| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website http://www.thejh.org |
Case Report
Volume 6, Number 4, October 2017, pages 96-100
A Case of Therapy-Related Acute Myeloid Leukemia in a Patient With Heterozygous Mutations in the Ataxia Telangiectasia Mutated Gene
Andrew Shieha, Ali A. Mohameda, b
aPenn State Milton S. Hershey Medical Center, 500 University Dr., Hershey, PA 17033, USA
bCorresponding Author: Ali A. Mohamed, Department of Hematology/Oncology, Penn State Cancer Institute, 500 University Drive, CH46, Hershey, PA 17033, USA
Manuscript submitted June 13, 2017, accepted June 29, 2017
Short title: t-AML in a Patient With ATM Mutations
doi: https://doi.org/10.14740/jh330w
| Abstract | ▴Top |
Use of adjuvant chemotherapy has improved survival for many patients with breast cancer. Unfortunately, such treatment can come at a price, in particular, malignancies. We present a case of a 36-year-old woman with heterozygous mutations in the ataxia telangiectasia mutated (ATM) gene who was admitted to the hospital for fatigue and diffusely scattered bruises. She was diagnosed with invasive ductal carcinoma of the left breast and had bilateral mastectomy with axillary node clearance followed by adjuvant chemotherapy 3 years prior. Her vitals were stable. Lab tests revealed thrombocytopenia, leukocytosis, and anemia. Peripheral blood smear and bone marrow biopsy revealed numerous myeloblasts. After flow cytometry and FISH analysis were performed, a diagnosis of therapy-related acute myeloid leukemia (t-AML) was made. The patient was treated with induction chemotherapy and a bone marrow biopsy revealed residual disease. Re-induction therapy was given and a bone marrow biopsy revealed complete remission. She subsequently received an allogenic stem cell transplant and was cured. Her treatment course was uncomplicated. We raise the question as to whether certain chemotherapy agents should be avoided in patients with mutations in DNA repair genes. Furthermore, it is essential for physicians to educate patients on the risk of secondary malignancies arising from chemotherapeutic treatment.
Keywords: Therapy-related leukemia; Acute myeloid leukemia; Breast cancer; Adjuvant chemotherapy; Ataxia telangiectasia mutated gene; Hematology; Oncology
| Introduction | ▴Top |
Breast cancer is the second leading cause of cancer deaths among women of all races. Adjuvant therapy for breast cancer is proven to decrease risk of relapse and improve survival. Unfortunately, for some patients, chemotherapy may lead to leukemic syndromes in the future. Treatment with agents that cause DNA damage in patients with known genetic mutations in genes responsible for the DNA repair process may increase the risk of developing malignancies. Therapy-related acute myeloid leukemia (t-AML) represents a risk that patients undergoing certain chemotherapy face and unfortunately carry a poorer prognosis than de novo disease [1]. Even though a causal relationship between cytotoxic agents and leukemia is implied, the mechanism remains to be unproven. However, in this case of t-AML, the patient’s mutations in the ATM gene must also be emphasized. This case highlights the potential dangers from adjuvant therapy in patients with susceptible genetic mutations and why it is essential for patients and physicians to understand the risks and benefits of adjuvant chemotherapeutic treatment for breast cancer. Furthermore, we question whether such patients should receive alternative chemotherapy regimens to reduce the risk for t-AML, specifically anthracycline-induced AML.
| Case Report | ▴Top |
A 36-year-old woman had bilateral mastectomy and axillary node clearance for a T2N1M0 invasive ductal carcinoma of the left breast which was grade 3 and ER/PR positive, HER-2/neu receptor negative. Her family history revealed that her maternal grandmother, paternal grandmother, and paternal grandfather were diagnosed with breast cancer at the age of 70 years, ovarian cancer at the age of 83 years, and lymphoma at the age of 79 years, respectively. On genetic screen after her diagnosis, she was found to have c.1229T>C (p.Val410Ala) and c.6067G>A (p.Gly2023Arg) heterozygous mutations in the ataxia telangiectasia mutated (ATM) gene. She received adjuvant chemotherapy that consisted of four doses of doxorubicin and cyclophosphamide followed by paclitaxel and trastuzumab. She was put on adjuvant hormone therapy. Approximately 3 years after beginning treatment for breast cancer, the patient presented to the hospital with extreme fatigue and easy bruising. She was found to have platelet count of 21,000/µL (150,000 - 350,000), WBC of 13,380/µL (4,000 - 10,400), and hemoglobin of 6.5 g/dL (11.7 - 15.0). Blood smear showed approximately 20% of WBC composed of myeloblasts of high nucleus-to-chromatin ratio and hypolobular and hypogranular neutrophils. Flow cytometry of peripheral blood demonstrated 24.7% blasts positive for CD45 and co-expression of CD33, CD13, CD117, CD34, CD64, CD14, CD11c, CD10, CD2, and MPO. Bone marrow aspirate revealed 34.5% myeloblasts and 41.5% segmented and band neutrophils in a hypercellular background (Figs. 1 and 2). FISH analysis showed rearrangement in the mixed-lineage leukemia gene (MLL) with t(11;19)(q23;p13.1) in 76.4% of nuclei. The patient was diagnosed with therapy-related acute myeloid leukemia (t-AML) and was treated with the “7+3” induction chemotherapy regimen that consisted of a combination of daunorubicin (60 mg/m2 per day) for 3 days combined with cytarabine (100 mg/m2 per day) for 7 days without complications.
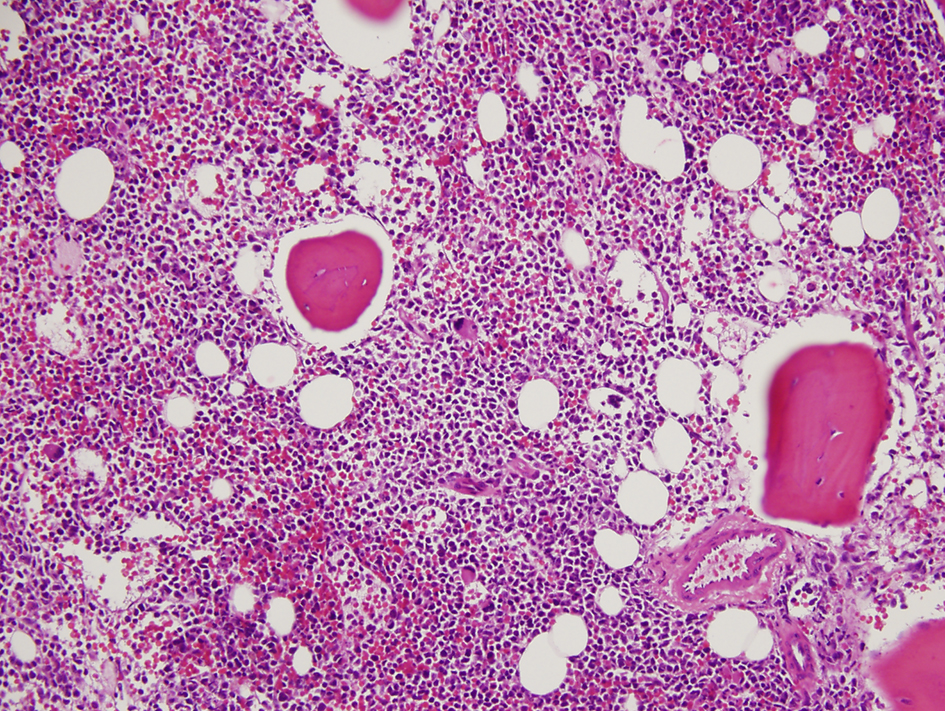 Click for large image | Figure 1. Hypercellular bone marrow with myeloblasts (× 20 magnification). |
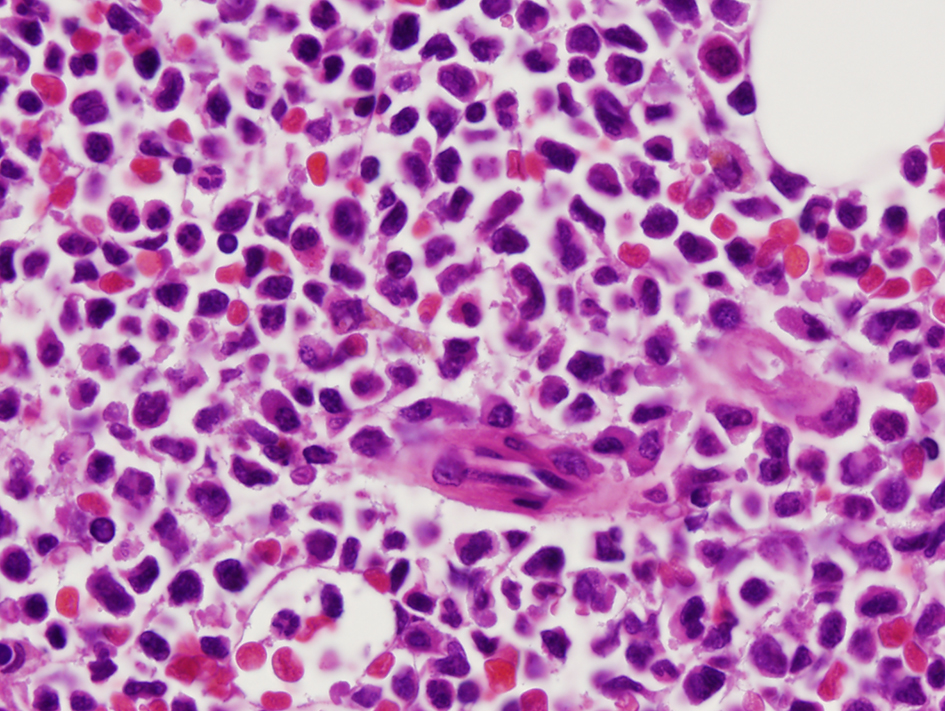 Click for large image | Figure 2. Myeloblasts with high nucleus to cytoplasm ratio (×100 magnification). |
After 16 days, a bone marrow biopsy was performed, which revealed a hypercellular marrow with 68% myeloblasts and 15% erythroblasts. Peripheral blood smear revealed leukopenia without circulating blasts. She subsequently underwent CLAG re-induction therapy, which consisted of 6 days of filgrastim (G-CSF) 300 mg and cladribine (5 mg/m2) for 5 days followed by one dose of cytarabine (2,000 mg/m2). Mitoxantrone was held due to her increased lifetime exposure to anthracyclines. A bone marrow biopsy performed after 30 days revealed normal maturation of all cellular components with 2.0% blast cells and a peripheral blood smear showed no circulating blast cells. FISH analysis no longer revealed rearrangements in the MLL gene. Complete remission of acute leukemia was achieved in this patient. She subsequently underwent an allogenic stem cell transplant without complications and was cured.
| Discussion | ▴Top |
The ATM gene is located on chromosome band 11q22.3 and belongs to a protein family known as the PI3K-protein-related protein kinases (PIKK). ATM has multiple functions through the p53 tumor-suppressor protein including recognition of damaged DNA, recruitment of repair proteins, repair of DNA regulation of transcription, and activation of apoptosis. Biallelic inactivation of the ATM gene causes ataxia-telangiectasia, a neurodegenerative disorder characterized by cerebellar ataxia, dysarthria, and 10-20% increased risk of cancers, including leukemias, lymphomas, and various carcinomas including breast, liver, and lung [2, 3]. Specifically, it has recently been estimated that ATM mutation heterozygosity is associated with breast cancer with relative risk of 2.37 (95% CI: 1.51 - 3.78) [4]. A variety of ATM germline variants have been documented in patients with a family history of breast and hematologic malignancy, especially T-cell acute lymphocytic leukemia (T-ALL) and prolymphocytic leukemia (T-PLL) [5]. For example, the p.Gly2023Arg mutation has been documented previously in T-PLL and is known to be a highly penetrant, deleterious missense mutation leading to a truncated protein [6]. However, p.Val410Ala, likely to be a missense variant as seen in this patient, is of unknown significance to date. More importantly, genes involved in DNA repair influence both a patient’s cancer susceptibility and response to chemotherapy treatment.
Most cases of AML occur de novo and approximately 50-60% patients present with chromosome abnormalities [6, 7]. Approximately 10-15% of AML cases arise after chemotherapy treatment and/or radiation for other primary malignancies [8]. Overall, patients exposed to previous chemotherapy for other malignancies are much more likely than the general population to develop leukemia [9]. Regarding breast cancer specifically, compared to breast cancer cases treated with surgery alone, the subset of breast cancer survivors treated with adjuvant chemotherapy and radiotherapy have an increased risk of developing myelodysplastic syndrome (MDS) or AML after 3 years [10]. Young age survivors, especially those aged 20 - 49, have been found to have the highest relative risk among all women [11]. While the overall risk is small when compared to the general population, MDS/AML is a serious condition that requires the patient’s attention, especially younger women.
Unfortunately, the overall prognosis of t-AML is poor. The median survival is less than 1 year and shorter than patients with de novo AML [1]. In t-AML, 85-90% patients have detectable chromosome abnormalities similar to AML de novo and oftentimes, these cytogenetic changes can be directed to previous exposure of chemotherapeutic agents with well-understood mechanisms of action [12-14]. In other studies, it has been reported that patients with t-AML tend to have more unfavorable cytogenetics more often compared to patients with de novo AML [1]. Deletions or loss of chromosome band 7q and abnormalities in chromosome 5 along with additional chromosome aberrations have been observed following therapy with alkylating agents in t-AML and generally carry a poor prognosis (Table 1) [12-16]. Mutations of the TP53 gene are very common in these pathways. Other mutations such as inv(16), chimeric rearrangement between the PML and the RARA genes, and rearrangement of the AMLI (CBFA) on chromosome 21q22 and CBFB at 16q22 have been observed in therapy related to topoisomerase II inhibitors, specifically anthracyclines, and carry varying prognosis [17-19]. Other regimens that include an anthracycline or cyclophosphamide carry an increased risk for MDS/AML [10]. Even though the risk is small, t-AML still warrants consideration when adjuvant therapy is required for breast cancer patients.
 Click to view | Table 1. Therapy-Related AML Caused by Previous Chemotherapy |
Unfavorable balanced translocations involving the KMT2A proto-oncogene on chromosome band 11q23, which codes for the mixed-lineage leukemia (MLL) enzyme, have been documented to be more frequent in t-AML than de novo AML, especially in woman younger than 60 years of age [20, 21]. The mixed-lineage leukemia enzyme is a histone methyltransferase that is critical for regulating gene expression during hematopoiesis. The fusion of MLL with other proteins results in oncogenic gene products that trigger faulty self-renewal of stem cells leading to leukemia. Cases of t-AML with MLL rearrangement are rarely preceded by t-MDS and often occur within 2 - 3 years of cytotoxic therapy, as in our case. Our patient had doxorubicin-induced balanced translocation, t(11;19)(q23;p13.1), which has only been previously observed in myeloid malignancies in adults over 40 years of age [20, 21]. Such abnormality in t-AML often presents with rapidly progressive leukemia that is usually more responsive to initial induction chemotherapy, but the prognosis is poor compared to those with t(9;11) mutations. According to a study conducted on chromosome band 11q23 abnormalities in hematological malignancies, 50% of patients t(11;19)(q23;p13.1) died within 2 years of diagnosis [22]. Allogeneic bone marrow transplantation (BMT) may be an option. In a long-term study of 70 patients who underwent BMT for t-MDS or t-AML, the estimated mortality rate within 2 years was 49% (95% CI: 36-62%) [23]. Currently, there is limited evidence, and more studies are required to evaluate the success rate of allogeneic HCT to cure t-AML.
Induction of tumor death by radiation and chemotherapy can cause DNA double-strand breaks (DSBs), leading to genetic arrangements in patients with mutations in genes for the DNA repair process, such as the ATM gene, that are difficult to repair. Recently, ATM abnormalities have been found in chronic lymphocytic leukemia (CLL) de novo [24]. In a previous study, it was reported that ATM mutations were detected in 16% patients, and all mutated CLL cells demonstrated ATM defects (impaired regulation in the p53 pathway) after doxorubicin exposure or radiation exposure. ATM mutations were detected in patients with 11q deletion after doxorubicin treatment, further demonstrating that ATM dysfunction may have a functional impact on other genes [24]. Hindered repair events in MLL translocations may result in a full spectrum of oncogenic events commonly identified in leukemic samples such as tandem duplications, deletions, inversions and insertions [25]. Since ATM is normally responsible for activating the p53 protein and up-regulating genes involved in apoptosis and repairing double-strand breaks, mutations in the ATM gene, as seen in our patient, increase the risk for cancers with gene rearrangements. If we do not use topoisomerase II inhibitors, DSBs may be repaired and chromosomal rearrangements may be suppressed. Thus, we raise the question as to whether certain chemotherapeutic agents such as topoisomerase II inhibitors should be avoided in patients with mutations in the ATM gene.
In conclusion, topoisomerase II inhibitors, anthracyclines, and alkylating agents have been linked to a complex framework of cytogenetic abnormalities and genetic mutations characteristic of therapy-related leukemia. The effectiveness of adjuvant chemotherapy for breast cancer should not be overlooked, as the benefit often outweighs the risk of developing t-AML. Nevertheless, with the increased use of adjuvant chemotherapy in the treatment of breast cancer, it is essential for patients to understand the potential risks of secondary malignancies, especially younger patients with documented mutations in repair genes that may confer a higher risk. While more studies are needed to illustrate this hypothesis, and standard treatment protocol frequently yields favorable outcomes for patients, additional evaluation for patients undergoing cytotoxic therapies for breast cancer may be warranted.
Consent
Consent was obtained.
Funding Source
No funding was secured for this study.
Financial Disclosure
The authors have indicated they have no financial relationships relevant to this article to disclose.
Conflicts of Interest
The authors have indicated they have no potential conflicts of interest to disclose.
Author Contributions
Shieh and Mohamed drafted the initial manuscript, revised the manuscript, and approved the final manuscript as submitted.
| References | ▴Top |
- Schoch C, Kern W, Schnittger S, Hiddemann W, Haferlach T. Karyotype is an independent prognostic parameter in therapy-related acute myeloid leukemia (t-AML): an analysis of 93 patients with t-AML in comparison to 1091 patients with de novo AML. Leukemia. 2004;18(1):120-125.
doi pubmed - Suarez F, Mahlaoui N, Canioni D, Andriamanga C, Dubois d'Enghien C, Brousse N, Jais JP, et al. Incidence, presentation, and prognosis of malignancies in ataxia-telangiectasia: a report from the French national registry of primary immune deficiencies. J Clin Oncol. 2015;33(2):202-208.
doi pubmed - Thompson D, Duedal S, Kirner J, McGuffog L, Last J, Reiman A, Byrd P, et al. Cancer risks and mortality in heterozygous ATM mutation carriers. J Natl Cancer Inst. 2005;97(11):813-822.
doi pubmed - Renwick A, Thompson D, Seal S, Kelly P, Chagtai T, Ahmed M, North B, et al. ATM mutations that cause ataxia-telangiectasia are breast cancer susceptibility alleles. Nat Genet. 2006;38(8):873-875.
doi pubmed - Vorechovsky I, Luo L, Dyer MJ, Catovsky D, Amlot PL, Yaxley JC, Foroni L, et al. Clustering of missense mutations in the ataxia-telangiectasia gene in a sporadic T-cell leukaemia. Nat Genet. 1997;17(1):96-99.
doi - Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G, Sanz M, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89(6):2079-2088.
pubmed - Grimwade D, Walker H, Oliver F, Wheatley K, Harrison C, Harrison G, Rees J, et al. The importance of diagnostic cytogenetics on outcome in AML: analysis of 1,612 patients entered into the MRC AML 10 trial. The Medical Research Council Adult and Children's Leukaemia Working Parties. Blood. 1998;92(7):2322-2333.
pubmed - Brunning RD, Matutes E, Harris NL, Jaffe ES, Stein H, Vardiman JW, Stein H, et al. World Health Organization classification of tumours: pathology and genetics of tumours of haematopoietic and lymphoid tissues. IARC Press, Lyons, France. 2001:71-108.
- Morton LM, Dores GM, Tucker MA, Kim CJ, Onel K, Gilbert ES, Fraumeni JF, Jr., et al. Evolving risk of therapy-related acute myeloid leukemia following cancer chemotherapy among adults in the United States, 1975-2008. Blood. 2013;121(15):2996-3004.
doi - Calip GS, Malmgren JA, Lee WJ, Schwartz SM, Kaplan HG. Myelodysplastic syndrome and acute myeloid leukemia following adjuvant chemotherapy with and without granulocyte colony-stimulating factors for breast cancer. Breast Cancer Res Treat. 2015;154(1):133-143.
doi pubmed - Kaplan HG, Malmgren JA, Li CI, Calip GS. Age related risk of myelodysplastic syndrome and acute myeloid leukemia among breast cancer survivors. Breast Cancer Res Treat. 2013;142(3):629-636.
doi pubmed - Le Beau MM, Albain KS, Larson RA, Vardiman JW, Davis EM, Blough RR, Golomb HM, et al. Clinical and cytogenetic correlations in 63 patients with therapy-related myelodysplastic syndromes and acute nonlymphocytic leukemia: further evidence for characteristic abnormalities of chromosomes no. 5 and 7. Journal of Clinical Oncology. 1986;4(3):325-345.
doi pubmed - Pedersen-Bjergaard J, Andersen MT, Andersen MK. Genetic pathways in the pathogenesis of therapy-related myelodysplasia and acute myeloid leukemia. Hematology Am Soc Hematol Educ Program. 2007;2007:392-397.
doi pubmed - Johansson B, Mertens F, Heim S, Kristoffersson U, Mitelman F. Cytogenetics of secondary myelodysplasia (sMDS) and acute nonlymphocytic leukemia (sANLL). Eur J Haematol. 1991;47(1):17-27.
doi pubmed - Vardiman JW, Harris NL, Brunning RD. The World Health Organization (WHO) classification of the myeloid neoplasms. Blood. 2002;100(7):2292-2302.
doi pubmed - Arber DA, Slovak ML, Popplewell L, Bedell V, Ikle D, Rowley JD, International Workshop on Leukemia K, et al. Therapy-related acute myeloid leukemia/myelodysplasia with balanced 21q22 translocations. Am J Clin Pathol. 2002;117(2):306-313.
doi pubmed - Dissing M, Le Beau MM, Pedersen-Bjergaard J. Inversion of chromosome 16 and uncommon rearrangements of the CBFB and MYH11 genes in therapy-related acute myeloid leukemia: rare events related to DNA-topoisomerase II inhibitors? J Clin Oncol. 1998;16(5):1890-1896.
doi pubmed - Quesnel B, Kantarjian H, Bjergaard JP, Brault P, Estey E, Lai JL, Tilly H, et al. Therapy-related acute myeloid leukemia with t(8;21), inv(16), and t(8;16): a report on 25 cases and review of the literature. J Clin Oncol. 1993;11(12):2370-2379.
doi - Pedersen-Bjergaard J, Andersen MK, Johansson B. Balanced chromosome aberrations in leukemias following chemotherapy with DNA-topoisomerase II inhibitors. J Clin Oncol. 1998;16(5):1897-1898.
doi pubmed - Andersen MK, Christiansen DH, Kirchhoff M, Pedersen-Bjergaard J. Duplication or amplification of chromosome band 11q23, including the unrearranged MLL gene, is a recurrent abnormality in therapy-related MDS and AML, and is closely related to mutation of the TP53 gene and to previous therapy with alkylating agents. Genes Chromosomes Cancer. 2001;31(1):33-41.
doi pubmed - Schoch C, Schnittger S, Klaus M, Kern W, Hiddemann W, Haferlach T. AML with 11q23/MLL abnormalities as defined by the WHO classification: incidence, partner chromosomes, FAB subtype, age distribution, and prognostic impact in an unselected series of 1897 cytogenetically analyzed AML cases. Blood. 2003;102(7):2395-2402.
doi pubmed - Moorman AV, Hagemeijer A, Charrin C, Rieder H, Secker-Walker LM. The translocations, t(11;19)(q23;p13.1) and t(11;19)(q23;p13.3): a cytogenetic and clinical profile of 53 patients. European 11q23 Workshop participants. Leukemia. 1998;12(5):805-810.
doi pubmed - Yakoub-Agha I, de La Salmoniere P, Ribaud P, Sutton L, Wattel E, Kuentz M, Jouet JP, et al. Allogeneic bone marrow transplantation for therapy-related myelodysplastic syndrome and acute myeloid leukemia: a long-term study of 70 patients-report of the French society of bone marrow transplantation. J Clin Oncol. 2000;18(5):963-971.
doi pubmed - Navrkalova V, Sebejova L, Zemanova J, Kminkova J, Kubesova B, Malcikova J, Mraz M, et al. ATM mutations uniformly lead to ATM dysfunction in chronic lymphocytic leukemia: application of functional test using doxorubicin. Haematologica. 2013;98(7):1124-1131.
doi pubmed - Libura J, Slater DJ, Felix CA, Richardson C. Therapy-related acute myeloid leukemia-like MLL rearrangements are induced by etoposide in primary human CD34+ cells and remain stable after clonal expansion. Blood. 2005;105(5):2124-2131.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


