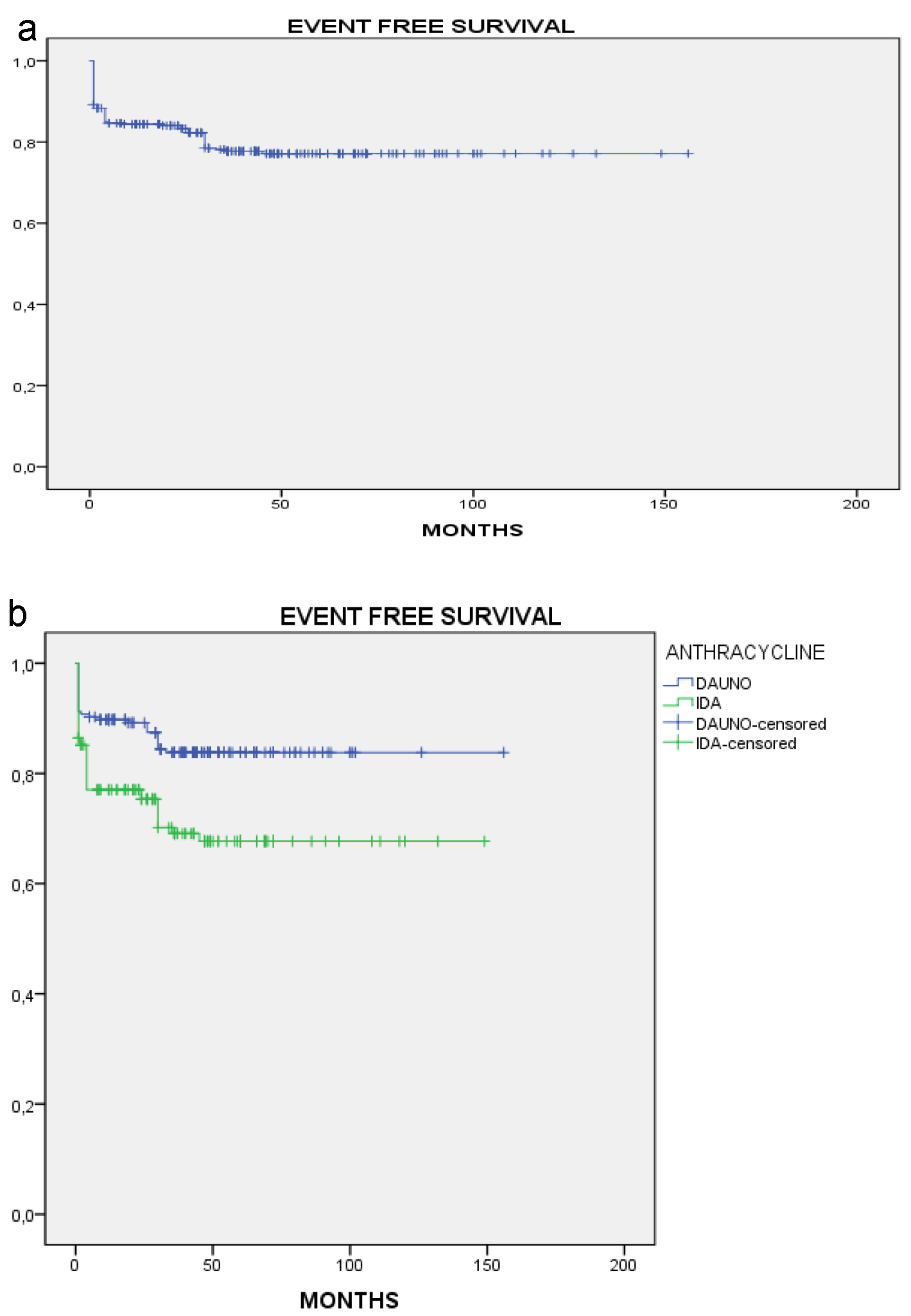 Click for large image | Figure 2. Event-free survival. (a) All patients. (b) By anthracycline. |
Fifty (13.9%) early deaths were reported: 18 (8.8%) among patients treated with daunorubicin vs. 32 (20.6%) in the idarubicin group (P = 0.001). All early deaths were attributed to either DIC (33 patients: 10 in the daunorubicin group and 23 in the idarubicin group, P = 0.002) or infection (17 patients: eight patients treated with daunorubicin and nine treated with idarubicin, P = 0.250).
Uni- and multivariate analyses
Uni- and multivariate analyses identified age, risk category at diagnosis, the type of anthracycline used, ATRA treatment and the timing of its initiation, and DIC as significant prognostic factors for OS (Table 3). No significant association was found between differentiation syndrome (DS) and OS.
 Click to view | Table 3. Uni- and Multivariate Analysis of Factors Influencing Overall Survival (N = 360) |
Complications
While receiving induction treatment, 336 (93.3%) and 66 (18.3%) patients developed febrile neutropenia (FN) and DS, respectively (Table 4). FN was treated with ceftazidime/amikacin, imipenem, or levofloxacin. Similar rates of both FN (94.1% vs. 92.3%; P = 0.44) and DS (16.1% vs. 21.3%; P = 0.22) were reported among patients treated with daunorubicin vs. idarubicin (Table 4). Ceftazidime/amikacin was prescribed for similar percentages of patients with FN in the two anthracycline groups (42.9% daunorubicin vs. 46.5% idarubicin). However, 29.8% and 21.5% of patients in the daunorubicin group who presented with FN received imipenem and levofloxacin, respectively, compared with 40.6% and 5.2% of patients with FN in the idarubicin group.
 Click to view | Table 4. Reported Complications and Antibiotic Treatment Received |
| Discussion | ▴Top |
APL is a medical emergency that requires prompt diagnosis and management, and effective monitoring of measurable residual disease (MRD) [5, 6]. For many years, APL was associated with high rates of bleeding complications and early death [2, 16-20]. The first major breakthrough in its treatment was achieved in 1973, when it was shown that high CR rates could be achieved with daunorubicin monotherapy [21]. Nevertheless, the prognosis for patients with APL remained poor until the introduction of ATRA in the 1980s [5, 22, 23] and ATO in the following decade [5, 24, 25]. Numerous studies have since demonstrated the beneficial effects of adding ATO to ATRA, either with or without chemotherapy [26-35], leading to the approval of this combination in Europe [14] and the US [13] for the treatment of newly diagnosed, low-to-intermediate risk APL. ATO is not currently available in Mexico; however, hence the preferred treatment for APL in this country is anthracycline and ATRA-based induction and consolidation, followed by ATRA-based maintenance. Using this treatment approach, patients in our study achieved OS and EFS rates of 84% and 79%, respectively, after a median follow-up of 150 months post-diagnosis. These survival rates are generally similar to those reported in previously published clinical trials of anthracycline plus ATRA-based induction and consolidation followed by at least 1 year of maintenance therapy (Table 5) [31, 33, 34, 36-40], although follow-up times in the majority of those trials were substantially shorter than the 150-month median follow-up in our study.
 Click to view | Table 5. Survival Rates in Published Clinical Trials of Anthracycline Plus ATRA-Based Induction and Consolidation Treatment for APL |
Adding ATO to ATRA induction has been shown to improve outcomes versus an anthracycline plus ATRA. Patients included in the phase 3 APL0406 trial were randomized to either ATRA plus ATO as induction and consolidation therapy, or idarubicin plus ATRA-based induction and consolidation followed by ATRA-based maintenance for 2 years [33]. After a median follow-up of 40.6 months, 50-month OS was estimated at 99% in the ATO arm vs. 93% in the idarubicin arm (P = 0.0073), and 50-month EFS at 97% vs. 80% (P < 0.001), respectively [34]. In the AML17 trial, in which patients received induction and consolidation treatment based on ATRA plus either idarubicin or ATO, 4-year EFS was significantly prolonged in the ATO arm vs. the idarubicin arm (91% vs. 70%, P = 0.002) [41]. In the light of such findings, it seems reasonable to suggest that access to ATO may have prolonged OS and EFS rates in our study population also.
Treatment with ATRA should be initiated as soon as APL is suspected, even before the diagnosis is confirmed [6]. However, if the diagnosis is not corroborated either genetically or molecularly, ATRA should be discontinued [6]. In patients with WBC ≤ 10 × 109/L, administration of ATO or chemotherapy may be delayed until the diagnosis of APL is confirmed; in those with WBC > 10 × 109/L, chemotherapy should be initiated without delay [6]. Wherever possible, bone marrow samples should be used to confirm the genetic diagnosis of APL [6]. Although the PML/RARA fusion gene can be identified using any of a variety of techniques, including conventional karyotyping or fluorescence in situ hybridization (FISH), only RT-PCR or real-time quantitative (RQ-) PCR will enable definition of the type of PML-RARA isoform and its quantification for subsequent MRD measurement [6, 42].
In a study of 217 patients with newly diagnosed PML/RARA-positive APL who received ATRA plus chemotherapy as maintenance following induction and consolidation treatment, multivariate regression analysis identified initial WBC and platelet counts as the only variables that were independently prognostic for relapse-free survival (RFS) [43]. On the basis of these findings, the authors developed a predictive model that defined three risk groups, as follows: low risk - WBC ≤ 10 × 109/L and platelet count > 40 × 109/L; intermediate risk - WBC ≤ 10 × 109/L and platelet count ≤ 40 × 109/L; and high risk - WBC > 10 × 109/L. In the approach taken by the NCCN, risk stratification is based solely on WBC counts, leading to patients being classified simply as either low (WBC ≤ 10 × 109/L) or high risk (WBC > 10 × 109/L). According to these risk stratification methods, 40% of the patients in our study were categorized as high risk at diagnosis. This is somewhat higher than the 20-28% reported in trials of patients from Europe, Australia, New Zealand, Canada, or the USA [30-32, 36, 37, 41]. In the International Consortium on APL (IC-APL) 2006 trial of daunorubicin plus ATRA-based induction and consolidation followed by 2 years of ATRA-based maintenance therapy, 34% of patients were categorized as high risk at diagnosis [38]. Although Mexico was one of the participating countries in IC-APL 2006, Mexican patients accounted for only 16% of the patient population, with the remainder coming from South American nations. It is possible, therefore, that not only ethnicity, but also geographical factors contribute to the risk profile of patients with APL. Ethnicity has previously been found to be associated with both the incidence of APL and the survival of patients with APL. In separate studies, Douer et al [44] and Estey et al [45] reported an increased incidence of APL among Latinos versus non-Latinos with AML. Although Matasar et al found no significant difference in the lifetime incidence of APL in Hispanic versus non-Hispanic white populations, they did observe significantly higher APL incidence rates in Hispanic versus non-Hispanic white children (age 1 - 19 years, P = 0.02) and adults aged 20 - 44 years (P = 0.004) [46]. A retrospective Surveillance, Epidemiology, and End Results (SEER) database analysis that included 2,962 patients diagnosed with APL from 2000 through 2014 found that, compared with other ethnic groups, Hispanic ethnicity was associated with significant increases in both APL incidence per 100,000 population and early mortality rates [47]. Additionally, in a retrospective study of patients residing at the border between the USA and Mexico, age-adjusted OS was significantly worse among Hispanic patients with APL vs. non-Hispanic white patients [48].
In our study, high-risk patients accounted for 34% of daunorubicin recipients and 47% of those treated with idarubicin. The 91% CR rate that we observed following induction with daunorubicin plus ATRA is similar to the 90% and 85% CR rates reported among patients who received daunorubicin and ATRA-based induction in North American Leukemia Intergroup Study C9710 [31] and the IC-APL 2006 trial [38], respectively. Twenty-three percent of the C9710 population were high risk, compared with 32% of the IC-APL 2006 population, as already noted. Following daunorubicin and ATRA-based consolidation and ATRA-based maintenance therapy, patients randomized to the standard-therapy arm of C9710 achieved 3-year OS and EFS rates of 81% and 63%, respectively [31], while 2-year OS and EFS rates in IC-APL 2006 were 80% and 77%, respectively [38]. Although not obtained in head-to-head trials, the 150-month OS and EFS rates of 84% and 79% in the daunorubicin group in our study appear quite favorable by comparison with these outcomes. Rates of CR, 50-month OS and 50-month EFS in the 137 low-to-intermediate risk patients randomized to the idarubicin arm in the APL0406 trial were 97%, 93% and 80%, respectively [33, 34]. The AIDA 0493 study, in which 28% of the 807 patients were categorized as high risk, reported a 94% CR rate following induction with idarubicin plus ATRA [36]. While that figure is higher than the 79% CR rate in the idarubicin group in our study, EFS rates in the two studies were similar following three intensive consolidation cycles and maintenance treatment: 72% at 12.5 years among idarubicin recipients in our study vs. 69% at 12 years in AIDA 0493 [36]. Taken together, these results demonstrate that, in the absence of ATO, ATRA and anthracycline-based induction followed by intensive consolidation and maintenance therapy is a highly effective treatment strategy for patients with APL of any risk category.
We observed a higher CR rate among patients who received daunorubicin- vs. idarubicin-based induction and consolidation therapy. Furthermore, both OS and EFS rates at 150 months were significantly prolonged in the daunorubicin group vs. the idarubicin group. DS and FN were reported in similar percentages of patients in the two groups, however, indicating no difference in toxicity profiles. It should be noted that the two patient groups in our study differed in a few important aspects. A significantly higher proportion of patients in the idarubicin group vs. the daunorubicin group had DIC at diagnosis, while a higher percentage of daunorubicin recipients received ATRA induction, started ATRA treatment on day 1, and achieved a CR following induction treatment. Each of these factors could have contributed to the improved outcomes observed in the daunorubicin group vs. the idarubicin group, therefore no firm conclusions regarding the relative efficacy of daunorubicin- vs. idarubicin-based induction and consolidation can be drawn from our data. Prospective, randomized, comparative studies are required to clarify whether differences do, indeed, exist in the efficacy of these two anthracyclines when combined with ATRA in the treatment of APL.
As this was a retrospective chart review, and access to diagnostic tests and tools at the participating institutions was limited, the results reported in this paper must be interpreted with caution. Nevertheless, they provide valuable information about the incidence of APL in Mexico, the characteristics of Mexican patients at diagnosis and the efficacy of treatment strategies currently followed in this country.
Conclusions
Guidelines published by the NCCN identify ATRA plus ATO as the preferred treatment option for patients with APL of all risk categories. Where ATO is either unavailable or contraindicated, anthracycline-based treatment is recommended as an alternative. The findings of the present study demonstrate that, in newly diagnosed Mexican patients with APL, treatment with either daunorubicin or idarubicin plus ATRA results in high CR rates following induction, and long-term OS and EFS rates of around 80% after intensive consolidation and maintenance. These results are in line with those of previously published clinical trial data, and confirm the benefits of treatment with anthracycline plus ATRA-based regimens in patients with APL of any risk category when ATO is contraindicated or otherwise unavailable.
Acknowledgments
The results reported in this manuscript were presented in part at the 24th Congress of the European Hematology Association, June 13 - 16, 2019, Amsterdam, the Netherlands. Medical writing assistance was provided by Sandralee Lewis PhD, UK.
Financial Disclosure
None to declare.
Conflict of Interest
The authors have no potential conflict of interest to disclose.
Informed Consent
Written informed consent was obtained from all study participants.
Author Contributions
NZ-C and MA designed the study. LA produced the first draft of the manuscript. MA-I analyzed the data. All the authors collected patient data, and read and approved the final manuscript.
Data Availability
The data analyzed during the study are available from the corresponding author on reasonable request.
| References | ▴Top |
- Barbui T, Finazzi G, Falanga A. The impact of all-trans-retinoic acid on the coagulopathy of acute promyelocytic leukemia. Blood. 1998;91(9):3093-3102.
doi pubmed - Ryan MM. Acute promyelocytic leukemia: a summary. J Adv Pract Oncol. 2018;9(2):178-187.
doi - Sainty D, Liso V, Cantu-Rajnoldi A, Head D, Mozziconacci MJ, Arnoulet C, Benattar L, et al. A new morphologic classification system for acute promyelocytic leukemia distinguishes cases with underlying PLZF/RARA gene rearrangements. Blood. 2000;96(4):1287-1296.
- Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, Bloomfield CD, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391-2405.
doi pubmed - Thomas X. Acute Promyelocytic Leukemia: A History over 60 Years-From the Most Malignant to the most Curable Form of Acute Leukemia. Oncol Ther. 2019;7(1):33-65.
doi pubmed - Sanz MA, Fenaux P, Tallman MS, Estey EH, Lowenberg B, Naoe T, Lengfelder E, et al. Management of acute promyelocytic leukemia: updated recommendations from an expert panel of the European LeukemiaNet. Blood. 2019;133(15):1630-1643.
doi pubmed - Tallman MS, Kwaan HC. Reassessing the hemostatic disorder associated with acute promyelocytic leukemia. Blood. 1992;79(3):543-553.
doi - Fenaux P, Le Deley MC, Castaigne S, Archimbaud E, Chomienne C, Link H, Guerci A, et al. Effect of all transretinoic acid in newly diagnosed acute promyelocytic leukemia. Results of a multicenter randomized trial. European APL 91 Group. Blood. 1993;82(11):3241-3249.
doi pubmed - Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, Gralnick HR, Sultan C. Proposals for the classification of the acute leukaemias. French-American-British (FAB) co-operative group. Br J Haematol. 1976;33(4):451-458.
doi pubmed - McKenna RW, Parkin J, Bloomfield CD, Sundberg RD, Brunning RD. Acute promyelocytic leukaemia: a study of 39 cases with identification of a hyperbasophilic microgranular variant. Br J Haematol. 1982;50(2):201-214.
doi pubmed - Bassan R, Battista R, Viero P, d'Emilio A, Buelli M, Montaldi A, Rambaldi A, et al. Short-term treatment for adult hypergranular and microgranular acute promyelocytic leukemia. Leukemia. 1995;9(2):238-243.
- Schiffer CA, Stone RM. Acute myeloid leukemia in adults. In: Kufe DW, Pollock RE, Weichselbaum RR et al, eds. Holland-Frei Cancer Medicine. 6th ed. Hamilton (ON): BC Decker Inc., 2003. Available from: https://www.ncbi.nlm.nih.gov/books/NBK13452. Accessed December 11, 2020.
- Cephalon Inc. Trisenox prescribing information. June 2019.
- European Medicines Agency. EMA/CHMP/580349/2019. September 19, 2019.
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology (NCCN guidelines®). Acute myeloid leukemia. Version 2.2021 - November 12, 2020. Accessed January 7, 2021. Available at: https://www.nccn.org/professionals/physician_gls/pdf/aml.pdf.
- Hillestad LK. Acute promyelocytic leukemia. Acta Med Scand. 1957;159(3):189-194.
doi pubmed - Cooperberg AA. Acute promyelocytic leukemia. Can Med Assoc J. 1967;97(2):57-63.
- Cordonnier C, Vernant JP, Brun B, Heilmann MG, Kuentz M, Bierling P, Farcet JP, et al. Acute promyelocytic leukemia in 57 previously untreated patients. Cancer. 1985;55(1):18-25.
doi - Kantarjian HM, Keating MJ, Walters RS, Estey EH, McCredie KB, Smith TL, Dalton WT, Jr., et al. Acute promyelocytic leukemia. M.D. Anderson Hospital experience. Am J Med. 1986;80(5):789-797.
doi - Cunningham I, Gee TS, Reich LM, Kempin SJ, Naval AN, Clarkson BD. Acute promyelocytic leukemia: treatment results during a decade at Memorial Hospital. Blood. 1989;73(5):1116-1122.
doi pubmed - Bernard J, Weil M, Boiron M, Jacquillat C, Flandrin G, Gemon MF. Acute promyelocytic leukemia: results of treatment by daunorubicin. Blood. 1973;41(4):489-496.
doi pubmed - Huang ME, Ye YC, Chen SR, Chai JR, Lu JX, Zhoa L, Gu LJ, et al. Use of all-trans retinoic acid in the treatment of acute promyelocytic leukemia. Blood. 1988;72(2):567-572.
doi pubmed - Castaigne S, Chomienne C, Daniel MT, Ballerini P, Berger R, Fenaux P, Degos L. All-trans retinoic acid as a differentiation therapy for acute promyelocytic leukemia. I. Clinical results. Blood. 1990;76(9):1704-1709.
doi pubmed - Shen ZX, Chen GQ, Ni JH, Li XS, Xiong SM, Qiu QY, Zhu J, et al. Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL): II. Clinical efficacy and pharmacokinetics in relapsed patients. Blood. 1997;89(9):3354-3360.
doi pubmed - Niu C, Yan H, Yu T, Sun HP, Liu JX, Li XS, Wu W, et al. Studies on treatment of acute promyelocytic leukemia with arsenic trioxide: remission induction, follow-up, and molecular monitoring in 11 newly diagnosed and 47 relapsed acute promyelocytic leukemia patients. Blood. 1999;94(10):3315-3324.
doi pubmed - Hu J, Shen ZX, Sun GL, Chen SJ, Wang ZY, Chen Z. Long-term survival and prognostic study in acute promyelocytic leukemia treated with all-trans-retinoic acid, chemotherapy, and As2O3: an experience of 120 patients at a single institution. Int J Hematol. 1999;70(4):248-260.
- Estey E, Garcia-Manero G, Ferrajoli A, Faderl S, Verstovsek S, Jones D, Kantarjian H. Use of all-trans retinoic acid plus arsenic trioxide as an alternative to chemotherapy in untreated acute promyelocytic leukemia. Blood. 2006;107(9):3469-3473.
doi pubmed - Aribi A, Kantarjian HM, Estey EH, Koller CA, Thomas DA, Kornblau SM, Faderl SH, et al. Combination therapy with arsenic trioxide, all-trans retinoic acid, and gemtuzumab ozogamicin in recurrent acute promyelocytic leukemia. Cancer. 2007;109(7):1355-1359.
doi pubmed - Hu J, Liu YF, Wu CF, Xu F, Shen ZX, Zhu YM, Li JM, et al. Long-term efficacy and safety of all-trans retinoic acid/arsenic trioxide-based therapy in newly diagnosed acute promyelocytic leukemia. Proc Natl Acad Sci U S A. 2009;106(9):3342-3347.
doi pubmed - Ravandi F, Estey E, Jones D, Faderl S, O'Brien S, Fiorentino J, Pierce S, et al. Effective treatment of acute promyelocytic leukemia with all-trans-retinoic acid, arsenic trioxide, and gemtuzumab ozogamicin. J Clin Oncol. 2009;27(4):504-510.
doi pubmed - Powell BL, Moser B, Stock W, Gallagher RE, Willman CL, Stone RM, Rowe JM, et al. Arsenic trioxide improves event-free and overall survival for adults with acute promyelocytic leukemia: North American Leukemia Intergroup Study C9710. Blood. 2010;116(19):3751-3757.
doi pubmed - Iland HJ, Bradstock K, Supple SG, Catalano A, Collins M, Hertzberg M, Browett P, et al. All-trans-retinoic acid, idarubicin, and IV arsenic trioxide as initial therapy in acute promyelocytic leukemia (APML4). Blood. 2012;120(8):1570-1580; quiz 1752.
doi pubmed - Lo-Coco F, Avvisati G, Vignetti M, Thiede C, Orlando SM, Iacobelli S, Ferrara F, et al. Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N Engl J Med. 2013;369(2):111-121.
doi pubmed - Platzbecker U, Avvisati G, Cicconi L, Thiede C, Paoloni F, Vignetti M, Ferrara F, et al. Improved outcomes with retinoic acid and arsenic trioxide compared with retinoic acid and chemotherapy in non-high-risk acute promyelocytic leukemia: final results of the randomized Italian-German APL0406 trial. J Clin Oncol. 2017;35(6):605-612.
doi pubmed - Abaza Y, Kantarjian H, Garcia-Manero G, Estey E, Borthakur G, Jabbour E, Faderl S, et al. Long-term outcome of acute promyelocytic leukemia treated with all-trans-retinoic acid, arsenic trioxide, and gemtuzumab. Blood. 2017;129(10):1275-1283.
doi pubmed - Avvisati G, Lo-Coco F, Paoloni FP, Petti MC, Diverio D, Vignetti M, Latagliata R, et al. AIDA 0493 protocol for newly diagnosed acute promyelocytic leukemia: very long-term results and role of maintenance. Blood. 2011;117(18):4716-4725.
doi pubmed - Burnett AK, Hills RK, Grimwade D, Jovanovic JV, Craig J, McMullin MF, Kell J, et al. Inclusion of chemotherapy in addition to anthracycline in the treatment of acute promyelocytic leukaemia does not improve outcomes: results of the MRC AML15 trial. Leukemia. 2013;27(4):843-851.
doi pubmed - Rego EM, Kim HT, Ruiz-Arguelles GJ, Undurraga MS, Uriarte Mdel R, Jacomo RH, Gutierrez-Aguirre H, et al. Improving acute promyelocytic leukemia (APL) outcome in developing countries through networking, results of the International Consortium on APL. Blood. 2013;121(11):1935-1943.
doi pubmed - Sanz MA, Montesinos P, Vellenga E, Rayon C, de la Serna J, Parody R, Bergua JM, et al. Risk-adapted treatment of acute promyelocytic leukemia with all-trans retinoic acid and anthracycline monochemotherapy: long-term outcome of the LPA 99 multicenter study by the PETHEMA Group. Blood. 2008;112(8):3130-3134.
doi pubmed - Sanz MA, Montesinos P, Rayon C, Holowiecka A, de la Serna J, Milone G, de Lisa E, et al. Risk-adapted treatment of acute promyelocytic leukemia based on all-trans retinoic acid and anthracycline with addition of cytarabine in consolidation therapy for high-risk patients: further improvements in treatment outcome. Blood. 2010;115(25):5137-5146.
doi pubmed - Burnett AK, Russell NH, Hills RK, Bowen D, Kell J, Knapper S, Morgan YG, et al. Arsenic trioxide and all-trans retinoic acid treatment for acute promyelocytic leukaemia in all risk groups (AML17): results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2015;16(13):1295-1305.
doi - Sanz MA, Grimwade D, Tallman MS, Lowenberg B, Fenaux P, Estey EH, Naoe T, et al. Management of acute promyelocytic leukemia: recommendations from an expert panel on behalf of the European LeukemiaNet. Blood. 2009;113(9):1875-1891.
doi pubmed - Sanz MA, Lo Coco F, Martin G, Avvisati G, Rayon C, Barbui T, Diaz-Mediavilla J, et al. Definition of relapse risk and role of nonanthracycline drugs for consolidation in patients with acute promyelocytic leukemia: a joint study of the PETHEMA and GIMEMA cooperative groups. Blood. 2000;96(4):1247-1253.
- Douer D, Preston-Martin S, Chang E, Nichols PW, Watkins KJ, Levine AM. High frequency of acute promyelocytic leukemia among Latinos with acute myeloid leukemia. Blood. 1996;87(1):308-313.
doi pubmed - Estey E, Thall P, Kantarjian H, Pierce S, Kornblau S, Keating M. Association between increased body mass index and a diagnosis of acute promyelocytic leukemia in patients with acute myeloid leukemia. Leukemia. 1997;11(10):1661-1664.
doi pubmed - Matasar MJ, Ritchie EK, Consedine N, Magai C, Neugut AI. Incidence rates of acute promyelocytic leukemia among Hispanics, blacks, Asians, and non-Hispanic whites in the United States. Eur J Cancer Prev. 2006;15(4):367-370.
doi pubmed - Guru Murthy GS, Szabo A, Michaelis L, Carlson KS, Runaas L, Abedin S, Atallah E. Improving outcomes of acute promyelocytic leukemia in the current era: analysis of the SEER database. J Natl Compr Canc Netw. 2020;18(2):169-175.
- Bencomo AE, Rubio AJ, Gonzalez MA, Olivas IM, Lara JJ, Ghimire K, Padilla O, et al. Retrospective study of incidence and survival for patients with hematological malignancies residing at the U.S./Mexico border. Cancer Res. 2020;80(16 Suppl):4343.
doi
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


