| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website http://www.thejh.org |
Case Report
Volume 5, Number 2, June 2016, pages 60-66
Should the Dose of Recombinant Activated Factor VII Be Adjusted for Morbidly Obese Patients? Balancing Risk of Bleeding With Thrombosis
Nick W. Lonardoa, c, Lynn F. Lonardob, Mary C. Moneb, Heidi J. Simonsa, Richard G. Bartonb
aDepartment of Pharmacy, University of Utah Health Sciences Center, Salt Lake City, UT, USA
bDepartment of Surgery, University of Utah Health Sciences Center, Salt Lake City, UT, USA
cCorresponding Author: Nick W. Lonardo, Pharmacy Department, University of Utah, 50 North Medical Drive, A050, Salt Lake City, UT 84132, USA
Manuscript accepted for publication March 25, 2016
Short title: Dosing Coagulation Factors for Obesity
doi: http://dx.doi.org/10.14740/jh256w
| Abstract | ▴Top |
This case report compared and contrasted calculations for dosing recombinant activated factor VII (rFVIIa) in a morbidly obese patient using estimation of blood volume rather than actual weight or ideal body weight (IBW). A specific case report in a morbidly obese, critically ill patient with new diagnosis of acquired hemophilia A (AHA) was presented. A 64-year-old morbidly obese patient with AHA required emergent surgery for perforated diverticulitis and peritonitis. The patient required bypass treatment with rFVIIa and was initially dosed based on actual weight of 167 kg (body mass index 61). The patient had a history of deep vein thrombosis and new onset of atrial fibrillation. Based on numerous risk factors for both bleeding and the potential for thromboembolism, the rFVIIa dose was adjusted based on calculations using adjusted body weight and blood volume principles, which decreased dosage by 40%, from 15 to 9 mg. With no active bleeding, rFVIIa dosage was adjusted and rotating thromboelastography testing was added to coagulation parameter monitoring. With this change in dosage, hemostasis was maintained and 132 mg of rFVIIa was saved over an 8-day treatment period. There is very limited literature to direct clinicians on rFVIIa dosing in morbidly obese patients. Since obesity is prevalent in all patient populations, clinicians should consider an estimation of blood volume as a means to dose rFVIIa. Adjusting the dose of rFVIIa to account for changes in blood volume is a physiologic target that should help balance the risk of underdosing using IBW and the risk of overdosing using actual body weight.
Keywords: Coagulation factors; Morbid obesity; Blood volume; Recombinant activated factor VII; Ideal body weight; Adjusted body weight; Body mass index
| Introduction | ▴Top |
Acquired hemophilia A (AHA) is a rare disease caused by the spontaneous development of autoantibodies to endogenous factor eight (FVIII) [1]. These autoantibodies neutralize the function of endogenous FVIII, resulting in acute bleeding episodes and the need for prompt hemostatic control [1]. Patients who develop AHA have been found to be at risk for more severe bleeding episodes than those with congenital hemophilia having comparable FVIII levels [1]. Due to the presence of the inhibitor autoantibodies, replacement with FVIII concentrate is generally not effective. The medical management approach of AHA is twofold: first is to control bleeding with factor eight inhibitor bypassing agent (FEIBA) or recombinant activated factor VII (rFVIIa) and second is to control inhibitor antibodies via plasmapheresis, steroids, cyclophosphamide or rituximab [1, 2].
In a critically ill patient with AHA, the risk for bleeding presents a challenge since hematologic product use may not guarantee hemostasis [2]. Normally, factor VIIa and FEIBA replacement is dosed on a per-kilogram basis of actual body weight and there is no mention of exceptions for patients with extreme weight or body mass index (BMI) [3, 4]. However, since blood volume and therefore plasma volume do not increase proportionately with the increase in fatty tissue, the dose calculation may better correlate to lean body mass [5, 6]. Use of actual body weight in morbidly obese patients may result in overdosing, which can potentially lead to unintended thrombosis in the way of pulmonary embolism or stroke.
Very limited literature exists to direct clinicians on clotting factors or bypass agent dosing in obese patients. Since obesity (defined as BMI ≥ 30) occurs in 35.1% of adults over age 20 in the United States [7], and 34.5% of adults in the hemophilia population [8], clinicians need dosing tools based on pharmacokinetic principles to help them optimize the dosing of these lifesaving medications. The need for judicious dosing is underscored in obese patients with a BMI ≥ 40 (grade 3), which now makes up 6.4% of the US population [7].
| Case Report | ▴Top |
A 64-year-old female was seen at an emergency department (ED) for significant bleeding from a small laceration. Relevant history includes idiopathic thrombocytopenic purpura 15 years earlier with splenectomy, morbid obesity, diet-controlled type II diabetes, essential hypertension, deep vein thrombosis (DVT), chronic kidney disease, hypothyroidism, and multiple antibiotic allergies. The ED obtained a normal prothrombin time (PT) and a partial thromboplastin time (PTT) > 100 s. Follow-up laboratories showed a PTT 240 s, PT 11.5 s, von Willebrand panel antigen 385% (activity level 334%) and factor VIII level < 1%. The patient was diagnosed with acquired factor VIII deficiency with inhibitors or AHA. Prednisone was started and a hematology referral was made to our tertiary academic center. Initial exam showed fading ecchymosis, Bethesda titer 2,400 units and flow cytometry negative for monoclonal population. Glucocorticoids were started, 1 mg/kg, with omeprazole for gastritis coverage. At 10 days, there was continued bruising and Bethesda titer 1,030 units. At 2 weeks, the patient was seen for urinary frequency, fatigue, and malaise. Bruising worsened with laboratories: WBC 12,130/mm3, platelets 104,000/mm3, glucose 798 mg/dL, sodium 133 mEq/L, and creatinine 1.84 mg/dL. The patient was admitted for hyperglycemia and possible urinary tract infection. The patient developed atrial fibrillation, which was treated with intravenous metoprolol. During the 5-day stay, the patient received rituximab (1,000 mg) and discharge medications included insulin, oral hypoglycemics, and a prednisone taper.
Symptoms of abdominal pain, vomiting, and spontaneous bleeding to trunk and extremities prompted a return to the hospital within 8 h of discharge. On arrival, the patient was tachycardic with atrial fibrillation and laboratories: WBC 1,720/mm3, platelets 78,000/mm3, and glucose 240 mg/dL. Abdominal CT scan showed free air and diffuse mesenteric stranding. Prior to operating, rFVIIa 15 mg was given (90 µg/kg) using admission body weight of 167 kg (BMI 61). An emergent sigmoid colectomy was performed, during which 8,176 units of FEIBA (50 units/kg), albumin (500 mL), and 2 units of platelets were given. Operative blood loss was estimated at 150 mL. Operative pathology confirmed acute diverticulitis with perforation.
The patient was admitted to the surgical intensive care unit (SICU) and started on rFVIIa 15 mg every 6 h. Broad spectrum antibiotics were started with ventilatory and cardiovascular support with norepinephrine. Over the next 24 h, the patient received five doses of rFVIIa (15 mg each) and FEIBA (2,555 units). On postoperative day 1, the patient developed atrial fibrillation, and was treated with intravenous amiodarone. Per the new AHA diagnosis and atrial fibrillation along with a calculated CHADS2 score of 2, the decision was made not to anticoagulate.
By postoperative day 2, the pharmacy team, in collaboration with SICU physicians and hematology service, discussed the relative and potential risk of thromboembolism for this patient who could not be anticoagulated. The possibility of basing the rFVIIa dosage on ideal body weight (IBW) or using an adjusted body weight with a correction factor was discussed. Literature was consulted, but nothing was found to guide rFVIIa dosing for morbid obesity. In addition, literature was sought to estimate changes in blood volume for the morbidly obese. An article by Lemmens et al, which estimated blood volume based on the patient’s BMI, was found and a proportional dose was calculated that was equal to a dose calculated using adjusted body weight with a correction factor of 0.4 [5]. Since the patient had no active bleeding, the decision was made to reduce rFVIIa dosage (Table 1) and follow with standard coagulation surveillance adding rotating thromboelastography (ROTEM).
 Click to view | Table 1. Calculations of rFVIIa Dosage Based on Pertinent Weight Values |
Over the ensuing SICU stay, the following coagulopathy treatment was delivered: 1,000 mg dose rituximab, 4 units platelets, 1 unit packed red blood cells, and 22 rFVIIa doses of 9 mg (Fig. 1). On postoperative day 3, the patient requested “do not resuscitate” status; on day 7, insisted on comfort care; and expired on day 8. Throughout admission, hemostasis was maintained and there were no thrombosis-related complications.
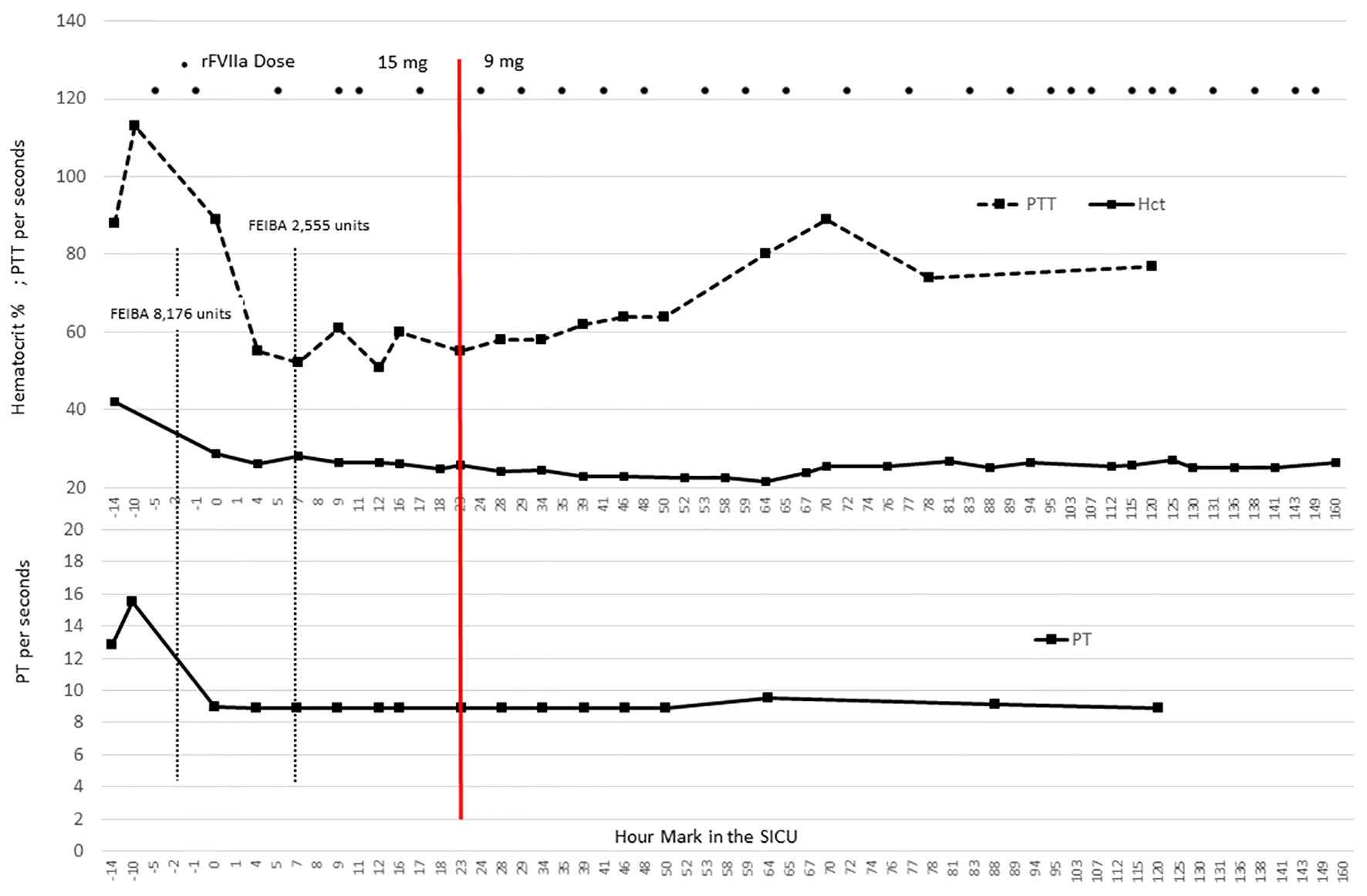 Click for large image | Figure 1. Coagulation parameters in relation to factor dosing. The graphic illustrates the coagulation parameters for this case in relation to the dose of FEIBA and rFVIIa. Notably, the partial thromboplastin time (PTT) remains elevated, but the prothrombin time (PT) specimens were < 10 s, suggesting the consistent presence of rFVIIa throughout the ICU stay [4]. The hematocrit remains stable and transfusion needs included 1 unit of packed red blood cells on postoperative day 3. The red line at 23 h designates the change in rFVIIa dose from 15 to 9 mg. |
| Discussion | ▴Top |
Recombinant Factor VIIa is a coagulation factor and, therefore, resides primarily in plasma with a very small volume of distribution of 0.108 L/kg [9]. Neither total blood volume nor plasma volume increases proportionately with total body weight [5, 6]. The standard preoperative indexed blood volume assessment for non-obese adult patients is 70 ± 5 mL/kg; however, this equation markedly overestimates total blood volume in morbidly obese patients [5]. Lemmens et al developed an equation that estimated indexed blood volume for morbidly obese patients based on BMI values [5]. In the present case, the blood volume using the standard estimate of 70 mL/kg was calculated to be 11,690 mL, but using Lemmens’ formula (Table 1), the corrected blood volume of 42 mL/kg was calculated to be 7,014 mL.
Using a simple proportion based on the corrected blood volume (15 mg × (42 mL/kg/70 mL/kg)), an adjusted rFVIIa dose of 9 mg would approximate a similar blood concentration, and therefore, closely approximate the same pharmacologic effects. In the present case, both the proportional calculation using the corrected blood volume and an adjusted body weight using a correction factor of 0.4 approximated a dose of 9 mg (Table 1). The recommended dose for rFVIIa when used as a bypass agent is 70 - 90 µg/kg every 2 - 3 h or until hemostasis is achieved [4]. Our patient achieved hemostasis quickly and the dosage interval was extended to every 6 h.
Although PTs have not been shown to directly predict bleeding outcomes, a shortening of the PT after administration of rFVIIa does suggest the presence of pharmacologic activity [4]. In the present case, we obtained standard coagulation parameters, as well as ROTEM testing (Fig. 1, Table 2). We are unaware of studies that have established ROTEM monitoring to assess the dosing of rFVIIa; however, because rFVIIa exerts influence on the extrinsic coagulation pathway, we speculated that persistently abnormal values in the Extem portion of the ROTEM analysis would imply inadequate rFVIIa dosing. As shown in Table 2, the initial Extem revealed a prolonged clotting time (CT) on postoperative day 1, but all subsequent Extem parameters were consistently in the therapeutic ranges, even though Intem parameters were reflective of an abnormal intrinsic coagulation pathway (i.e. acquired factor VIII deficiency). It is also notable that with the adjusted dose of rFVIIa, PT levels were maintained at < 0.9 - 0.95 s, suggesting a robust presence of pharmacologic activity from rFVIIa (Fig. 1).
 Click to view | Table 2. ROTEM With Highlighted Bolded Cells Designating Abnormal Values |
The proportional calculation we employed highlights several important points. Firstly, the blood volume calculation using the standard 70 mL/kg for non-obese patients results in a 40% larger blood volume than the blood volume corrected for BMI. Secondly, the reduced dose of 9 mg should achieve the same concentration and the same pharmacologic effect if the blood volume estimate is accurate. Thirdly, if the uncorrected dose of 15 mg were used, and the blood volume was 40% lower, this would result in a 40% higher rFVIIa concentration, potentially increasing the risk for thrombosis. Of note, we have not discussed the effect of morbid obesity on change in elimination of rFVIIa. Using pharmacokinetic principles, large volumes of distribution with no change in clearance prolong the elimination half-life and allow longer dosing intervals to achieve the same concentration [10]. Lastly, using IBW for the rFVIIa calculation results in a dose of only 5 mg. This calculation may significantly underestimate the blood volume contribution of morbid obesity, resulting in significant underdosing.
This case presents several important and difficult therapeutic dilemmas with multiple complicating factors. This case involves the diagnosis of recent AHA with inhibitors and a high Bethesda titer, which eliminated the option for factor VIII concentrate. Secondly, initial treatment consisted of rituximab and prednisone, but the patient suffered from significant hyperglycemia and an abdominal catastrophe and sepsis, complicated by cardiac arrhythmias. Combining a history of DVT, age, morbid obesity, and recent abdominal surgery, the risk for thromboembolism was very high. Because of the combined risks for bleeding and thrombosis, it was crucial that the bypass treatment be carefully dosed and monitored closely.
Over the last two decades, there has been a large influx of Food & Drug Administration-approved biological products, but there is still no requirement for manufacturers to perform pharmacokinetic studies in the obese population [11]. With the rate of obesity in the hemophilia population paralleling the general population [8], this is an important issue for all treating clinicians. Most biological products, by nature of their large molecular size and relatively small volume of distribution, reside primarily in blood and plasma. There is a deficit of current standards to assist clinicians in dosing of medications like coagulation factors in obese patients [11]. Given that neither blood nor plasma volume increases proportionately with total body weight, we believe a proportional blood volume calculation using Lemmens et al’s formula [5] is a reasonable approach to effectively dose coagulation factors in morbidly obese patients (Fig. 2).
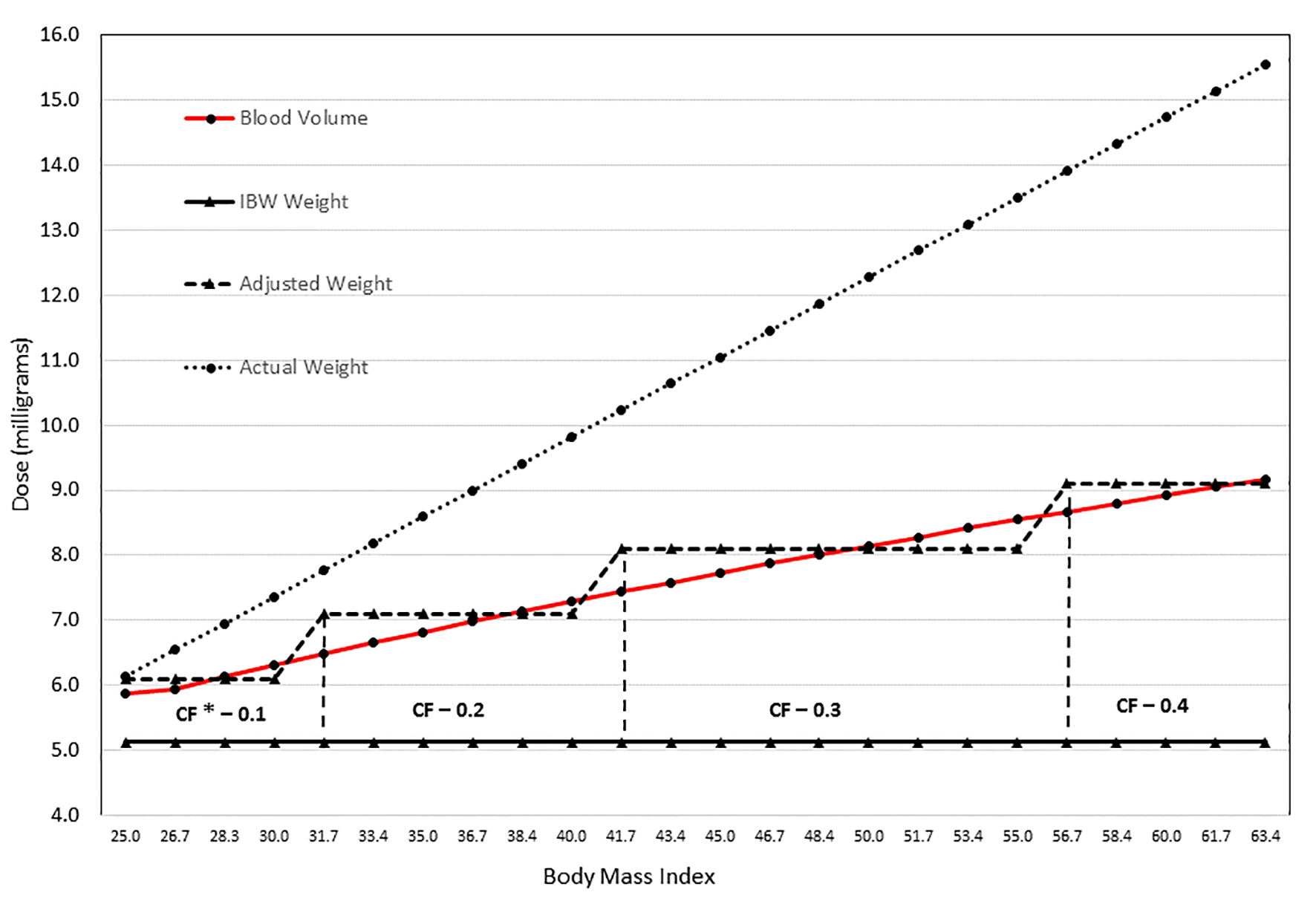 Click for large image | Figure 2. Simulation illustrating dosing (mg) with varying BMI using blood volume, IBW, adjusted body weight with CF, and actual weight. The graphic illustrates the significant variability in dose depending on the weight used to calculate the rFVIIa dose. The values were calculated based on a theoretical case: female of 66 inches in height with increasing weight as noted with BMI values ranging from 25 to 63 kg/m2. Weight increase for each increment was 10 pounds. The graph highlights the substantial difference between using the actual body weight and ideal body weight (IBW). Because blood volume increases with increasing BMI, IBW may result in underdosing a morbidly obese patient. Using an estimation of blood volume to calculate the rFVIIa dose still results in a significant difference compared to actual body weight; however, the dose increases gradually with increasing weight, as does the blood volume. Adjusted body weight was included in the graphic, but it should be noted that the correction factor was calculated based on the percent change in blood volume. *Correction factor. |
In addition to those patients with coagulopathies being treated in an ICU setting, there are other critical care populations for which rFVIIa is being used, which primarily include trauma and cardiac surgery patients. The data examining dosing and the risks and complications reported, particularly thromboembolic, are conflicting and often call for more research into causality [12-17].
Conclusion
In summary, we achieved successful hemostasis in a morbidly obese, ICU patient using an adjusted body weight with a 0.4 correction factor, which correlated precisely with a proportional dose based on a corrected blood volume. In addition to establishing a safe and effective dosing regimen, a total of 132 mg of rFVIIa was saved. Considering that rFVIIa costs $1.53±20% per/µg, the result led to significant cost savings of approximately $200,000 [18]. In the current climate of medication shortages and increasing healthcare costs, this case report serves to highlight the need to be mindful of resource allocation and conservation when able. We caution clinicians that this by no means suggests that treatment should be changed based on this one example. However, given the increased rate of obesity, manufacturers should investigate the effect of obesity on the pharmacokinetics and safety of drug dosing [11]. We hope this case will encourage critical care and hematologic disciplines to address dosing standards in the obese population based on pharmacokinetic principles.
Financial Support
None.
Conflicts of Interest
None.
| References | ▴Top |
- Kessler CM, Knobl P. Acquired haemophilia: an overview for clinical practice. Eur J Haematol. 2015;95(Suppl 81):36-44.
doi pubmed - Hay CR, Brown S, Collins PW, Keeling DM, Liesner R. The diagnosis and management of factor VIII and IX inhibitors: a guideline from the United Kingdom Haemophilia Centre Doctors Organisation. Br J Haematol. 2006;133(6):591-605.
doi pubmed - FEIBA(R) [package insert]. Baxter Healthcare Corporation, Westlake Village, CA; November 2013. [3]
- NovoSeven(R) [package insert]. Novo Nordisk Pharmaceuticals, Inc., Princeton, NJ; September, 1999. http://factorviia.com/pi.pdf. [accessed November 15, 2015].
- Lemmens HJ, Bernstein DP, Brodsky JB. Estimating blood volume in obese and morbidly obese patients. Obes Surg. 2006;16(6):773-776.
doi pubmed - Graham A, Jaworski K. Pharmacokinetic analysis of anti-hemophilic factor in the obese patient. Haemophilia. 2014;20(2):226-229.
doi pubmed - Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA. 2014;311(8):806-814.
doi pubmed - Wong TE, Majumdar S, Adams E, Bergman S, Damiano ML, Deutsche J, Recht M. Overweight and obesity in hemophilia: a systematic review of the literature. Am J Prev Med. 2011;41(6 Suppl 4):S369-375.
doi pubmed - Lindley CM, Sawyer WT, Macik BG, Lusher J, Harrison JF, Baird-Cox K, Birch K, et al. Pharmacokinetics and pharmacodynamics of recombinant factor VIIa. Clin Pharmacol Ther. 1994;55(6):638-648.
doi pubmed - Winter ME. Basic Clinical Pharmacokinetics, 3rd ed. Vancouver: Applied Therapeutics, Inc.; 1994.
- American Society Health-System Pharmacists Policy Positions 1982-2015: Research on Drug Use in Obese Patients; Policy 1515.23-24. http://www.ashp.org/DocLibrary/BestPractices/ASHP-Policy-Positions-2015.pdf. [accessed December 2, 2015].
- Tritapepe L, De Santis V, Vitale D, Nencini C, Pellegrini F, Landoni G, Toscano F, et al. Recombinant activated factor VII for refractory bleeding after acute aortic dissection surgery: a propensity score analysis. Crit Care Med. 2007;35(7):1685-1690.
doi pubmed - von Heymann C, Redlich U, Jain U, Kastrup M, Schroeder T, Sander M, Grosse J, et al. Recombinant activated factor VII for refractory bleeding after cardiac surgery--a retrospective analysis of safety and efficacy. Crit Care Med. 2005;33(10):2241-2246.
doi pubmed - Gill R, Herbertson M, Vuylsteke A, Olsen PS, von Heymann C, Mythen M, Sellke F, et al. Safety and efficacy of recombinant activated factor VII: a randomized placebo-controlled trial in the setting of bleeding after cardiac surgery. Circulation. 2009;120(1):21-27.
doi pubmed - Mohr AM, Holcomb JB, Dutton RP, Duranteau J. Recombinant activated factor VIIa and hemostasis in critical care: a focus on trauma. Crit Care. 2005;9(Suppl 5):S37-42.
doi pubmed - Thomas GO, Dutton RP, Hemlock B, Stein DM, Hyder M, Shere-Wolfe R, Hess JR, et al. Thromboembolic complications associated with factor VIIa administration. J Trauma. 2007;62(3):564-569.
doi pubmed - O'Connell KA, Wood JJ, Wise RP, Lozier JN, Braun MM. Thromboembolic adverse events after use of recombinant human coagulation factor VIIa. JAMA. 2006;295(3):293-298.
doi pubmed - Earnshaw SR, Graham CN, McDade CL, Spears JB, Kessler CM. Factor VIII alloantibody inhibitors: cost analysis of immune tolerance induction vs. prophylaxis and on-demand with bypass treatment. Haemophilia. 2015;21(3):310-319.
doi pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


