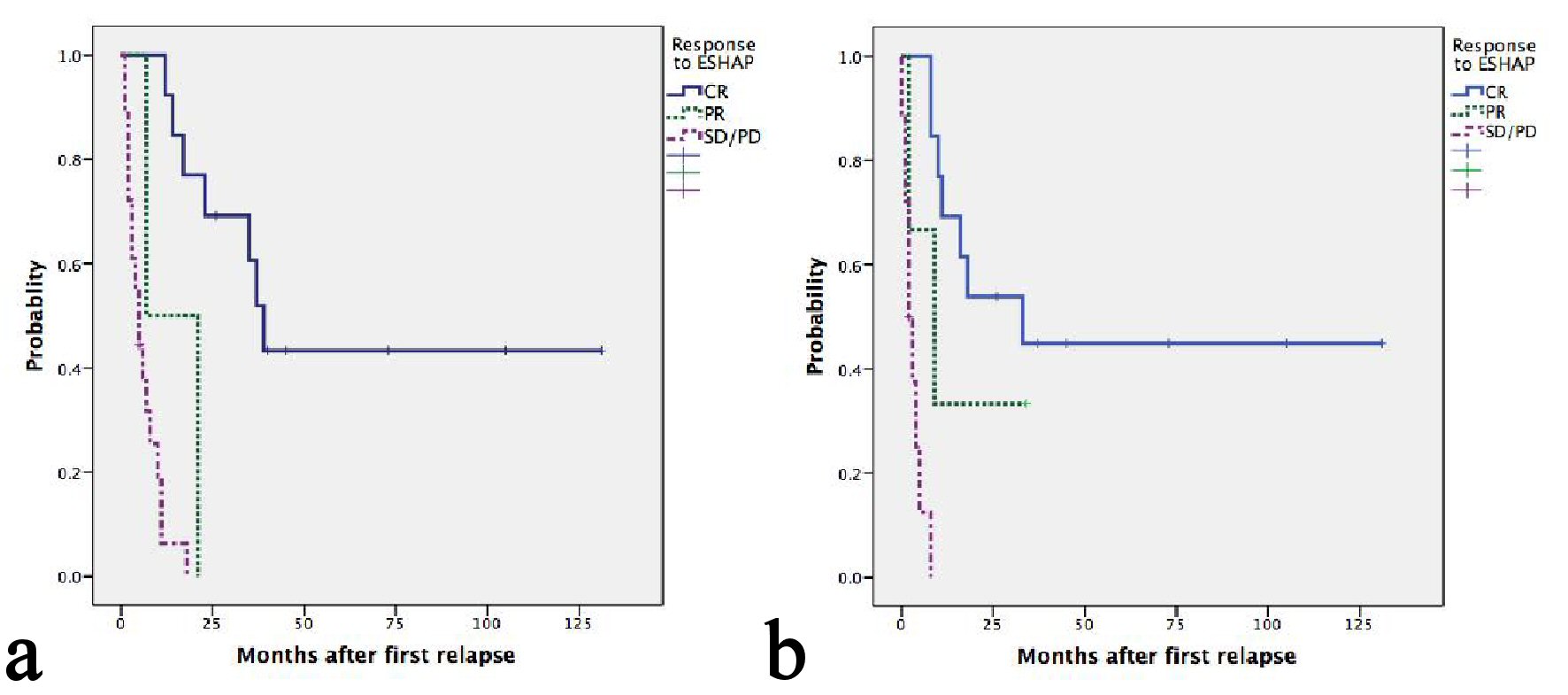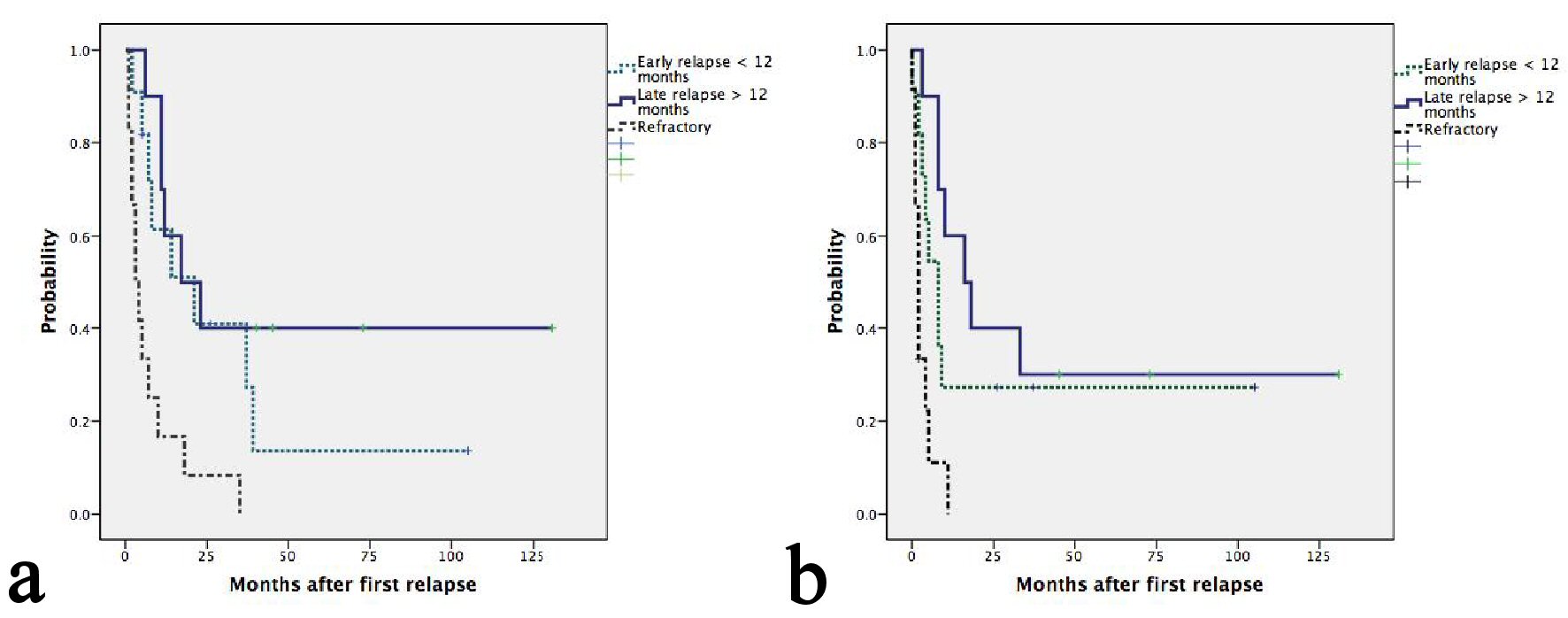
Figure 1. (a) Kaplan-Meier plots of OS according to relapse or refractory disease after frontline chemotherapy. The median OS for patients with late, early relapses and refractory disease were 21, 17 and 3 months, respectively (P = 0.001). (b) Kaplan-Meier plots of second PFS according to relapse or refractory disease after frontline chemotherapy. The median PFS for patients with late, early relapses and refractory disease were 16, 8 and 2 months, respectively (P = 0.001).