| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website http://www.thejh.org |
Original Article
Volume 7, Number 4, December 2018, pages 131-139
Efficacy of ESHAP Regimen in Transplant Ineligible Patients With Relapsed/Refractory T-Cell Lymphoma
Lalita Norasetthadaa, b, Adisak Tantiworawita, Thanawat Rattanathammetheea, Chatree Chai-Adisaksophaa, Thanapat Chaipoha, Ekarat Rattarittamronga
aDivision of Hematology, Department of Internal Medicine, Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand
bCorresponding Author: Lalita Norasetthada, Division of Hematology, Department of Internal Medicine, Faculty of Medicine, Chiang Mai University, Chiang Mai, 50200, Thailand
Manuscript submitted August 21, 2018, accepted September 17, 2018
Short title: Efficacy of ESHAP Regimen in R/RPTCLs Patients
doi: https://doi.org/10.14740/jh459w
| Abstract | ▴Top |
Background: Salvage chemotherapy is the mainstay for the treatment of relapsed/refractory peripheral T-cell lymphomas (R/R PTCLs). ESHAP regimen, consisting of etoposide, methylprednisolone, high-dose Ara-C, and cisplatin is considered one of the well-accepted regimens for R/R lymphoma. Though, the evidence of long-term efficacy of ESHAP on R/R PTCLs is limited. This study aims to determine the efficacy and safety of ESHAP as a first salvage regimen, not followed by autologous stem cell transplantation (ASCT), in R/R PTCLs.
Methods: Patients with PTCLs, who progressed after one prior therapy and received ESHAP as a salvage treatment without subsequent ASCT, were recruited from the prospective observational study in the patients with lymphoma.
Results: From January 2005 to April 2015, 33 patients with R/R PTCLs received ESHAP as first salvage regimen at Chiang Mai University Hospital. The overall response rate was 46% (complete remission (CR) 39%). The median duration of response was 18 months. Median second progression-free survival (PFS) and overall survival (OS) were 8.0 and 11.0 months, respectively. Patients having late relapse had more favorable OS than those having early relapsed or refractory disease with a median OS of 21, 17 and 3 months, respectively (P = 0.001). Patients achieving CR after ESHAP had significantly better median OS (39, 7 and 5 months, P < 0.0001) and second PFS (33, 2 and 2 months, P < 0.0001) than those achieving PR or having progressive disease. Grade 3-4 neutropenia (45.5%) and thrombocytopenia (33.4%) were common but manageable.
Conclusions: ESHAP offers a long-term survival in some transplant ineligible patients with PTCLs who were chemosensitive with late relapse after front-line therapy. These results require further investigation in a prospective study.
Keywords: Peripheral T-cell lymphoma; ESHAP; Relapsed lymphoma; Chemotherapy
| Introduction | ▴Top |
Peripheral T-cell lymphomas (PTCLs) are accounting for 10% of non-Hodgkin lymphoma (NHL) in western countries [1] and in Thailand [2]. The outcome of patients with PTCL is inferior to those with aggressive B-cell lymphoma [3]. Depending on subtypes of T-cell lymphoma, the outcome of patients with ALK-positive anaplastic large cell lymphoma (ALCL) is more favorable than those with other subtypes for which long-term survival is less than 30% [4]. Despite an initial response to CHOP or CHOP-like chemotherapy, the majority of patient experiences disease progression thereafter [5]. Attempt to improve a durable response by upfront autologous stem transplantation (ASCT) appears to provide a favorable outcome [6]. When disease relapses, salvage chemotherapy followed by ASCT is considered the standard of care [7]. In the absence of ASCT, the survival of patients with relapsed or refractory (R/R) PTCLs is dismal with median overall survival of 5 months [8]. On the contrary to the success of novel therapies in the treatment of B-cell counterpart [9, 10], the recently approved therapies for R/R PTCLs including pralatrexate [11], romidepsin [12], and belinostat [13] provided a response rate of less than 30%. The optimal treatment of PTCLs remains challenging as the recommendation of treatment for R/R PTCLs derives from retrospective and phase II studies. Thus, combined chemotherapy regimens remain the established therapy for R/R PTCLs.
However, the salvage regimen of choice is still undefined due to the lack of large randomized controlled trials. ESHAP regimen, consisting of etoposide, methylprednisolone, high-dose Ara-C, and cisplatin is considered one of the well-accepted regimens for R/R lymphoma [14], though the evidence of long-term efficacy of ESHAP on PTCLs is limited. The purpose of this study was to determine the efficacy of ESHAP regimen, not followed by ASCT, as a first salvage therapy for patients with R/R PTCLs.
| Patients and Methods | ▴Top |
Study design and patient selection
According to our institute’s treatment guidelines to uniform treatment for reducing error during chemotherapy prescription, handling and administration, ESHAP is recommended as a first-line salvage regimen for patients with R/R PTCLs, excluding cutaneous T-cell lymphoma (CTCL) and subcutaneous panniculitis-like T-cell lymphoma (SPTL), who are ineligible for ASCT. While patients who are eligible for ASCT receive DICE regimen consisting of dexamethasone, ifosfamide, carboplatin and etoposide, then responding patients will be transferred for ASCT at the transplant center. After the year of 2011, the recommendation for R/R extranodal NK/T cell lymphoma (ENKTL) has been changed to L-asparaginase with methotrexate and dexamethasone (Asp-Met-Dex). From the prospective registry of lymphoma patients, we recruited adult patients with R/R PTCLs after anthracycline-based chemotherapy who were ineligible for ASCT and received ESHAP as salvage therapy between January 2005 and April 2015 at Chiang Mai University Hospital. All patients had given informed consent to receive treatment per institutional standards. Histological diagnoses were made by hematopathologist according to WHO classification. At relapse or progression, tissue biopsy of the tumor was routinely performed when sites of involvement were surgical accessible. Patients were restaged with computed tomography (CT) and bone marrow (BM) biopsy. Time of relapse from diagnosis, clinical characteristics, including International Prognostic Index (IPI) [15] at diagnosis and secondary IPI at relapse were recorded. The study was approved by the Research Ethics Committee of the Faculty of Medicine, Chiang Mai University and conducted in accordance with the Declaration of Helsinki.
Treatment plan
Each 21-day cycle consisted of etoposide at a dose of 40 mg/m2/day on days 1 - 4 (intravenous (IV) infusion over 1 h), methylprednisolone at 500 mg/day on days 1 - 5 (IV infusion in 15 min), Ara-C at 2 g/m2 on day 5 (IV infusion in 2 h) and cisplatin at 25 mg/m2 as a continuous infusion from day 1 to day 4. For patients older than 65 years of age, etoposide and Ara-C doses were reduced by 50% according to the institute’s guideline. Patients were hospitalized and observed throughout each course of ESHAP. Filgrastim (G-CSF) at a dose of 300 µg/day subcutaneous injection for 7 days was administered to every patient as primary neutropenic prophylaxis. Patients who developed febrile neutropenia had etoposide and Ara-C doses reduced by 20% and 50%, respectively, in subsequent cycles. A 25% and 50% reduction of the cisplatin dose was made for serum creatinine of 1.5 - 2.0 mg/dL and 2.1 - 3.0 mg/dL, respectively. Cisplatin was omitted if the serum creatinine was greater than 3 mg/dL. Co-trimoxazole and acyclovir were given to every patient according to institutional standard of practice. Clinical response and toxicity were assessed before each cycle to determine whether or not to continue treatment. CT scan was scheduled after three cycles and after the completion of ESHAP. BM reevaluation was performed only in those with previous positive study at baseline. After three cycles of ESHAP, patients who achieved complete remission (CR) or partial remission (PR) received further ESHAP treatment at a maximum of eight cycles.
Response and toxicity evaluation and definitions
Responses were determined by the treating physician and retrospectively revised from clinical and imaging records based on the Response Criteria for Malignant Lymphoma [16]. Toxicity was classified and graded according to the National Cancer Institute Common Toxicity Criteria (CTC) Version 3.0. Responses to primary therapy were categorized as refractory disease defined as response less than PR after frontline chemotherapy, early relapsed disease (relapsed within 12 months after the completion of frontline treatment) and late relapsed disease (relapsed 12 months after the completion of frontline treatment).
Statistical methods
Patients who received at least one cycle of ESHAP were analyzed. The primary endpoint was overall response rate (ORR), defined as the sum of patients with CR, unconfirmed CR (CRu), and PR. Secondary endpoints were second progression-free survival (PFS) and overall survival (OS). Duration of response (DOR) was measured for patients with CR, CRu and PR from the first date of documentation of response until progression or date of the last assessment. Chi-squared and Fisher exact test were used to compare responses between groups according to baseline characteristics at relapse and multivariable analysis for factors predicting response were analyzed by the logistic regression model. OS was measured from date of relapse/progression to date of last follow-up or death from any cause. Second PFS was calculated from date of first relapse/progression to date of last follow-up, second relapse/progression or death from any cause. Probabilities of OS and second PFS were estimated by using the Kaplan and Meier method and using log-rank test for survival comparison. Cox regression analysis was used for multivariable analysis, including factors with P < 0.1 in univariate analysis. All statistical analyses were performed using SPSS 16.0 for Windows (SPSS, Chicago, IL).
| Results | ▴Top |
Patients
Of 102 patients with PTCLs, excluding CTCL and SPTL, diagnosed between January 2005 and April 2015, 62 patients relapsed. Thirty-four patients received ESHAP, only one of them underwent ASCT. Fourteen ENKTL received Asp-Met-Dex (n = 12) and SMILE (n = 2) as salvage regimens. Five transplant-eligible patients received DICE followed by ASCT (n = 3). The rest of the patients (n = 9) were too frail to be treated with salvage chemotherapy, and therefore, received palliative treatment. There were 33 patients received ESHAP as first salvage regimens, not followed by stem cell transplantation. The reasons of transplant ineligibility were elderly (n = 12), poor performance status (n = 10), comorbidities (n = 4), socioeconomic problems (n = 4) and active infection (n = 3). The baseline characteristics of 33 patients at first relapse according to histologic subtypes are shown in Table 1. The median age of patients at first relapse was 54 years (range: 27 to 79). The majority of patients had nodal PTCLs (n = 27, 81%), including 18 (54.5%) with peripheral T-cell lymphoma, not otherwise specified (PTCL-NOS), five with angioimmunoblastic T-cell lymphoma (AITL), one with ALK-positive (ALK+) ALCL, three with ALK-negative (ALK-) ALCL, while another six patients had extranodal PTCLs, including three with ENKTL, two with hepatosplenic T-cell lymphoma (HSTL) and one with monomorphic epitheliotropic intestinal T-cell lymphoma (MEITL). At relapse, majority of patients had high LDH (n = 22, 67%), advanced stage (n = 27, 82%), good performance status (ECOG 0-1) (n = 21, 64%) and extranodal involvement ≤ one site (n = 20, 61%). Secondary IPI was distributed as followed, nine with low (27%), eight with low-intermediate (24%), five with high-intermediate (15%) and 11 with high-risk IPI (33%). Six patients had chronic viral hepatitis infection, including three (10%) with viral hepatitis B (HBV) and another three with viral hepatitis C (HCV) infection while none of them had HBV and HCV coinfection. All patients had relapsed following prior anthracycline therapy, consisting of CHOP (n = 22, 67%), CHOEP (n = 10, 30%) and EPOCH (n = 1). Twelve patients (36%) were categorized as refractory disease after their frontline chemotherapy while 21 patients (64%) had relapsed disease, including 10 patients whose disease progressed over 12 months after the completion of frontline treatment. The median prior duration of remission after frontline chemotherapy was 4 months (range: 0 months to 6 years) (Table 1).
 Click to view | Table 1. Baseline Patient Characteristics at First Relapse According to Histological Subtypes |
Therapy delivered and response
One hundred thirty-six cycles of ESHAP were administered with a median of four cycles (range: one to eight) per patient. All patients received G-CSF prophylaxis. Sixteen of the 33 patients (48%) required either dose reduction or delay of at least one cycle. All three patients with ENKTL received involved field radiation after ESHAP. The overall response rate (ORR) was 46%, with 13 patients (39%) achieving CR/CRu. Median DOR was 18 months (range, 1 - 131 months). There was much difference in DOR between those achieving CR (25 months) and PR (4.5 months). The ORR of ESHAP in patients receiving etoposide-containing regimens (n = 11) as first-line chemotherapy was slightly lower than those receiving CHOP regimen (n = 22) (36.4% vs. 50.0%, P=0.4).
Patients with PTCL-NOS, the most common subtype treated in this cohort, had ORR of 50% (CR/CRu 44%). None of the patients with HSTL and MEITL responded to ESHAP. The details of the responses by histological subtypes are described in Table 2.
 Click to view | Table 2. Response Rates and Median Survival According to Histological Subtypes |
Overall and progression free survival
With a median follow-up of 45 months, seven patients were still alive, five of whom were progression free. Median OS and second PFS were 11 months (95%CI: 5.5 - 16.4) and 8 months (95%CI: 4.5 - 11.5), respectively. Causes of death included progression of lymphoma in 26 patients and treatment-related mortality in one patient. The median second PFS of ENKTL, ALK- ALCL, PTCL-NOS, AITL and ALK+ ALCL were 33, 9, 8, 3 and 2 respectively (P = 0.017) (Table 2). Two patients with HSTL and one with MEITL progressed during ESHAP and subsequently died at 1, 4 and 7 months, respectively. Patients having late relapsed disease after frontline treatment had more favorable OS than those having early relapsed or refractory disease with a median OS of 21, 17 and 3 months, respectively(P = 0.001) (Fig. 1a). The corresponding figures for median second PFS were 16, 8 and 2 months, respectively (P = 0.001) (Fig. 1b). Patients achieving CR after ESHAP had significantly better median OS (39, 7 and 5 months, P < 0.0001) and PFS (33, 2 and 2 months, P < 0.0001) than those achieving PR or having progressive disease (Fig. 2a-b, Table 3).
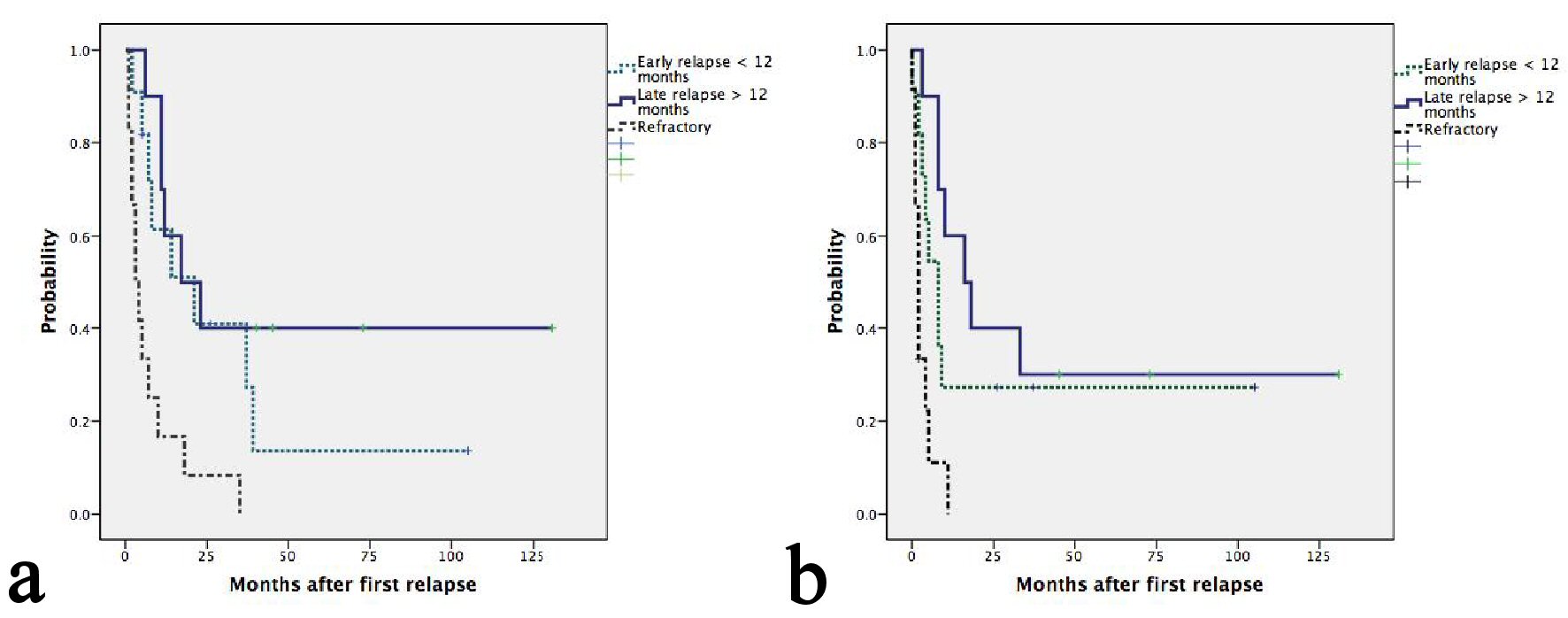 Click for large image | Figure 1. (a) Kaplan-Meier plots of OS according to relapse or refractory disease after frontline chemotherapy. The median OS for patients with late, early relapses and refractory disease were 21, 17 and 3 months, respectively (P = 0.001). (b) Kaplan-Meier plots of second PFS according to relapse or refractory disease after frontline chemotherapy. The median PFS for patients with late, early relapses and refractory disease were 16, 8 and 2 months, respectively (P = 0.001). |
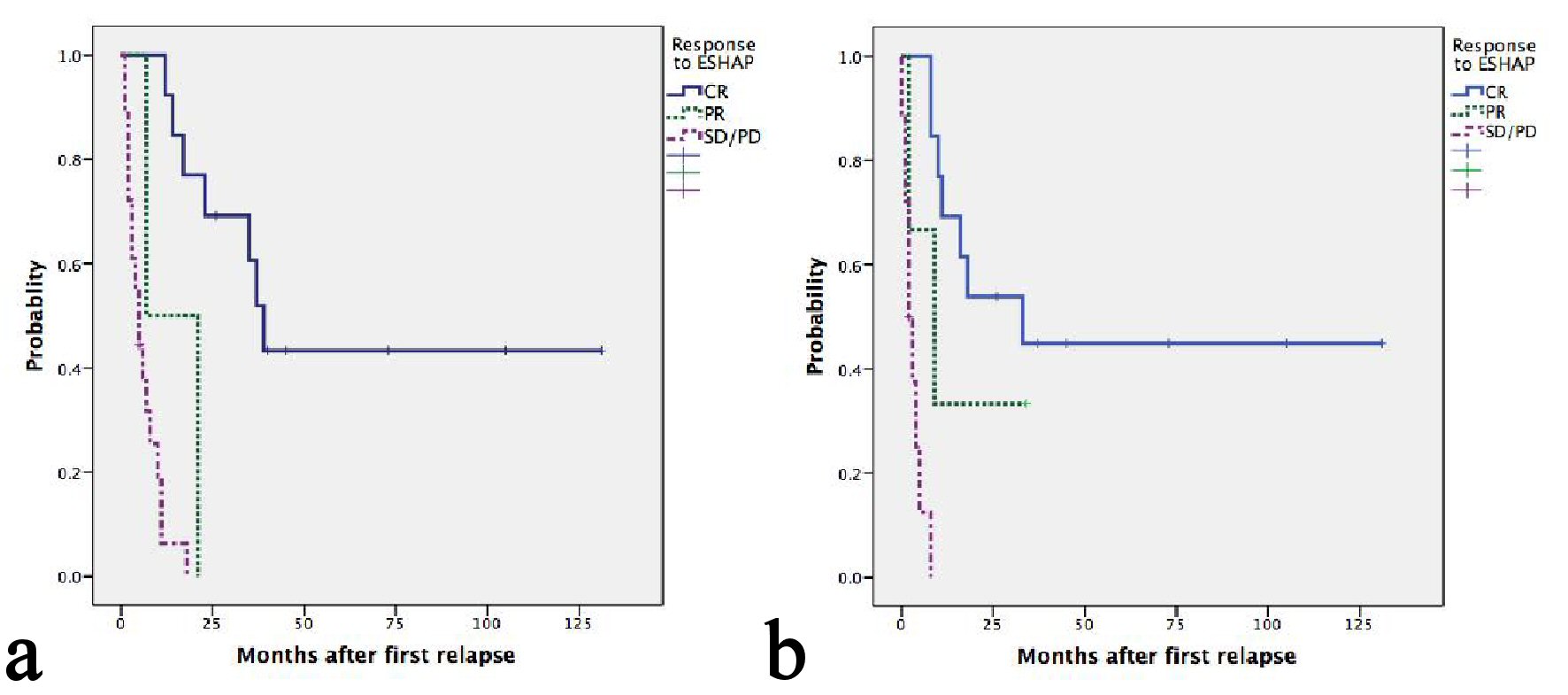 Click for large image | Figure 2. (a) Kaplan-Meier plots of OS according to response after ESHAP. Median OS of patients achieving CR, PR and SD/PD were 39, 7 and 5 months, respectively (P < 0.0001). (b) Kaplan-Meier plots of second PFS according to response after ESHAP. Median second PFS of patients achieving CR, PR and SD/PD were 33, 2 and 2 months, respectively (P < 0.0001). |
 Click to view | Table 3. Univariate Analysis ff Factor Affecting OS and Second PFS |
Adverse events
The most common Grade 3 and 4 hematologic toxicities were anemia observed in 42.2% and 24.2% of patients, respectively (Table 4). Grade 3 - 4 neutropenia and thrombocytopenia were frequently found in 45.5% and 33.4%, respectively. Febrile neutropenia was observed in 27.2% of patients despite receiving G-CSF prophylaxis. One patient with advanced stage refractory PTCL-NOS developed febrile neutropenia (FN) and died due to uncontrolled infection on day 17 after the first cycle of ESHAP. Grade I - II rising of serum creatinine was frequently occurred in 36.4% of patients, though most events were transient and correctable after intravenous hydration. Acute kidney failure occurred in a previously described patient after developing septic shock as a consequence of FN.
 Click to view | Table 4. Adverse Events During ESHAP Therapy |
| Discussion | ▴Top |
Due to the inferior outcome of PTCLs compared to aggressive B-cell lymphoma, the majority of patients with PTCLs subsequently relapsed after frontline therapy [4]. Salvage chemotherapy followed by ASCT has become a standard of care [7]. In the absence of transplantation, the outcome was dismal with a median survival of 5 months after the first relapse [8]. ESHAP has been introduced for over two decades and currently has become one of the recommended salvage regimens for relapsed NHL [14]. However, the efficacy of ESHAP has been examined in the cohorts of various subtypes of relapsed or refractory NHL, not specifically describing the outcome in those with PTCLs [14, 17-20]. In this study, we reported the efficacy and safety of ESHAP as the first salvage regimen for patients with PTCLs. To focus on the long-term outcomes after ESHAP, we recruited only patients who did not undergo high dose therapy and ASCT as a result of age, comorbidities, physical fitness or having the chemorefractory disease. We observed the response rate of 46% (CR 39%) with a median DOR of 18 months. In particularly, patients with PTCL-NOS had favorable response with ORR of 50% (CR 44%) while patients with HSTL and MEITL were refractory to ESHAP. Patients, who previously received etoposide containing regimens (CHOEP/EPOCH) as the first-line therapy, may harbor drug resistance to previously exposed chemotherapy, including etoposide. When treated with ESHAP, they might not respond as well as etoposide naive patients. Due to the limited number of patients, we could not demonstrate the significant difference in the response rate after ESHAP between these two groups receiving different first-line regimens (36.4% for CHOEP/EPOCH vs. 50.0% for CHOP, P = 0.4). Regardless of survival less than 1 year for the whole cohort, patients with late relapsed disease after frontline chemotherapy and achieving CR after ESHAP could maintain disease remission and entertained long-term survival with a median OS of 39 months even without salvage ASCT. Hematologic toxicities were quite common as previously reported [14, 17-20], but manageable with only one treatment-related death (TRD) due to FN which occurred in a patient with advanced disease progression after refractory to frontline treatment.
The direct comparison of ESHAP’s efficacy with other regimens would be difficult due to imbalances in patient population and differences of histological subtypes of T-cell lymphoma in each study. Nevertheless, the outcomes after ESHAP in this study were more favorable than those of recent approved agents for R/R PTCLs including pralatrexate [11], romidepsin [12] and belinostat [13] due to the fact that the majority of patients in the aforementioned studies had received novel agents as subsequent lines of treatment while all patients in this study received ESHAP as first salvage regimen (Table 5 [11-13, 21-26]). When focusing on studies included the majority of patients receiving one prior therapy, gemcitabine combined with dexamethasone and cisplatin (GDP) [21] provided an encouraging outcome with ORR of 64% (CR 32%) with low toxicity profile. However, the median PFS after GDP when four transplanted patients also included was 9.3 months which appeared to be comparable with that of our study when none underwent ASCT. This may reflect the more durable response after ESHAP than that of GDP due to the higher intensity of chemotherapy in ESHAP regimen. In BENTLY study [22], single agent bendamustine provided a promising efficacy on R/R PTCLs with ORR of 50%, which was comparable to the response to ESHAP. It is of note that the median DOR in that study was 3.5 months, which compared unfavorably to a longer median DOR of 18 months in this study. This may explain by the better quality of response after ESHAP as there were a higher proportion of patients achieving CR (39%) after ESHAP than that after bendamustine (28%). To reduce the nephrotoxicity of cisplatin, modified ESHAP [23] was introduced by substituting cisplatin with carboplatin. The results were featured by negligible nephrotoxicity but lower response rate (ORR 32%, CR18%) as well as median PFS (2.5 months) when compared to the standard ESHAP regimen in this study. This may be explained by the difference in drug uptake and pharmacokinetics at the cellular level between cisplatin and carboplatin leading to the different sensitivity of tumor cells to these platinum-based regimens [27, 28].
 Click to view | Table 5. The Results of Previously Reported Salvage Regimens for PTCLs [11-13, 21-26] |
There was a small proportion (n = 4, 6.5%) of patients with relapsed PTCLs (n = 62) in our cohort proceeding to stem cell transplantation, different from which has previously been reported by the British Columbia Cancer Agency (20%) [8]. The discrepancy rate could be partly explained by the difference in clinical characteristics of the patients in each cohort. First, patients with relapsed ENKTL who received L-asparaginase containing salvage regimens in this cohort (n = 14, 22.5%), achieved a durable response after salvage treatment without undergoing ASCT. Second, at the time of relapse, many patients in our cohort (n = 19, 30%) were either too frail to receive further chemotherapy (n = 9) or not fit enough for ASCT (n = 10). Furthermore, the eligible age for transplantation in our institution is younger (< 60 years) than the transplant eligibility criteria (< 65 - 70 years) in other well-experienced centers. Lastly, the socioeconomic problem is still the major issue for not being transplanted in developing countries, including Thailand, where the expense of transplantation is not under the coverage of the universal health care system. Only those who could pay out of their pockets, or under the coverage of health care insurance and the civil servant health care schema would be able to access to transplantation.
The major limitation of this study is the patient recruitment from the lymphoma registry which has the inherent limitation regarding data collection and outcome criteria. However, the results of this study reflect the real-life practice for those who are ineligible for salvage ASCT. In our experience, there was only one patient receiving ESHAP followed by ASCT who has continued in remission for 3 years after transplantation. To focus on the long-term efficacy of ESHAP, we did not include this patient in this cohort. The other transplant ineligible patients (n = 9), who were too frail to receive ESHAP, were supported by either corticosteroids or palliative radiotherapy. None of them survived over 6 months after relapse. The limitation of data collection and outcome criteria has been minimized by using the institute’s electronic data capture system which provided most of the patient characteristics, laboratory results and imaging studies for review and uniform assessment of response. Additionally, we did not perform a multivariate analysis to identify independent prognostic factors due to the small sample size of this cohort.
The results of this study provided the characteristics of patients who would have favorable outcomes after ESHAP therapy. Notably, patients who experienced relapse later than 12 months after frontline therapy and achieved CR after ESHAP could have sustained remission contributing to extended survival even without salvage ASCT. Recognizing that majority of R/R PTCLs patients are the non-transplant candidates, our analysis provides the prognostic characteristics for those who may entertain the durable remission after ESHAP without salvage ASCT. However, ASCT is still the standard of care for eligible patients. Given the wide varieties of salvage therapy for R/R PTCLs, the results from this study with a favorable response rate of 46% after ESHAP would provide the information for selecting salvage chemotherapy before proceeding to ASCT. Of note, these data highlight the unmet medical need to find the therapies for specific subtypes of PTCLs, especially HSTL and MEITL, which may harbor distinct drug sensitivities and tend to refractory to currently available treatments.
In conclusion, ESHAP offers a long-term survival in some transplant ineligible patients who were chemosensitive with late relapse after frontline therapy. The results of this cohort require further investigation in a prospective study.
Grant Support
None.
Financial Support
We have no financial arrangement related to the product figures in the submitted manuscript.
Conflict of Interest
The authors declare that they have no conflict of interest.
| References | ▴Top |
- Anderson JR, Armitage JO, Weisenburger DD. Epidemiology of the non-Hodgkin's lymphomas: distributions of the major subtypes differ by geographic locations. Non-Hodgkin's Lymphoma Classification Project. Ann Oncol. 1998;9(7):717-720.
doi pubmed - Bunworasate U SN, Khuhapinant A, Lekhakula L, Rujirojindakul P, Sirijeracha C, Chansung K, et al. A nationwide prospective multicenter study of clinical features and outcomes of non-Hodgkin lymphoma in Thailand: An analysis of 939 Cases. Blood. 2011;118:abstract 2064.
- Armitage JO, Vose JM, Weisenburger DD. Towards understanding the peripheral T-cell lymphomas. Ann Oncol. 2004;15(10):1447-1449.
doi pubmed - Vose J, Armitage J, Weisenburger D, International TCLP. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26(25):4124-4130.
doi pubmed - Schmitz N, Trumper L, Ziepert M, Nickelsen M, Ho AD, Metzner B, Peter N, et al. Treatment and prognosis of mature T-cell and NK-cell lymphoma: an analysis of patients with T-cell lymphoma treated in studies of the German High-Grade Non-Hodgkin Lymphoma Study Group. Blood. 2010;116(18):3418-3425.
doi pubmed - d'Amore F, Relander T, Lauritzsen GF, Jantunen E, Hagberg H, Anderson H, Holte H, et al. Up-front autologous stem-cell transplantation in peripheral T-cell lymphoma: NLG-T-01. J Clin Oncol. 2012;30(25):3093-3099.
doi pubmed - Philip T, Armitage JO, Spitzer G, Chauvin F, Jagannath S, Cahn JY, Colombat P, et al. High-dose therapy and autologous bone marrow transplantation after failure of conventional chemotherapy in adults with intermediate-grade or high-grade non-Hodgkin's lymphoma. N Engl J Med. 1987;316(24):1493-1498.
doi pubmed - Mak V, Hamm J, Chhanabhai M, Shenkier T, Klasa R, Sehn LH, Villa D, et al. Survival of patients with peripheral T-cell lymphoma after first relapse or progression: spectrum of disease and rare long-term survivors. J Clin Oncol. 2013;31(16):1970-1976.
doi pubmed - Coiffier B, Haioun C, Ketterer N, Engert A, Tilly H, Ma D, Johnson P, et al. Rituximab (anti-CD20 monoclonal antibody) for the treatment of patients with relapsing or refractory aggressive lymphoma: a multicenter phase II study. Blood. 1998;92(6):1927-1932.
pubmed - Wang ML, Rule S, Martin P, Goy A, Auer R, Kahl BS, Jurczak W, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2013;369(6):507-516.
doi pubmed - O'Connor OA, Pro B, Pinter-Brown L, Bartlett N, Popplewell L, Coiffier B, Lechowicz MJ, et al. Pralatrexate in patients with relapsed or refractory peripheral T-cell lymphoma: results from the pivotal PROPEL study. J Clin Oncol. 2011;29(9):1182-1189.
doi pubmed - Coiffier B, Pro B, Prince HM, Foss F, Sokol L, Greenwood M, Caballero D, et al. Romidepsin for the treatment of relapsed/refractory peripheral T-cell lymphoma: pivotal study update demonstrates durable responses. J Hematol Oncol. 2014;7:11.
doi pubmed - O'Connor OA, Horwitz S, Masszi T, Van Hoof A, Brown P, Doorduijn J, Hess G, et al. Belinostat in patients with relapsed or refractory peripheral t-cell lymphoma: results of the pivotal phase II BELIEF (CLN-19) study. J Clin Oncol. 2015;33(23):2492-2499.
doi pubmed - Velasquez WS, McLaughlin P, Tucker S, Hagemeister FB, Swan F, Rodriguez MA, Romaguera J, et al. ESHAP—an effective chemotherapy regimen in refractory and relapsing lymphoma: a 4-year follow-up study. J Clin Oncol. 1994;12(6):1169-1176.
doi pubmed - The International Non-Hodgkin's Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin's lymphoma. N Engl J Med. 1993;329(14):987-994.
doi pubmed - Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, Lister TA, et al. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999;17(4):1244.
doi pubmed - Choi CW, Paek CW, Seo JH, Kim BS, Shin SW, Kim YH, Kim JS. ESHAP salvage therapy for relapsed or refractory non-Hodgkin's lymphoma. J Korean Med Sci. 2002;17(5):621-624.
doi pubmed - Park SH, Kim S, Ko OB, Koo JE, Lee D, Jeong YP, Huh J, et al. ESHAP salvage therapy for refractory and relapsed non-Hodgkin's lymphoma: a single center experience. Korean J Intern Med. 2006;21(3):159-164.
doi pubmed - Sweetenham JW, Johnson PW. ESHAP chemotherapy for relapsed/refractory non-Hodgkin's lymphoma. J Clin Oncol. 1994;12(12):2766.
pubmed - Wang WS, Chiou TJ, Liu JH, Fan FS, Yen CC, Tung SL, Chen PM. ESHAP as salvage therapy for refractory non-Hodgkin's lymphoma: Taiwan experience. Jpn J Clin Oncol. 1999;29(1):33-37.
doi pubmed - Park BB, Kim WS, Suh C, Shin DY, Kim JA, Kim HG, Lee WS. Salvage chemotherapy of gemcitabine, dexamethasone, and cisplatin (GDP) for patients with relapsed or refractory peripheral T-cell lymphomas: a consortium for improving survival of lymphoma (CISL) trial. Ann Hematol. 2015;94(11):1845-1851.
doi pubmed - Damaj G, Gressin R, Bouabdallah K, Cartron G, Choufi B, Gyan E, Banos A, et al. Results from a prospective, open-label, phase II trial of bendamustine in refractory or relapsed T-cell lymphomas: the BENTLY trial. J Clin Oncol. 2013;31(1):104-110.
doi pubmed - Kogure Y, Yoshimi A, Ueda K, Nannya Y, Ichikawa M, Nakamura F, Kurokawa M. Modified ESHAP regimen for relapsed/refractory T cell lymphoma: a retrospective analysis. Ann Hematol. 2015;94(6):989-994.
doi pubmed - Mikesch JH, Kuhlmann M, Demant A, Krug U, Thoennissen GB, Schmidt E, Kessler T, et al. DexaBEAM versus ICE salvage regimen prior to autologous transplantation for relapsed or refractory aggressive peripheral T cell lymphoma: a retrospective evaluation of parallel patient cohorts of one center. Ann Hematol. 2013;92(8):1041-1048.
doi pubmed - Zinzani PL, Venturini F, Stefoni V, Fina M, Pellegrini C, Derenzini E, Gandolfi L, et al. Gemcitabine as single agent in pretreated T-cell lymphoma patients: evaluation of the long-term outcome. Ann Oncol. 2010;21(4):860-863.
doi pubmed - Yao YY, Tang Y, Zhu Q, Zhuang Y, Cheng YM, Wang L, Zou LF. Gemcitabine, oxaliplatin and dexamethasone as salvage treatment for elderly patients with refractory and relapsed peripheral T-cell lymphoma. Leuk Lymphoma. 2013;54(6):1194-1200.
doi pubmed - Los G, Verdegaal E, Noteborn HP, Ruevekamp M, de Graeff A, Meesters EW, ten Bokkel Huinink D, et al. Cellular pharmacokinetics of carboplatin and cisplatin in relation to their cytotoxic action. Biochem Pharmacol. 1991;42(2):357-363.
doi - Dilruba S, Kalayda GV. Platinum-based drugs: past, present and future. Cancer Chemother Pharmacol. 2016;77(6):1103-1124.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


