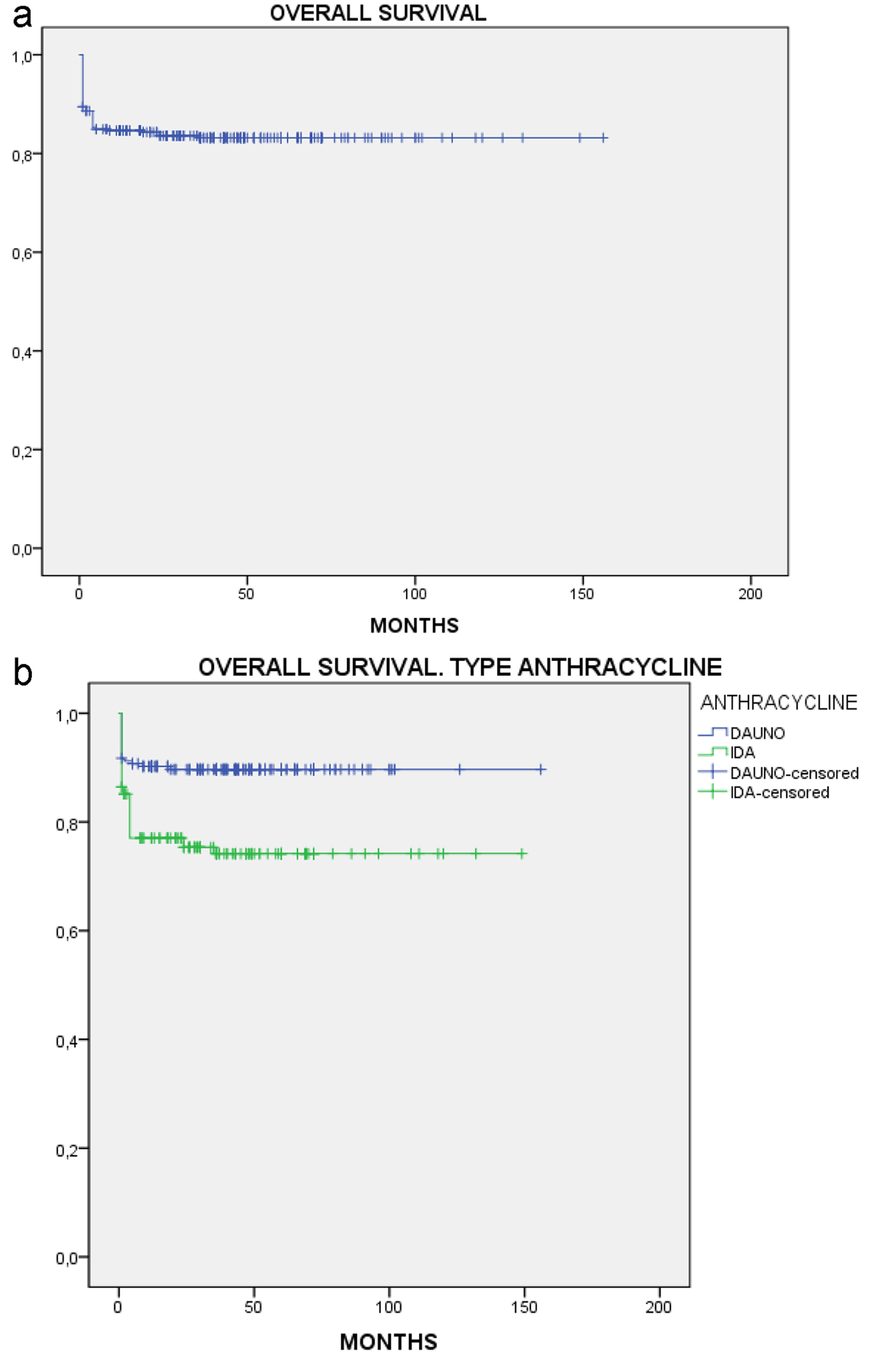
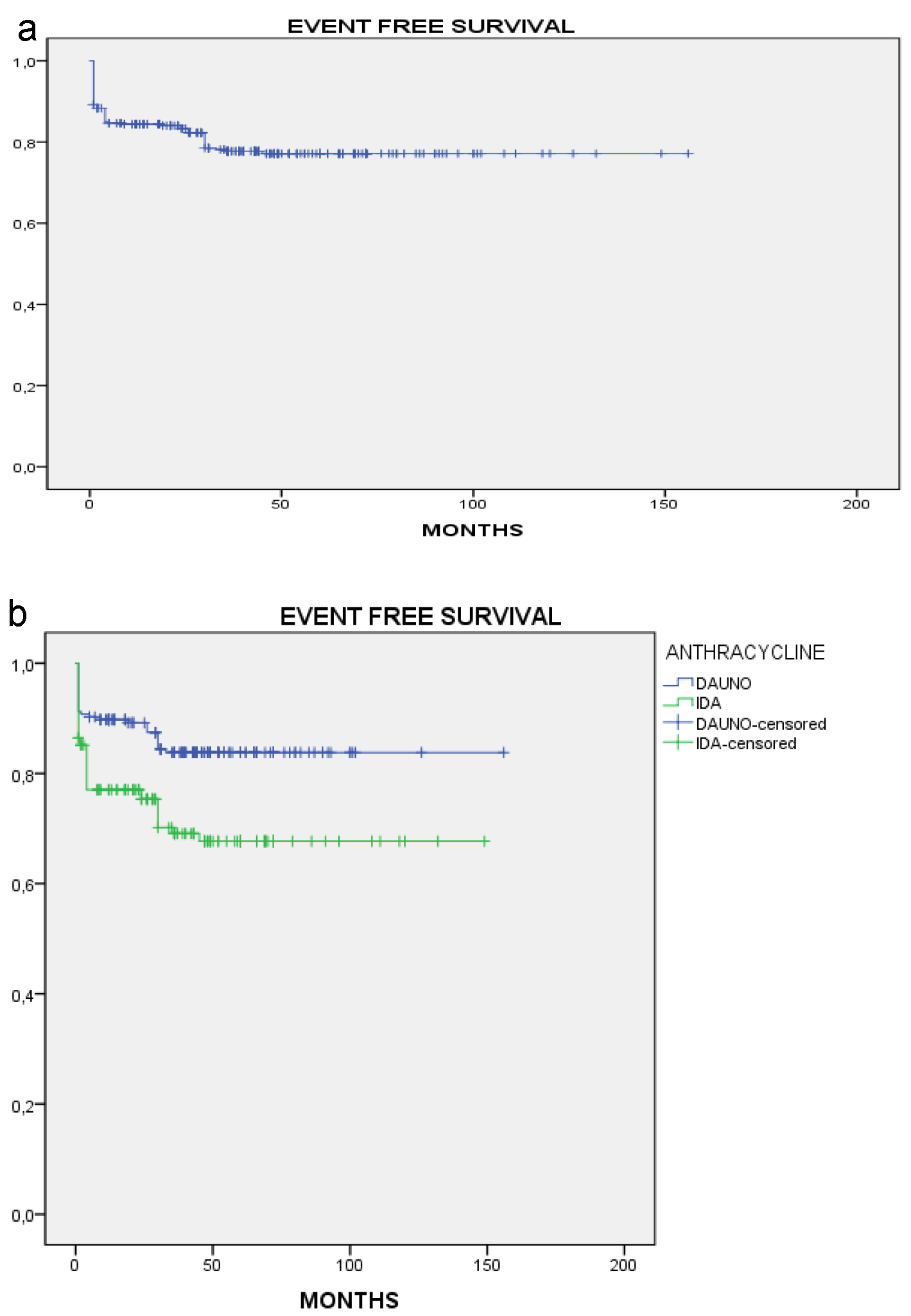
Figure 1. Overall survival. (a) All patients. (b) By anthracycline.
| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Original Article
Volume 10, Number 2, April 2021, pages 53-63
Acute Promyelocytic Leukemia: A Long-Term Retrospective Study in Mexico
Figures

Tables
| Characteristics | All patients (N = 360) | Daunorubicin recipients (n = 205) | Idarubicin recipients (n = 155) | P |
|---|---|---|---|---|
| BM: bone marrow; CNS: central nervous system; COPD: chronic obstructive pulmonary disease; DIC: disseminated intravascular coagulation; NA: not available. | ||||
| Median age, years (range) | 37 (15 - 73) | 36 (15 - 73) | 35 (16 - 67) | 0.65 |
| Male sex, n (%) | 182 (50.6) | 110 (53.7) | 72 (46.5) | 0.202 |
| Hemorrhage, n (%) | 76 (21.1) | 39 (19.0) | 37 (23.9) | 0.49 |
| CNS | 8 (2.2) | 5 (2.4) | 3 (1.9) | 0.39 |
| Digestive system | 18 (5.0) | 8 (3.9) | 10 (6.5) | 0.41 |
| Genitourinary system | 15 (4.2) | 7 (3.4) | 8 (5.2) | 0.50 |
| Integumentary system | 35 (9.7) | 15 (7.3) | 20 (12.9) | 0.44 |
| DIC, n (%) | 112 (31.1) | 43 (21.0) | 71 (45.8) | 0.02 |
| Infection, n (%) | 44 (12.2) | 21 (10.2) | 23 (14.8) | 0.48 |
| Comorbidities, n (%) | 46 (12.8) | 23 (11.2) | 23 (14.8) | NA |
| Arterial hypertension | 7 (1.9) | 4 (2.0) | 3 (1.9) | |
| Asthma/COPD | 1 (0.3) | 0 (0.0) | 1 (0.6) | |
| Chronic renal disease | 1 (0.3) | 0 (0.0) | 1 (0.6) | |
| Collagenopathy | 2 (0.6) | 1 (0.5) | 1 (0.6) | |
| Diabetes mellitus | 35 (9.7) | 18 (8.8) | 17 (11.0) | |
| Laboratory parameters, median (range) | ||||
| Leukocytes, per µL | 22 (1 - 450) | 6.2 (2 - 434) | 8.0 (1 - 450) | 0.34 |
| Hematocrit, % | 24 (10 - 42) | 25 (11 - 42) | 22 (10 - 41) | 0.50 |
| Platelets, per µL | 40 (2 - 318) | 40 (3 - 118) | 38 (2 - 315) | 0.18 |
| Partial thromboplastin time, s | 32 (18 - 255) | 30 (18 - 250) | 33 (19 - 255) | 0.21 |
| Prothrombin time, s | 17 (9 - 163) | 16 (9 - 151) | 19 (11 - 163) | 0.26 |
| Thrombin time, s | 21 (17 - 190) | 24 (19 - 190) | 20 (17 - 179) | 0.29 |
| Fibrinogen concentration, mg/dL | 190 (22 - 825) | 175 (22 - 789) | 198 (35 - 825) | 0.47 |
| D-dimer concentration, ng/mL | 167 (2 - 81,000) | 172 (4 - 81,000) | 161 (2 - 69,000) | 0.35 |
| Promyelocytes in BM, % | 71 (30 - 100) | 72 (4 - 100) | 68 (30 - 90) | 0.40 |
| Risk category, n (%) | 0.042 | |||
| Low | 136 (37.8) | 86 (42.0) | 50 (32.3) | |
| Intermediate | 81 (22.5) | 49 (23.9) | 32 (20.6) | |
| High | 143 (39.7) | 70 (34.1) | 73 (47.1) | |
| All patients (N = 360) | Daunorubicin recipients (n = 205) | Idarubicin recipients (n = 155) | P | |
|---|---|---|---|---|
| ATRA: all-trans retinoic acid; CR: complete response. | ||||
| Treated with ATRA, n (%) | 339 (94.2) | 201 (98.0) | 138 (89.0) | 0.001 |
| Initiated at diagnosis | 284 (83.8) | 184 (91.5) | 100 (72.5) | 0.0001 |
| Initiated > 1 day after diagnosis | 55 (16.2) | 17 (8.5) | 38 (27.5) | 0.02 |
| Median time to start of ATRA, days (range) | 4 (1 - 27) | 2 (1 - 18) | 5 (3 - 27) | 0.32 |
| Median duration of ATRA treatment, days (range) | 75 (45 - 95) | 73 (45 - 89) | 77 (52 - 95) | 0.41 |
| Response to induction therapy, n (%) | ||||
| CR | 310 (86.1) | 187 (91.2) | 123 (79.4) | 0.04 |
| Variables | Univariate HR (95% CI) | P | Multivariate HR (95% CI) | P |
|---|---|---|---|---|
| ATRA: all-trans retinoic acid; CI: confidence interval; DIC: disseminated intravascular coagulation; DS: differentiation syndrome; HR: hazard ratio. | ||||
| Age (< 35 years vs. ≥ 35 years) | 1.02 (1.00 - 1.04) | 0.016 | 1.01 (0.96 - 1.36) | 0.01 |
| Risk category (low vs. intermediate vs. high) | 2.20 (1.56 - 3.27) | 0.001 | 1.92 (1.32 - 2.79) | 0.01 |
| Anthracycline type (daunorubicin vs. idarubicin) | 1.55 (1.18 - 2.04) | 0.001 | 1.17 (0.87 - 1.58) | 0.02 |
| DS (yes vs. no) | 1.81 (0.77 - 4.23) | 0.160 | 1.10 (0.46 - 2.65) | 0.82 |
| ATRA treatment (yes vs. no) | 1.00 (0.57 - 1.76) | 0.002 | 0.45 (0.33 - 0.89) | 0.01 |
| Timing of ATRA initiation (day 0 vs. after day 1) | 1.40 (0.81 - 2.44) | 0.020 | 0.76 (0.55 - 1.10) | 0.04 |
| DIC (yes vs. no) | 0.99 (0.73 - 1.00) | 0.030 | 0.81 (0.66 - 1.12) | 0.02 |
| All patients (N = 360) | Daunorubicin recipients (n = 205) | Idarubicin recipients (n = 155) | P | |
|---|---|---|---|---|
| Values are expressed as n (%). DS: differentiation syndrome; FN: febrile neutropenia. | ||||
| DS | 66 (18.3) | 33 (16.1) | 33 (21.3) | 0.22 |
| FN | 336 (93.3) | 193 (94.1) | 143 (92.3) | 0.44 |
| Antibiotic treatment for FN | 0.098 | |||
| Ceftazadime/amikacin | 160 (44.4) | 88 (42.9) | 72 (46.5) | |
| Imipenem | 124 (34.4) | 61 (29.8) | 63 (40.6) | |
| Levofloxacin | 52 (14.4) | 44 (21.5) | 8 (5.2) | |
| Trials | Treatment regimen | Number of patients | OS | EFS |
|---|---|---|---|---|
| APL: acute promyelocytic leukemia; ATRA: all-trans retinoic acid; EFS: event-free survival; NR: not reported; OS: overall survival. | ||||
| AIDA 0493 [36] | Idarubicin and ATRA-based induction and consolidation; maintenance for 2 years | 828 | 77% at 12 years | 69% at 12 years |
| APL0406 [33, 34] | Idarubicin and ATRA-based induction and consolidation; maintenance for 2 years | 137 | 93% at 50 months | 80% at 50 months |
| IC-APL 2006 [38] | Daunorubicin and ATRA-based induction and consolidation; maintenance for 2 years | 183 | 80% at 2 years | 77% at 2 years |
| PETHEMA LPA 99 [39] | Idarubicin and ATRA-based induction and consolidation; maintenance for 2 years | 560 | 82% at 5 years | NR |
| PETHEMA LPA 2005 [40] | Idarubicin and ATRA-based induction and consolidation; maintenance for 2 years | 404 | 88% at 4 years | NR |
| MR AML15 [37] | Idarubicin and ATRA-based induction and consolidation; maintenance for 2 years | 146 | 84% at 5 years | NR |
| North American Leukemia Intergroup Study C9710 [31] | Daunorubicin and ATRA-based induction and consolidation; maintenance for 1 year | 237 | 81% at 3 years | 63% at 3 years |