| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website http://www.thejh.org |
Original Article
Volume 6, Number 4, October 2017, pages 81-86
Regulatory and Memory B Lymphocytes in Children With Newly Diagnosed Immune Thrombocytopenia
Asmaa M. Zahrana, Sanaa Shaker Alyb, Ahmed Elabdc, Ismail Lotfy Mohamadd, e, Khalid I. Elsayhd
aClinical Pathology Department, South Egypt Cancer Institute, Assiut, Egypt
bClinical and Chemical Pathology Department, Faculty of Medicine, South Valley University, Qena, Egypt
cPediatric Department, Faculty of Medicine, South Valley University, Qena, Egypt
dPediatric Department, Faculty of Medicine, Assiut University, Assiut, Egypt
eCorresponding Author: Ismail Lotfy Mohamad, Pediatric Department, Faculty of Medicine, Assiut University, Assiut 71516, Egypt
Manuscript submitted July 13, 2017, accepted August 28, 2017
Short title: Memory B Lymphocytes in ITP
doi: https://doi.org/10.14740/jh336w
| Abstract | ▴Top |
Background: Immune (idiopathic) thrombocytopenic purpura (ITP) is a primary autoimmune disease. It is characterized by a diminished peripheral platelet count (< 100 × 109/L) caused by platelet destruction with an increased risk of mucocutaneous bleeding. The diagnosis of ITP depends on clinical characteristics and the laboratory examinations conducted, as well as the ability to exclude other diseases associated with thrombocytopenia. Antiplatelet autoantibodies are responsible for platelet destruction and probably for inhibition of megakaryopoiesis. B lymphocytes participate in immune responses through production of antibodies, antigen presentation to T cells, and cytokine secretion. The aims of this study were to investigate the levels of Bregs and memory B lymphocytes in newly diagnosed pediatric ITP patients and to correlate their levels with the course of the disease.
Methods: This study was a case-control study. The study included 30 patients with acute ITP. The patients were recruited from Pediatric Clinical Hematology Unit of Children Hospital, Assiut University. In addition, 20 healthy children of comparable age and sex were taken as controls. The institutional review board approved the study and informed consents were obtained.
Results: There is a significant alteration of B-cell homeostasis in patients with ITP.
Conclusion: Analysis of Bregs and memory B cells could serve as prognostic markers and might guide therapy in ITP patients in the future.
Keywords: B lymphocytes; Children; Immune; Thrombocytopenia
| Introduction | ▴Top |
Immune (idiopathic) thrombocytopenic purpura (ITP) is a primary autoimmune disease. It is characterized by a diminished peripheral platelet count (< 100 × 109/L) caused by platelet destruction with an increased risk of mucocutaneous bleeding [1]. It is usually a benign, self-limiting disease in children [2]. The diagnosis of ITP depends on clinical characteristics and the laboratory examinations conducted, as well as the ability to exclude other diseases associated with thrombocytopenia [3]. Antiplatelet autoantibodies are responsible for platelet destruction and probably for inhibition of megakaryopoiesis [4]. Two forms of ITP are distinguished: acute and chronic. Acute ITP occurs abruptly, often after viral infection. It usually resolves spontaneously within 6 months of diagnosis. However, approximately 20% of newly diagnosed children with ITP progress to a chronic form, defined as persistence of thrombocytopenia for more than 12 months [1].
B lymphocytes participate in immune responses through production of antibodies, antigen presentation to T cells, and cytokine secretion. The development of the normal B-cell repertoire is largely dependent upon the concerted expression of the receptors for cytokine B cell activating factor BAFF, with levels of expression varying over the different stages of B-cell differentiation [5]. B cells are divided into naive and memory B-cell subpopulations. Naive human B cells are most sensitive to prosurvival signals delivered by BAFF through its prior activation through the B-cell receptor [6]. The maturation of naive B cells to memory status is usually associated with gain of CD27 expression [7].
Memory B cells are divided into two main populations [8, 9]. Switched CD19+CD27+IgM-IgD- B cells which are involved in T cell-dependent immune responses, secrete IgG and IgA Abs, and maintain long-term serological memory. The second memory B cells are the IgM memory CD19+CD27+IgMhighIgDlowCD21high B cells which are important in T cell-independent immune responses and secrete high-affinity IgM in the early phase of infection to inhibit microbial replication in blood [10]. IgM memory B cells are the circulating counterparts of splenic marginal zone B cells [7].
An immature subset of B cells, termed regulatory B cells (Bregs), have recently been shown to mediate protective immune responses by producing regulatory cytokines such as IL-10, TGF-b, IL-35 and directly interacting with pathogenic T cells via cell-to-cell contact [11-16]. The identification of Bregs has been demonstrated in several studies in allergic [13] and autoimmune diseases such as systemic lupus erythematosus [17]. An exact definition of human Bregs by lineage-specific surface markers is lacking [16, 18]. A widely accepted phenotypic characterization of Bregs in humans was suggested by Blair et al, who reported the immature transitional CD19+CD24+hiCD38+hi B-cell subset was shown to enrich for IL-10+ Bregs and these cells suppressed CD3-mediated activation and differentiation of Th1-cells via both secretion of IL-10 and cell-cell interaction [17].
Little is known about the frequencies of Bregs and memory B lymphocytes in children with ITP. The aims of this study were to investigate the levels of Bregs and memory B lymphocytes in newly diagnosed pediatric ITP patients and to correlate their levels with the course of the disease.
| Patients and Methods | ▴Top |
This study was a case-control study, including 30 patients with acute ITP. The patients were recruited from Pediatric Clinical Hematology Unit of Children Hospital, Assiut University. In addition, 20 healthy children of comparable age and sex were taken as controls. The study was approved by the institutional review board and informed consents were obtained.
At diagnosis, all patients and controls were subjected to history and physical examination with stress upon disease duration, drug intake, preceding viral infection and bleeding manifestations, organomegaly and lymphadenopathy, in addition to the following investigations: full blood picture (Celltac E automated hematology analyzer, Tokyo, Japan), detection of memory B lymphocytes and Bregs by flow cytometry. The patients were managed according to the grade of severity [2]. According to the response of treatment, the patients were then classified into two group: patients with short duration, who recovered before 3 months, and patients with long duration, who did not recover before 3 months according to the clinical score developed by Edslev et al [19].
Inclusion criteria
The following patients were included: 1) children aged from 6 months to 16 years presented with newly diagnosed acute ITP; 2) platelet count < 50 × 109/L; 3) bleeding tendency < grade 4 [2]; and 4) no prior immunomodulating (intravenous immunoglobulin and corticosteroids) treatment before diagnosis.
Exclusion criteria
The exclusion criteria included: 1) clinical features those are not compatible with diagnosis of acute ITP, such as presence of organomegaly, other cytopenias besides thrombocytopenia, other autoimmune phenomena, or features suggestive of infectious disease like hepatitis, Epstein-Barr virus; 2) patients with chronic ITP; 3) immunomodulating treatment within 4 weeks before diagnosis; and 4) severe or life-threatening bleeding at presentation (grade 4) [2].
Flow cytometric detection of regulatory B cells and memory B lymphocytes
For detection of Bregs and memory B lymphocytes, 50 µL of blood sample was stained with 5 µL of fluoroisothiocyanate (FITC)-conjugated CD24, phycoerythrin (PE)-conjugated CD38, and peridinium-chlorophyll-protein (Per-CP)-conjugated CD19 in one tube. Per-CP-conjugated CD19, PE-conjugated IgM and FITC-conjugated CD27 in another tube, all monoclonal antibodies were purchased from Becton Dickinson (BD) Biosciences (San Jose, CA, USA). After incubation for 15 min at room temperature in the dark, RBC lysis was done. After one wash, the cells were suspended in phosphate buffer saline (PBS). Flow cytometric analysis was done by FACSCalibur flow cytometry with Cell Quest software (BD Biosciences, USA). Anti-human IgG was used as an isotype-matched negative control for each sample. Forward and side scatter histogram was used to define the lymphocyte population. Then, CD19+ (B lymphocytes) were gated for further analysis. The percentages of CD19+ (B lymphocytes), CD19+CD27- (naive B cells), CD19+CD27+ (total memory B cells), CD19+CD27+IgM+ (IgM memory B cells), CD19+CD27+IgM- (switched memory B cells), and CD19+CD24+highCD38+high (Bregs) were then detected (Fig. 1).
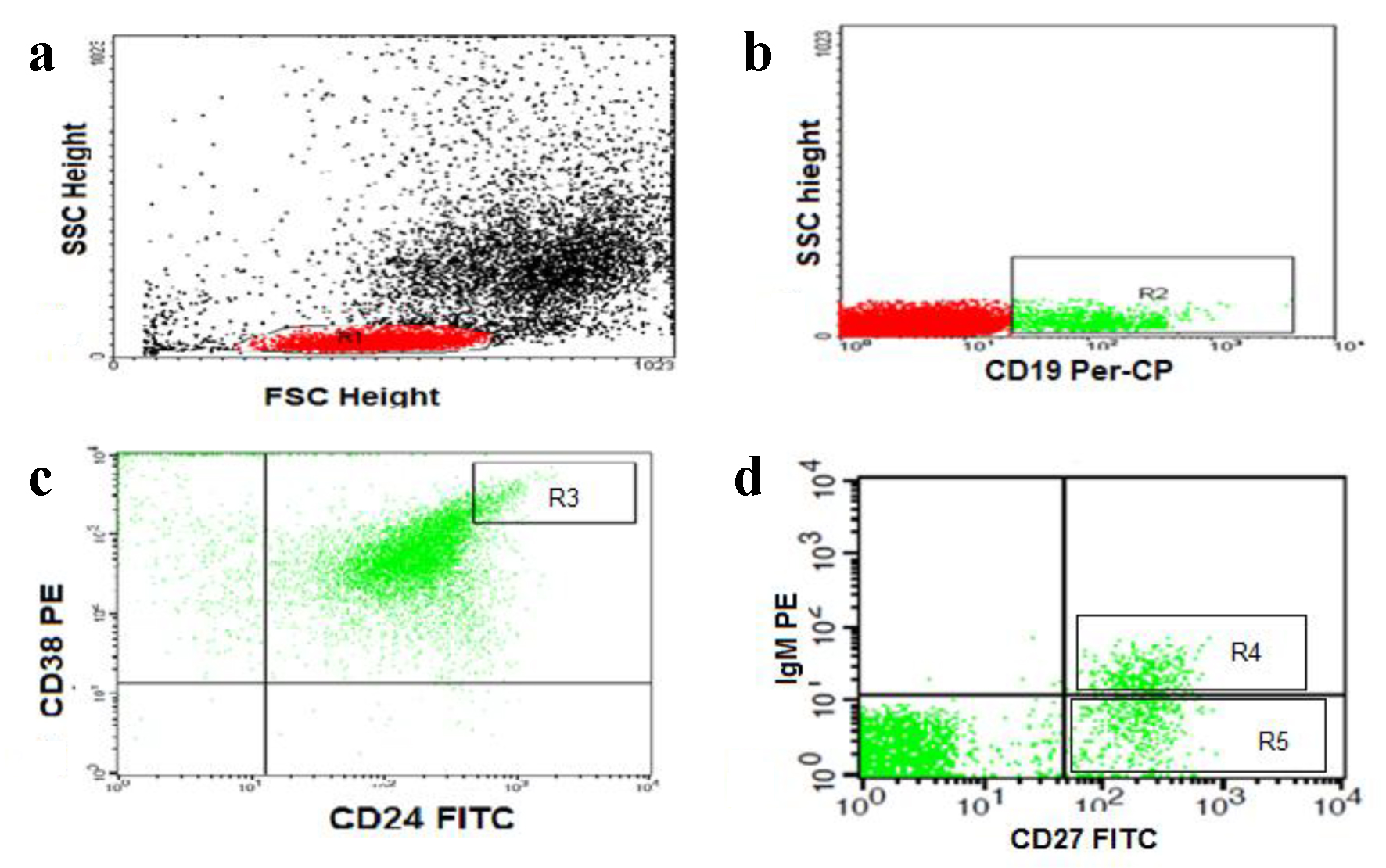 Click for large image | Figure 1. Flow cytometric detection of regulatory and memory B cells. (a) Forward and side scatter histogram was used to define the lymphocytes population (R1). (b) The CD19+ cells (B lymphocytes, R2) were then gated for further analysis. (c) The expression of CD24 and CD38 was assessed in CD19+ B cells to define CD19+CD24+HighCD38+High cells (regulatory B cells, R3). (d) The expression of IgM and CD27 in was assessed in CD19+ B cells to detect the CD19+CD27+IgM+ (IgM memory B cells, R4) and CD19+CD27+IgM- (switched memory B cells, R5). |
Statistical analysis
All statistical analyses were performed using SPSS version 18 (SPSS, Inc., Chicago, IL, USA). Qualitative data were expressed as number and percentage; quantitative data were expressed as mean ± standard error of mean (SEM). Mann-Whitney analysis was used to detect the statistical differences between the study groups. Spearman’s correlation was used to correlate the studied parameters. P value < 0.05 denoted significant difference.
| Results | ▴Top |
The baseline characteristics of ITP patients and the controls were shown in Table 1. The study included 30 children with age range from 1 to 12.5 years (5.95 ± 0.71), 17 males and 13 females. Cutaneous manifestations were present in all patients in the form of petechial hemorrhage and some bruising. Mild epistaxis was present in eight patients. Twenty patients (66.7%) experienced a preceding febrile illness. None of our patients presented with enlarged liver, spleen or significant lymphadenopathy. Twenty-one patients (70%) were recovered within 3 months and nine (30%) patients had duration of thrombocytopenia more than 3 months. There was no significant difference in white blood cell count, and hemoglobin concentration between ITP patients and controls. Platelet count was significantly decreased, and mean platelet volume (MPV) was significantly increased in the patients than the controls.
 Click to view | Table 1. Baseline Characteristics of ITP Patients and Controls |
Peripheral blood B-cell subpopulations in patients with ITP and the controls were shown in Table 2. The frequency of total B lymphocytes was significantly higher in ITP patients than the controls. There was no significant difference in the frequencies of naïve + B lymphocytes in ITP patients and the healthy controls. The frequencies of total memory B lymphocytes and switched memory B lymphocytes were significantly lower in ITP patients than the controls. While IgM+ memory B lymphocytes were significantly increased in ITP patients than the controls. The frequency of CD19+CD24+highCD38+high Bregs in patients with ITP was significantly lower in ITP patients than the controls.
 Click to view | Table 2. Peripheral Blood B-Cell Subpopulations in Patients With ITP and the Controls |
The comparison of some studied parameters at diagnosis in ITP patients with short and long duration of illness was shown in Table 3. The mean level of platelet count in peripheral blood of ITP patients with long duration of illness was significantly lower than that in patients with short duration of illness. The frequencies of total memory B cells and Bregs in ITP patients with long duration of illness were significantly lower than that level in patients with ITP with short duration of illness. There was no statistically significant difference between the other B lymphocytes subpopulation between ITP patients with long and short duration of illness. The correlations between some studied parameters in ITP patients were shown in Table 4.
 Click to view | Table 3. Comparison of Some Studied Parameters in ITP Patients With Short and Long Duration of Illness |
 Click to view | Table 4. Correlations Between Some Studied Parameters in ITP Patients |
| Discussion | ▴Top |
Primary ITP is an acquired immune-mediated disorder characterized by isolated thrombocytopenia and increased platelet destruction [20]. In patients with ITP, the maintenance of self-tolerance and the effective immune response seems to be altered. Circulating antibodies and/or immune complexes adsorb to the platelets, resulting in early destruction by macrophages. Impaired platelet production and T cell-mediated platelet destruction play significant roles in more than one step of the immune regulation in ITP [21, 22].
In the present study, we observed a significant distortion of B-cell homeostasis in patients with ITP. The percentage of total B cells was increased in ITP patients compared with the controls, with little difference between ITP patients with long and short duration of the illness. This might be expected, as B cells are the ultimate producers of anti-platelet antibodies, and patients with ITP have an increased generation of anti-platelet antibodies [23]. The pathogenic B cells are stimulated by platelet-specific helper T cells to proliferate into autoantibody-secreting cells and thus they may be responsible for decreased peripheral blood platelet count in patients with ITP [24]. Rituximab, an anti-CD20 monoclonal antibody that depletes B cells, has been used to treat ITP for about a decade and has shown its efficacy in a subpopulation of ITP patients [25, 26]. Our result, in agreement with previous studies [27-29], reported an increased frequency of B cells in ITP patients.
The decline of Bregs in the peripheral blood of our ITP patients compared with the healthy controls could indicate that the reduced Bregs play a role in the pathogenesis of ITP. Also, the more reduction of Bregs in ITP patients with long duration of the disease than those patients with short duration and the positive correlation between their level and the platelet count of the patients suggest that the number of Bregs might correlate with clinical outcome in ITP patients. Our results are in agreement with Kuwana et al and Li et al [28, 30], who reported decrease in the frequency of circulating Bregs in ITP patients compared to healthy controls.
Regulatory B cells inhibit T cell and monocyte activation through secretion of anti-inflammatory IL-10 which regulates the polarization, pro-inflammatory differentiation of other antigen presenting cells (APCs) and autoimmune responses [31]. B cells of ITP patients have impaired IL-10 response after stimulation and a reduced ability to dampen monocyte activation [30]. Bregs suppress CD4+ T cell responses and can prevent the induction of autoimmune disease in several mouse models [32, 33]. In our study, the mean levels of total memory B cells and the switched memory B cells in patients with ITP were lower than that of the normal controls. Both total memory B cells and the switched memory B cells were positively correlated with the platelet count in ITP patients. Also, the mean level of total memory B cells in patients with ITP with long duration of illness was lower than patients with ITP with short duration of illness. These findings could indicate the important role of the memory B cells in the development and prognosis of ITP.
The reduction in memory B cells may be due to continuous stimulation of the memory B cells with platelet autoantigens, which leads to differentiation of the memory B cells into plasma cells. Naive B cells differentiate into either memory or plasma cells. So another possibility of the reduction in memory B cells may be that there is more differentiation of the naive B cells to plasma cell and therefore less memory cells in ITP patients. Similar low numbers of peripheral memory B cells have been reported in patients with primary Sjogren syndrome, as well as in patients with severe chronic sarcoidosis and in a subgroup of patients with common variable immunodeficiency with autoimmune manifestations [34].
On the other hand, we found increased level of IgM memory B cells in our ITP patients and it was negatively correlated with their platelet count. This may suggest their role in the development of ITP. Their increased level could be explained by their increased production by the spleen of ITP patients, as IgM memory B cells develop in the marginal zone of the spleen and require the spleen for their survival and/or generation. The response of refractory ITP patients to splenectomy may be due to the deceased production of IgM memory B cells resulting from loss of germinal centers of the spleen. Decreased frequencies of memory B cells in splenectomized individuals were observed in ITP patients [35]. Kreutzman et al [36] reported that the patients, who had autoimmune disease as a precursor to splenectomy, had reduced numbers of circulating IgM+ memory B cells after splenectomy.
The correlation between Bregs and the memory B cells that was found in our results indicate that the human immune system is a complicated system in which all immune cells influence and control each other. The alterations in regulatory immune cells are to a great extent responsible for the disruption of immune homeostasis in autoimmune disorders, such as ITP.
Conclusion
The alteration of B-cell homeostasis in patients with ITP could play a role in the development of ITP. Analysis of Bregs and memory B cells could serve as prognostic markers and might guide the therapy in ITP patients in the future.
| References | ▴Top |
- Kuwana M. Helicobacter pylori-associated immune thrombocytopenia: clinical features and pathogenic mechanisms. World J Gastroenterol. 2014;20(3):714-723.
doi pubmed - Provan D, Stasi R, Newland AC, Blanchette VS, Bolton-Maggs P, Bussel JB, Chong BH, et al. International consensus report on the investigation and management of primary immune thrombocytopenia. Blood. 2010;115(2):168-186.
doi pubmed - Gernsheimer T. Chronic idiopathic thrombocytopenic purpura: mechanisms of pathogenesis. Oncologist. 2009;14(1):12-21.
doi pubmed - Sun L, Yu Z, Bu Y, Su J, Wang C, Cao L, Bai X, et al. [The clinical studies of 51 patients with thrombotic thrombocytopenic purpura]. Zhonghua Xue Ye Xue Za Zhi. 2014;35(2):147-151.
pubmed - Cancro MP. The BLyS/BAFF family of ligands and receptors: key targets in the therapy and understanding of autoimmunity. Ann Rheum Dis. 2006;65(Suppl 3):iii34-36.
doi pubmed - Tiller T, Tsuiji M, Yurasov S, Velinzon K, Nussenzweig MC, Wardemann H. Autoreactivity in human IgG+ memory B cells. Immunity. 2007;26(2):205-213.
doi pubmed - Weller S, Braun MC, Tan BK, Rosenwald A, Cordier C, Conley ME, Plebani A, et al. Human blood IgM "memory" B cells are circulating splenic marginal zone B cells harboring a prediversified immunoglobulin repertoire. Blood. 2004;104(12):3647-3654.
doi pubmed - Weller S, Reynaud CA, Weill JC. Vaccination against encapsulated bacteria in humans: paradoxes. Trends Immunol. 2005;26(2):85-89.
doi pubmed - Carsetti R, Rosado MM, Wardmann H. Peripheral development of B cells in mouse and man. Immunol Rev. 2004;197:179-191.
doi pubmed - Shi Y, Agematsu K, Ochs HD, Sugane K. Functional analysis of human memory B-cell subpopulations: IgD+CD27+ B cells are crucial in secondary immune response by producing high affinity IgM. Clin Immunol. 2003;108(2):128-137.
doi - Zhu XJ, Shi Y, Peng J, Guo CS, Shan NN, Qin P, Ji XB, et al. The effects of BAFF and BAFF-R-Fc fusion protein in immune thrombocytopenia. Blood. 2009;114(26):5362-5367.
doi pubmed - Lund FE, Randall TD. Effector and regulatory B cells: modulators of CD4+ T cell immunity. Nat Rev Immunol. 2010;10(4):236-247.
doi pubmed - Kalampokis I, Yoshizaki A, Tedder TF. IL-10-producing regulatory B cells (B10 cells) in autoimmune disease. Arthritis Res Ther. 2013;15(Suppl 1):S1.
doi pubmed - Shen P, Roch T, Lampropoulou V, O’Connor RA, Stervbo U, Hilgenberg E, Ries S, et al. IL-35-producing B cells are critical regulators of immunity during autoimmune and infectious diseases. Nature. 2014;507(7492):366-370.
doi pubmed - Fillatreau S. Cytokine-producing B cells as regulators of pathogenic and protective immune responses. Ann Rheum Dis. 2013;72(Suppl 2):ii80-84.
doi pubmed - Rosser EC, Mauri C. Regulatory B cells: origin, phenotype, and function. Immunity. 2015;42(4):607-612.
doi pubmed - Blair PA, Norena LY, Flores-Borja F, Rawlings DJ, Isenberg DA, Ehrenstein MR, Mauri C. CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic Lupus Erythematosus patients. Immunity. 2010;32(1):129-140.
doi pubmed - Nouel A, Simon Q, Jamin C, Pers JO, Hillion S. Regulatory B cells: an exciting target for future therapeutics in transplantation. Front Immunol. 2014;5:11.
doi pubmed - Edslev PW, Rosthoj S, Treutiger I, Rajantie J, Zeller B, Jonsson OG, Group NIW. A clinical score predicting a brief and uneventful course of newly diagnosed idiopathic thrombocytopenic purpura in children. Br J Haematol. 2007;138(4):513-516.
doi pubmed - Stasi R. Immune thrombocytopenia: pathophysiologic and clinical update. Semin Thromb Hemost. 2012;38(5):454-462.
doi pubmed - Beardsley DS. ITP in the 21st century. Hematology Am Soc Hematol Educ Program. 2006;2006(1):402-407.
doi pubmed - Cines DB, McMillan R. Pathogenesis of chronic immune thrombocytopenic purpura. Curr Opin Hematol. 2007;14(5):511-514.
doi pubmed - McMillan R. The pathogenesis of chronic immune thrombocytopenic purpura. Semin Hematol. 2007;44(4 Suppl 5):S3-S11.
doi pubmed - Kuwana M, Kaburaki J, Ikeda Y. Autoreactive T cells to platelet GPIIb-IIIa in immune thrombocytopenic purpura. Role in production of anti-platelet autoantibody. J Clin Invest. 1998;102(7):1393-1402.
doi pubmed - Patel VL, Mahevas M, Lee SY, Stasi R, Cunningham-Rundles S, Godeau B, Kanter J, et al. Outcomes 5 years after response to rituximab therapy in children and adults with immune thrombocytopenia. Blood. 2012;119(25):5989-5995.
doi pubmed - Zaja F, Baccarani M, Mazza P, Bocchia M, Gugliotta L, Zaccaria A, Vianelli N, et al. Dexamethasone plus rituximab yields higher sustained response rates than dexamethasone monotherapy in adults with primary immune thrombocytopenia. Blood. 2010;115(14):2755-2762.
doi pubmed - Chiang MR, Wei CC, Muo CS, Fu LS, Li TC, Kao CH. Association of primary immune thrombocytopenia and common allergic diseases among children. Pediatr Res. 2015;77(4):597-601.
doi pubmed - Kuwana M, Okazaki Y, Satoh T, Asahi A, Kajihara M, Ikeda Y. Initial laboratory findings useful for predicting the diagnosis of idiopathic thrombocytopenic purpura. Am J Med. 2005;118(9):1026-1033.
doi pubmed - Talaat RM, Elmaghraby AM, Barakat SS, El-Shahat M. Alterations in immune cell subsets and their cytokine secretion profile in childhood idiopathic thrombocytopenic purpura (ITP). Clin Exp Immunol. 2014;176(2):291-300.
doi pubmed - Li X, Zhong H, Bao W. Defective regulatory B-cell compartment in patients with immune thromboc. J Immunol. 2011;186:5569-5579.
- Moulin V, Andris F, Thielemans K, Maliszewski C, Urbain J, Moser M. B lymphocytes regulate dendritic cell (DC) function in vivo: increased interleukin 12 production by DCs from B cell-deficient mice results in T helper cell type 1 deviation. J Exp Med. 2000;192(4):475-482.
doi pubmed - Fillatreau S, Gray D, Anderton SM. Not always the bad guys: B cells as regulators of autoimmune pathology. Nat Rev Immunol. 2008;8(5):391-397.
doi pubmed - Mauri C, Ehrenstein MR. The ‘short’ history of regulatory B cells. Trends Immunol. 2008;29(1):34-40.
doi pubmed - Warnatz K, Denz A, Drager R, Braun M, Groth C, Wolff-Vorbeck G, Eibel H, et al. Severe deficiency of switched memory B cells (CD27(+)IgM(-)IgD(-)) in subgroups of patients with common variable immunodeficiency: a new approach to classify a heterogeneous disease. Blood. 2002;99(5):1544-1551.
doi pubmed - Martinez-Gamboa L, Mei H, Loddenkemper C, Ballmer B, Hansen A, Lipsky PE, Emmerich F, et al. Role of the spleen in peripheral memory B-cell homeostasis in patients with autoimmune thrombocytopenia purpura. Clin Immunol. 2009;130(2):199-212.
doi pubmed - Kruetzmann S, Rosado MM, Weber H, Germing U, Tournilhac O, Peter HH, Berner R, et al. Human immunoglobulin M memory B cells controlling Streptococcus pneumoniae infections are generated in the spleen. J Exp Med. 2003;197(7):939-945.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


