| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website http://www.thejh.org |
Case Report
Volume 9, Number 1-2, April 2020, pages 30-32
Prolonged Remission by Pembrolizumab and Brentuximab-Vedotin Combination Therapy in Heavily-Pretreated Relapsed/Refractory Hodgkin’s Lymphoma
Tsung-Ying Yua, Ming-Shen Daia, b
aDivision of Hematology/Oncology, Department of Internal Medicine, Tri-Service General Hospital, National Defense Medical Center, Taipei, Taiwan
bCorresponding Author: Ming-Shen Dai, Division of Hematology/Oncology, Department of Internal Medicine, Tri-Service General Hospital, National Defense Medical Center, No. 325, Sec 2, Cheng-Gong Road, Neihu District, Taipei 11490, Taiwan
Manuscript submitted January 7, 2020, accepted March 16, 2020
Short title: Pembrolizumab and BV in r/rHL
doi: https://doi.org/10.14740/jh596
| Abstract | ▴Top |
Hodgkin’s lymphoma (HL) is usually sensitive and curative to multi-agent chemotherapy, but may become refractory disease in a subset of relapsed patients. Recent novel agents, brentuximab-vedotin (BV) and immune checkpoint inhibitors have significantly improve the treatment outcome. We report the outcome by combination of BV with pembrolizumab in a patient with a relapsed/refractory HL in a remarkable and durable response, even previously failed to multiple lines of chemotherapy, or brentuximab-vedotin/pembrolizumab monotherapy. Further investigation of immunotherapy combination in relapsed/refractory HL is needed.
Keywords: Pembrolizumab; Brentuximab-vedotin; Hodgkin’s lymphoma
| Introduction | ▴Top |
Hodgkin’s lymphoma (HL) usually has an excellent outcome in the majority of patients and responds to multi-agent chemotherapy, with or without radiation therapy [1]. However, a subset of patients relapses after achieving an initial response, requiring subsequent salvage therapies. The antibody-drug conjugate brentuximab-vedotin (BV) and immune checkpoint inhibitors (ICIs) have been approved by the US Food and Drug Administration (FDA), transforming the therapeutic landscape of relapsed/refractory HL (r/rHL) [2, 3]. Re-challenge with a previously resistant agent is generally prohibited and does not elicit a strong response in clinical practice. We present a case of r/rHL that was previously resistant to monotherapy but responded to a protracted course of combined BV + ICI treatment.
| Case Report | ▴Top |
A 27-year-old male presented with multiple cervical and mediastinal lymphadenopathies. The excisional biopsy disclosed classical HL, nodular sclerosis subtype, characterized by architectural effacement by nodular collagenous bands, and a mixed-population inflammatory background including numerous eosinophils admixed with Hodgkin and Reed-Sternberg (HRS) cells. Computed tomography (CT) scan of chest and pelvis revealed lymphadenopathies in bilateral neck and superior mediastinum; multiple bone lesions in T1, 2, 12, and L4 vertebrae and right ischium. Whole-body positron emission tomography (PET) scan revealed multiple sites of bone and liver involvement. The diagnosis was classical HL, nodular sclerosis type, Ann Arbor stage IVEB (stage IV: diffuse or disseminated foci of involvement of one or more extralymphatic organs or tissues; E: extranodal organ involvement; B: presence of systemic symptoms like fever and loss of weight), with liver and bone metastasis. He experienced a short remission after 12 cycles of primary chemotherapy with ABVD (adriamycin, bleomycin, vinblastine, and dacarbazine) and then relapsed in para-aortic lymph nodes. After ESHAP (etoposide, methylprednisolone, cytarabine, and cisplatin) salvage chemotherapy, he received subsequent high-dose BEAM (carmustine, etoposide, cytarabine, and melphalan) chemotherapy conditioning and autologous peripheral blood stem cell transplantation. However, his disease relapsed 5 months thereafter. Subsequent chemotherapeutic regimens of vinorelbine + gemcitabine and DVIP (dexamethasone, etoposide, ifosfamide, and cisplatin) only achieved a limited period of partial clinical response. Re-biopsy of the chest wall soft-tissue mass confirmed classical HL with cluster of differentiation 30 (CD30) expression. BV was then administered, but after three cycles of treatment, rapid progression occurred (Fig. 1a). The patient experienced a severe skin reaction to subsequent lenalidomide treatment. Immunotherapy with pembrolizumab was then initiated, and his disease responded well for 16 months (Fig. 1b) until the PET scan showed progression with recurrent bony involvement and relapse (Fig. 1c). Because of the limited options for subsequent treatment and the patient being medically unfit for allogeneic stem cell transplantation, a combination of BV and pembrolizumab was initiated, and remission was achieved for > 15 months (Fig. 1d).
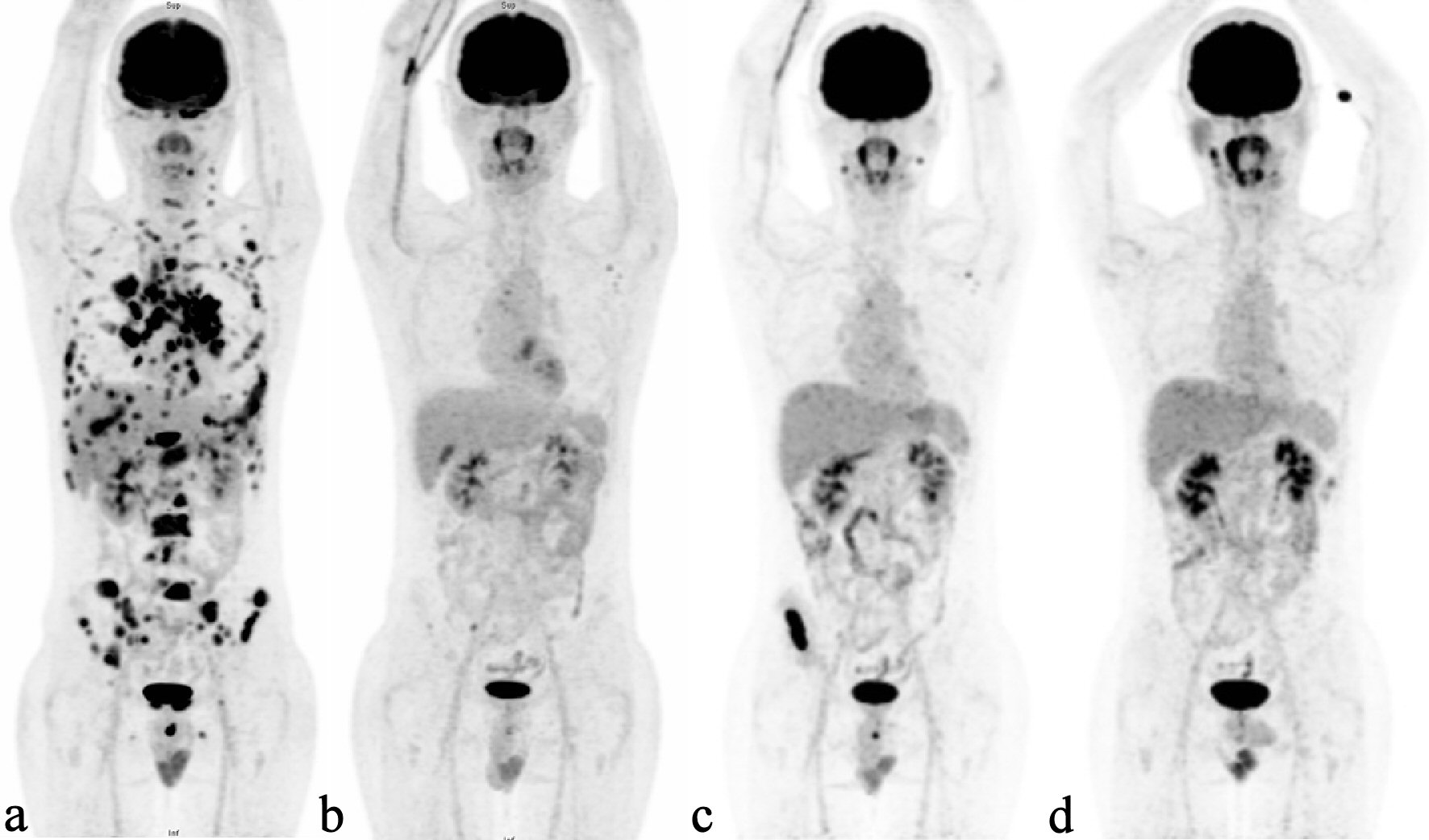 Click for large image | Figure 1. Serial PET scans demonstrating the relapsed Hodgkin’s lymphoma and treatment response. (a) PET scan showing disease progression with disseminated skeletal and visceral involvement after BV treatment. (b) Disease significantly controlled by anti-PD-1 treatment. (c) Multiple nodal and bony relapses following 16 months of remission. (d) Remission state regained after anti-PD-1 and BV combination treatment. PET: positron emission tomography; BV: brentuximab-vedotin; PD-1: programmed cell death protein-1. |
| Discussion | ▴Top |
BV is a CD30-monomethyl auristatin E (MMAE) conjugated antibody that is approved for r/rHL treatment. It exhibited significant clinical activity and achieved 61% overall response (OR) and 38% complete remission (CR) in a subset of patients as monotherapy [4]. Another two phase I/II studies reported high rates of OR (93% and 78%, respectively) and CR (74% and 43%, respectively) in r/rHL to BV + bendamustine [5, 6].
Patients with r/rHL who have failed multiple lines of therapy, including high-dose chemotherapy or BV, represent a clinical challenge and an unmet medical need. Anti-programmed cell death protein-1 (Anti-PD-1) antibody monotherapy has been effective and well tolerated in patients with r/rHL, with the majority experiencing a significant clinical response regardless of prior autologous hematopoietic cell transplantation or BV treatment. Based on these data, nivolumab and pembrolizumab were approved by the FDA for the treatment of advanced r/rHL [7].
With the increasing use of novel agents in r/rHL treatment, more clinical challenges are anticipated, mainly due to the failure of BV or ICIs. Evidence has emerged that patients with HL benefited from continued PD-1 blockade beyond disease progression according to traditionally defined immunotherapy response criteria, and that the addition of or switch to combination chemotherapy after anti-PD-1 antibody resistance could potentially re-induce a clinical response or re-sensitize the disease to previous treatment [8]. BV binds to CD30 on the HRS cell surface and enters the cell via endocytosis. Following cleavage of the cytotoxic MMAE from the anti-CD30 antibody, mitosis is interrupted. The anti-PD-1 antibodies nivolumab and pembrolizumab bind to PD-1 on T cells and block PD-L1/PD-1-mediated immune checkpoint signaling, allowing reactivation of T cells that exert a cytotoxic function against HRS cells [9].
More studies have evaluated novel anti-PD-1-based combination regimens as well as the use of anti-PD-1 antibody therapy earlier in the course of an HL patient’s therapy, including frontline therapy for r/rHL (e.g., nivolumab + BV) and even earlier combination treatment (e.g., nivolumab added to doxorubicin, vinblastine, and dacarbazine chemotherapy) [10].
Conclusions
Although it is unlikely that a clinical response would be obtained upon re-challenge to a previously resistant treatment agent, the applicability of combining the two regimens after failed earlier monotherapy has been successfully shown in this r/rHL patient and contributed to meaningfully synergistic effects. Based on the pronounced and durable response achieved, a combination of BV + ICIs is clinically applicable and required further mechanistic investigation in r/rHL patients.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Informed consent was obtained.
Author Contributions
Tsung-Ying Yu wrote the manuscript with support from Ming-Shen Dai. Ming-Shen Dai conceived the original idea and supervised the project.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, Stein KD, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66(4):271-289.
doi pubmed - Chen R, Gopal AK, Smith SE, Ansell SM, Rosenblatt JD, Savage KJ, Connors JM, et al. Five-year survival and durability results of brentuximab vedotin in patients with relapsed or refractory Hodgkin lymphoma. Blood. 2016;128(12):1562-1566.
doi pubmed - Turpin A, Michot JM, Kempf E, Mazeron R, Dartigues P, Terroir M, Boros A, et al. [Hodgkin lymphoma: Current and future therapeutic strategies]. Bull Cancer. 2018;105(1):81-98.
doi pubmed - Chen R, Wang F, Zhang H, Chen B. Brentuximab vedotin for treatment of relapsed or refractory malignant lymphoma: results of a systematic review and meta-analysis of prospective studies. Drug Des Devel Ther. 2015;9:2277-2283.
doi pubmed - LaCasce AS, Bociek RG, Sawas A, Caimi P, Agura E, Matous J, Ansell SM, et al. Brentuximab vedotin plus bendamustine: a highly active first salvage regimen for relapsed or refractory Hodgkin lymphoma. Blood. 2018;132(1):40-48.
doi pubmed - O'Connor OA, Lue JK, Sawas A, Amengual JE, Deng C, Kalac M, Falchi L, et al. Brentuximab vedotin plus bendamustine in relapsed or refractory Hodgkin's lymphoma: an international, multicentre, single-arm, phase 1-2 trial. Lancet Oncol. 2018;19(2):257-266.
doi - Chen R, Zinzani PL, Fanale MA, Armand P, Johnson NA, Brice P, Radford J, et al. Phase II study of the efficacy and safety of pembrolizumab for relapsed/refractory classic hodgkin lymphoma. J Clin Oncol. 2017;35(19):2125-2132.
doi pubmed - Rossi C, Gilhodes J, Maerevoet M, Herbaux C, Morschhauser F, Brice P, Garciaz S, et al. Efficacy of chemotherapy or chemo-anti-PD-1 combination after failed anti-PD-1 therapy for relapsed and refractory Hodgkin lymphoma: A series from Lysa centers. Am J Hematol. 2018;93(8):1042-1049.
doi pubmed - Wang Y, Nowakowski GS, Wang ML, Ansell SM. Advances in CD30- and PD-1-targeted therapies for classical Hodgkin lymphoma. J Hematol Oncol. 2018;11(1):57.
doi pubmed - Herrera AF. Where does PD-1 blockade fit in HL therapy? Hematology Am Soc Hematol Educ Program. 2018;2018(1):213-220.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


