| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website http://www.thejh.org |
Case Report
Volume 1, Number 2-3, June 2012, pages 58-60
Compassionate Use of Sorafenib in Primary Resistant Severely Progressed FLT3-ITD Positive AML in a Young Woman - A Danish Case Report
Anders K. Vistisena, b, c, Maria Kallenbachb, Marianne T. Severinsenb
aDepartment of Microbiology, Aalborg Hospital, Aarhus University Hospital, Hobrovej 18-22, Postboks 365, 9100 Aalborg, Denmark
bDepartment of Hematology, Aalborg Hospital, Aarhus University Hospital, Hobrovej 18-22, Postboks 365, 9100 Aalborg, Denmark
cCorresponding author: Anders K. Vistisen
Manuscript accepted for publication June 11, 2012
Short title: Compassionate Use of Sorafenib
doi: https://doi.org/10.4021/jh30w
| Abstract | ▴Top |
Acute myelogenous leukemia is a heterogeneous group of malignant diseases with high mortality. A continuous effort is being made in the attempt to find and describe the effects of novel treatment options and in recent years targeted therapy towards specific abnormalities in the malignant cell has increased. In this case report we describe the effect of FLT3-ITD targeting in the compassionate treatment of a primary resistant severely progressed FLT3-ITD positive AML in a young woman.
Keywords: Acute myelogenous leukemia; FLT3-ITD; Sorafenib
| Introduction | ▴Top |
A wide range of different genetic abnormalities can be seen in acute myelogenous leukemia (AML). Diagnosis, classification and prognosis are made based on phenotype, cytogenetic abnormalities and molecular abnormalities [1]. Several changes in the genetics of the cancerous cells, including acquired internal tandem duplication mutations in the FMS-like tyrosine kinase-3 receptor gene (FLT3-ITD), have been shown to influence prognosis [2]. Wild type (wd) FLT3 is present in healthy bone marrow cells and when binding to its ligands the receptor dimerizes and autophosphorylates thereby activating three major downstream pathways (STAT5, RAS/MAPK and PI3K/AKT) responsible for cell differentiation [3]. 20-25 % of patients with AML have been shown to harbour the FLT3-ITD mutation. The mutation itself is an independent negative prognostic marker and high mutant/wt ratio has been shown to correlate to a significantly poorer prognosis [2, 4].
Sorafenib is a multikinase inhibitor currently registered for use in advanced renal cell carcinoma and disseminated hepatocellular carcinoma. Sorafenib has also been investigated in the setting of colorectal and breast carcinomas [5]. It acts through an inhibition of FLT3 activity but also through inhibition of vascular endothelial growth factor receptor (VEGFR)-2, VEGFR-3, platelet-derived growth factor receptor β and c-KIT [6].
In the setting of hematologic malignancy both compassionate off-label use of sorafenib and phase I clinical trials for refractory and relapsed AML have been conducted showing promising but temporary effect, especially in FLT3-ITD positive patients, however data is sparse [7-9].
Here we present the first Danish AML patient receiving compassionate treatment with sorafenib.
| Case Report | ▴Top |
A 38 year old woman was diagnosed with AML, Fab type M1 positive for FLT3-ITD mutation after initial symptoms with three weeks of fever, sweats, fatigue, loss of appetite and malaise. Initial blood leukocyte count was 241 x 109/L with 55% blasts in peripheral blood. Acute bone marrow aspirate showed 90% blasts. Cytogenetic examination showed no chromosomal abnormalities.
The patient was treated initially in AML 17 protocol, randomised to treatment with ADE (cytarabine, daunorubicin and etoposide) in two induction treatments resulting in complete remission. The patient declined randomization for treatment with the experimental FLT3 inhibitor lestaurtinib (CEP-701) due to intense nausea. Afterwards the patient received consolidation treatment with high dose cytarabine. After consolidation treatment bone marrow aspirate unfortunately showed relapse with 40-50% blasts in bone marrow with unchanged morphology and phenotype. There were at this time 25-30% blasts in peripheral blood.
Treatment of the relapse was initiated with FLAG IDA (fludarabine, cytarabine, idarubicin, and G-CSF) as well as the search for a matching donor. However, the treatment had no effect on the disease and soon after there was evidence of severe progression with 90% blasts in peripheral blood. The patient’s condition was worsening; she was unable to eat and could not leave her bed.
The disease was thus chemotherapy resistant and further curative chemotherapy treatment was considered futile and unethical. The patient wished to be at home during palliation and she was discharged from the hospital in poor condition. She regretted that she did not participate in the lestaurtinib (CEP-701) randomisation during treatment and asked for experimental treatment with sorafenib.
Compassionate treatment with the experimental FLT3 inhibitor sorafenib, at an oral dose of 400 mg daily, was therefore initiated. Base line values before treatment showed a leukocyte count of 36.9 x 109/L (reference 3.5 - 10.0 x 109/L) and lactate dehydrogenase of 1187 U/l (reference 105 - 205 U/l), these values had been rising fast and were correlating to the patient’s clinical presentation after failed relapse treatment. A substantial decline in these values was seen in response to this treatment (Fig. 1). The patient was again able to leave the bed, have social activities and eat.
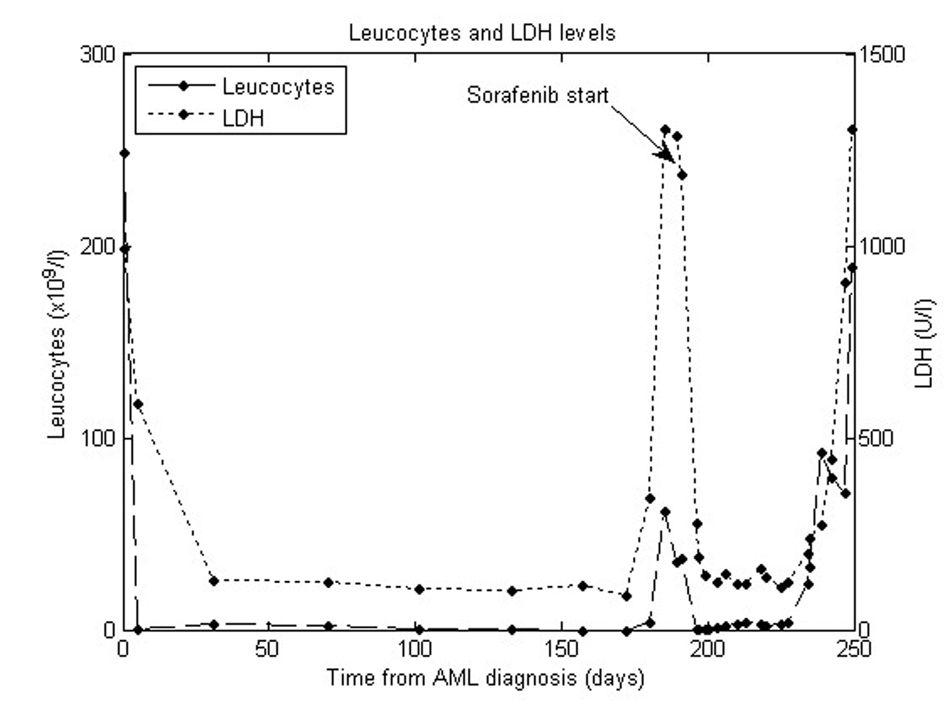 Click for large image | Figure 1. Leucocytes and LDH levels. |
Side effects unfortunately arose including pain, nose bleeds and anaemia resulting in a brief pause (6 days) of treatment with sorafenib and afterwards a reduced and better tolerated dose of 200 mg daily. Blood counts continued to be stable for six weeks but unfortunately there was finally an increase of the blood leukocyte count considered to represent a sorafenib resistant clone. Treatment with sorafenib was discontinued and monotherapy with hydroxyurea was instituted unfortunately without any substantial effect and the patient died in severely progressed leukaemia after approximately ten days discontinuation of sorafenib.
| Discussion | ▴Top |
This case demonstrated a transient but very clear effect of sorafenib monotherapy on severely progressed FLT3-ITD positive AML. The decision to treat this patient with sorafenib was made at a point of time where all other treatment options had been exhausted with the patient showing clear signs of imminent death due to severely progressed disease. We are convinced that the treatment gave the patient almost two extra months in good condition before she died, thereby gaining valuable time with her family.
The clinical impact of FLT3 inhibition in AML patients is not yet established. Our case report shows that FLT3 inhibition may be of value in the treatment of AML patients. Further studies will hopefully give more insight to the possible use of this group of drugs in the treatment of acute myelogenous leukemia.
Acknowledgments
We thank Simon Tilma Vistisen who helped making the graph.
| References | ▴Top |
- Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A, Harris NL, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114(5):937-951.
pubmed doi - Thiede C, Steudel C, Mohr B, Schaich M, Schakel U, Platzbecker U, Wermke M, et al. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: association with FAB subtypes and identification of subgroups with poor prognosis. Blood. 2002;99(12):4326-4335.
pubmed doi - Prescott H, Kantarjian H, Cortes J, Ravandi F. Emerging FMS-like tyrosine kinase 3 inhibitors for the treatment of acute myelogenous leukemia. Expert Opin Emerg Drugs. 2011;16(3):407-423.
pubmed doi - Frohling S, Schlenk RF, Breitruck J, Benner A, Kreitmeier S, Tobis K, Dohner H, et al. Prognostic significance of activating FLT3 mutations in younger adults (16 to 60 years) with acute myeloid leukemia and normal cytogenetics: a study of the AML Study Group Ulm. Blood. 2002;100(13):4372-4380.
pubmed doi - Wilhelm SM, Adnane L, Newell P, Villanueva A, Llovet JM, Lynch M. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol Cancer Ther. 2008;7(10):3129-3140.
pubmed doi - Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, Chen C, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64(19):7099-7109.
pubmed doi - Metzelder S, Wang Y, Wollmer E, Wanzel M, Teichler S, Chaturvedi A, Eilers M, et al. Compassionate use of sorafenib in FLT3-ITD-positive acute myeloid leukemia: sustained regression before and after allogeneic stem cell transplantation. Blood. 2009;113(26):6567-6571.
pubmed doi - Borthakur G, Kantarjian H, Ravandi F, Zhang W, Konopleva M, Wright JJ, Faderl S, et al. Phase I study of sorafenib in patients with refractory or relapsed acute leukemias. Haematologica. 2011;96(1):62-68.
pubmed doi - Pratz KW, Cho E, Levis MJ, Karp JE, Gore SD, McDevitt M, Stine A, et al. A pharmacodynamic study of sorafenib in patients with relapsed and refractory acute leukemias. Leukemia. 2010;24(8):1437-1444.
pubmed doi
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


