| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website http://www.thejh.org |
Original Article
Volume 5, Number 1, March 2016, pages 8-16
Associations Between Initial Presentation of Multiple Myeloma and Renal Function: the Experience of Two European Centers
Francesco Rainonea, Sayyid M. Ammar Razab, c, James Ritchieb, Lino Merlinoa, Helen Aldersonb, Diana Chiub, Mark Guyb, Magda Marcattia, Philip Kalrab
aNephrology, Dialysis and Hypertension, San Raffaele Hospital, Milan, Italy
bVascular Research Group, Department of Renal Medicine, Salford Royal Foundation Trust, UK
cCorresponding Author: S. M. Ammar Raza, Renal Department, Salford Royal Hospital, England, UK
Manuscript accepted for publication November 12, 2015
Short title: MM and Renal Function
doi: http://dx.doi.org/10.14740/jh229w
| Abstract | ▴Top |
Background: This study aimed to describe the baseline renal, histopathological and hematological characteristics and any clinical or biochemical associations of patients with a first coded diagnosis of multiple myeloma (MM). The incidence of renal replacement therapy (RRT) and association with mortality were also investigated.
Methods: A retrospective case review was performed to identify 287 MM patients from two European centers. Statistical analyses were performed using SPSS version 2.0 and SAS version 9.2.
Results: MM patients referred to renal centers were more likely to be elderly and male. The most common form of renal impairment was acute kidney injury (AKI). The most common paraprotein-associated lesions were myeloma cast nephropathy (MCN, 51%), light chain deposition disease (17%) and AL-amyloidosis (9.4%). MM with AKI was found to be a more aggressive disease, being associated with worse hematological features and increased risk of short-term death. Of the AKI patients requiring RRT, 80% required it at presentation. There was no increased risk of death in the RRT requiring vs. non-RRT requiring cohort. Monoclonal gammopathy of undetermined significance (MGUS) may predispose to renal damage and may increase likelihood of AKI in context of MM.
Conclusion: MM-related renal failure is a medical emergency with the need for rapid diagnosis and prompt supportive care, RRT and MM-directed therapy.
Keywords: Multiple myeloma; Acute kidney injury; Myeloma complications; Myeloma pathology
| Introduction | ▴Top |
Plasma cell dyscrasias are characterized by the clonal expansion of abnormal plasma cells. Disease severity ranges from the “benign” monoclonal gammopathy of undetermined significance (MGUS) to multiple myeloma (MM), and finally plasma cell leukemia [1]. In addition to representing a spectrum of disease severity, it is likely that these different diagnoses are representative of different stages in the natural history of plasma cell disease. MM is thought to be an evolution of MGUS [2, 3], with approximately 1% of cases converting annually [4].
MM is of greatest interest to nephrologists due to its frequent associations with hypercalcemia and renal impairment [5]. Renal damage is believed to be caused by serum free light chains (FLCs), proteins that can cause direct tubular damage [6-8]. Recognized patterns of tubulointerstitial injury in MM include isolated proximal tubule epithelial cell cytotoxicity, tubulointerstitial nephritis and cast nephropathy [9, 10]. However, the pathogenesis of kidney injury in MM is not completely defined, as although the risk of acute kidney injury (AKI) in patients with MM is increased when FLC proteinuria reaches > 500 mg/L [7], some patients never develop kidney disease, despite high FLC urine concentrations.
AKI in MM: causes, clinical relevance and potential early detecton
AKI is common in patients with MM. It constitutes an important cause of morbidity and mortality leading to interruptions in therapy, increased hospitalization stay and costs [11]. More importantly, AKI confers a higher risk for earlier death in myelomatous patients [12]. A number of factors appear associated with reversible acute tubular necrosis in patients with MM including dehydration, hypercalcemia, nephrotoxic drugs, diuretics, contrast medium usage and infection [7]. Even a minor reduction in kidney function is linked to adverse prognosis [13].
Early detection and treatment of AKI is crucial in order to improve patients’ outcomes [12]. For this reason, in 2012, the Kidney Disease: Improving Global Outcomes (KDIGO) AKI Guideline Work Group proposed a definition of AKI [14] aimed for practice, research and public health use that could help physicians detect AKI early, thereby helping to prevent serious complications.
Renal failure in MM has been correlated with FLCs, high serum lactate dehydrogenase (LDH), hypercalcemia, anemia, multiple bone lesions, and extensive marrow plasmacytosis [15]. Nevertheless, no previous literature has evaluated the clinical and histopathological characteristics of patients presenting with either AKI (diagnosed using current KDIGO guidelines) as compared with normal renal function at the time of the first myeloma diagnosis.
Aims
This study aims to describe 1) the baseline renal, hematologic and histopathological characteristics of patients with a first coded diagnosis of MM at two European renal centers; 2) clinical and biochemical factors associated with presentation with AKI; and 3) incidence of renal replacement therapy (RRT) and association with mortality.
| Materials and Methods | ▴Top |
Study population and follow-up
A retrospective case review was performed to identify patients from two European renal centers, Salford Royal NHS Foundation Trust, Manchester, UK and San Raffaele Hospital, Milan, Italy. All cases of newly diagnosed MM identified between inception of electronic health records (January 2002 Salford, January 2009 San Raffaele) and December 2012 were included. Patients were defined as having MM where all three of the following conditions were met: 1) > 10% monoclonal plasma cells in bone marrow; 2) monoclonal protein identified in serum and/or urine; 3) presence of one or more MM-related organ dysfunction, including hypercalcemia, anemia, lytic bone lesions, or kidney biopsy diagnosis of a paraprotein-associated lesion (myeloma cast nephropathy (MCN), amyloidosis or light chain deposition disease (LCDD)) [ 16].
Patients were followed until death or their most recent clinical follow-up prior to December 2012.
Clinical data
Baseline demographic (age and gender), comorbidity (diabetes, previous malignancy, macrovascular disease, and pre-existing renal disease), and laboratory (estimated glomerular filtration rate (eGFR), hemoglobin, proteinuria, and FLC) data were collected from patients’ electronic medical records and direct contact with referring physicians (Table 1). Pre-existing chronic kidney disease (CKD) was defined as the presence of renal insufficiency at the time of MM diagnosis (eGFR < 60 mL/min/1.73 m2) in association with one or more of the following: documentation of previously impaired renal function during a period of at least 4 months, small kidneys at ultrasound scan, histological evidence of major glomerular/tubular atrophy, and interstitial fibrosis.
 Click to view | Table 1. Demographic, Renal and Hematological Features |
The presence of AKI at the time of MM diagnosis was defined and staged according to KDIGO guidelines, there being evidence of previously documented normal renal function during a period of at least 4 months [14].
We used criteria based on eGFR measurements, proposed by Dimopoulos et al, for the definition of the reversibility of renal impairment [15].
Histology
Histological data were only available within the UK subgroup. Records were reviewed to identify all patients in whom renal biopsy had been performed, with detailed reports obtained from local consultant histopathologists. Standard processing of kidney biopsy specimens included light microscopy and immunofluorescence. Electron microscopy was not performed routinely. For light microscopy, all cases were stained with hematoxylin and eosin, periodic acid-Schiff and Masson trichrome. For immunofluorescence, 3-μ stained cryostat sections were stained with polyclonal fluorescein isothiocyanate-conjugated antibodies to IgG, IgM, IgA, C3, C1q, κ and λ light chains. Tubular atrophy and interstitial fibrosis were graded on a semi-quantitative scale based on the estimated percentage of renal cortex affected, and recorded as 0 (none), 1-25% (mild), 26-50% (moderate), or > 50% (severe).
Statistical analysis
Parametric continuous variables are expressed as mean ± standard deviation and non-parametric data as median (interquartile range). Categorical variables are expressed as number (percentage). Between group comparisons for continuous variables were made using ANOVA methodology appropriate to the distribution with categorical variables compared using χ2 test. Associations with RRT were assessed using logistic regression. Survival analysis was performed using Cox proportional hazards. Univariate analysis was performed on all baseline parameters, and variables with a plausible relationship to death and an alpha value of ≤ 0.1 in univariate analysis entered into a stepwise multivariate model. Survival curves were plotted using Kaplan-Meier analysis. In all cases, time zero was defined as date of diagnosis of MM. Unless otherwise stated, statistical significance was defined as P < 0.05.
Analyses were performed using SPSS version 20.0 (SPSS Inc, Chicago, IL) and SAS version 9.2 (SAS Institute, Cary, NC) under license to the University of Manchester.
| Results | ▴Top |
Demographics
A total of 287 cases were identified (218 from UK and 69 from Italy), with clinical and follow-up data available for all patients. The median observation period was 2.8 years (IQR 0.9 - 6.2) for the English cohort and 1.3 years (IQR 0.2 - 3.1) for the Italian cohort. At time of diagnosis, mean age was 66.3 ± 12.2 years, with 36% of patients aged > 70 years. The study cohort was almost entirely Caucasian and had a male preponderance (55.4% vs. 44.6%). The majority of patients (81%) were diagnosed with MM in a hematology unit or clinics, with 19% of patients diagnosed after admission to a renal unit for investigation of renal impairment.
When comparing the demographics of the two cohorts, there was a similar age and gender distribution, but the prevalence of comorbidities differed in the two groups. The Italian cohort had a higher prevalence of hypertension (54.2% vs. 37.2%, P = 0.052), previous history of cancer (20% vs. 6.9%, P = 0.038) and previous detection of MGUS (42% vs. 7%, P = 0.000). No differences were found with respect to diabetes, ischemic heart disease and atrial fibrillation prevalence (Table 2).
 Click to view | Table 2. Comparison Between the Two European Centers |
AKI vs. no renal impairment (NRI)
The AKI and NRI groups differed with respect to the mean age at presentation, with the AKI group being older than the NRI (67.03 ± 10.52 vs. 63.82 ± 13.06 years, P = 0.0438). No difference was found in terms of sex distribution, previous diagnosis of cardiovascular events, diabetes mellitus, hypertension, atrial fibrillation, MGUS, or previous cancer (Table 1). The subgroup of UK patients with MM-related AKI at presentation showed a significantly higher prevalence of MGUS in their past medical history than the NRI group (8.8% vs. 0.9%, P = 0.01). This finding was not replicated in the Italian cohort.
Renal and hematologic characteristics at baseline
At MM baseline presentation, 128 patients (44.6%) presented with normal renal function (i.e., eGFR ≥ 60 mL/min/1.73 m2). Renal impairment was present in 159 patients (55.4%), of whom 40 (25%) had a known history of CKD but no AKI, 105 (66%) presented with AKI in the setting of previously normal renal function, and 14 (9%) presented with AKI in the setting of known CKD. For subsequent analyses, comparison was made between patients presenting with AKI and patients presenting with NRI.
As expected, at baseline the AKI group had a lower eGFR (23.2 ± 21.3 vs. 85.5 ± 13.4 mL/min/1.73 m2, P < 0.001), higher urea (20.5 ± 13.8 vs. 6.1 ± 2.6 mmol/L, P < 0.001) and higher potassium (4.4 ± 0.7 vs. 4.2 ± 0.4 mmol/L, P = 0.002) than the NRI group (Table 1). Furthermore, the AKI cohort had higher serum calcium (2.54 ± 0.4 vs. 2.3 ± 0.2 mmol/L, P = 0.006), phosphate (1.6 ± 0.7 vs. 1.1 ± 0.4 mmol/L, P < 0.001) and proteinuria (1.9 ± 1.8 vs. 0.4 ± 0.6 g/24 h, P < 0.001) than the NRI group. On the contrary, AKI subjects presented with lower serum sodium (137.0 ± 4.7 vs. 138.4 ± 3.8 mmol/L, P = 0.01) and albumin (34.6 ± 7.7 vs. 38.7 ± 6.2 g/L, P < 0.001) concentrations.
At time of diagnosis, the AKI group had a higher κ/λ ratio (385 ± 962.7 vs. 36.1 ± 115.6, P = 0.02), a higher white blood cell count (7.9 ± 3.5 vs. 6.5 ± 2.7 × 109/L, P = 0.004), a higher percentage of plasma cells in the bone marrow (35.3 ± 26.5% vs. 25.1 ± 20.8%, P = 0.01) and lower hemoglobin (94.54 ± 17.5 vs. 118.98 ± 19.9 g/L, P < 0.001).
Histopathology data
Renal biopsy was performed in 54 patients presenting with AKI, representing 19% of the overall cohort and 51% of the AKI cohort. The median age of the biopsied patients was 68.0 (range 38 - 85) years, the mean eGFR was 16.9 ± 19.9 mL/min/1.73 m2 and 55% were male. Indications for kidney biopsy were AKI in 36 (68%) patients, acute on CKD in eight (15%) patients, chronic/progressive decrease in eGFR in six (11.3%) patients, nephrotic proteinuria without a decrease in eGFR in three (5.7%) patients.
Renal biopsy showed paraprotein-associated lesions in 77% of patients and non-paraprotein-associated lesions in 23%. The most common paraprotein-associated lesions were MCN (51%), LCDD (17%) and AL-amyloidosis (9.4%). The most common non-paraprotein-associated lesions were chronic tubulointerstitial nephritis (9.4%), acute tubular necrosis (3.8%), and focal segmental glomerulosclerosis (3.8%). We found one case of type 1 MM-associated cryoglobulinemic glomerulonephritis and one case in which diabetic glomerulosclerosis and MCN co-existed. Although not statistically significant, 24 h proteinuria was higher in amyloidosis patients than in MCD or LCDD (4.6 ± 2.9 vs. 2.3 ± 1.8 vs. 2.6 ± 2.1 g/24 h, respectively, P = 0.08). The percentage of globally sclerotic glomeruli did not significantly differ between MCN (8.6%), amyloidosis (11.4%) and LCDD (17.8%) (P = 0.403).
Clinical and pathological correlation
Table 3 describes the patients’ clinical and laboratory features according to the three most common paraprotein-related biopsy findings (MCN, LCDD, and AL-amyloidosis). The age (P = 0.1) and sex representation (P = 0.66) of the three subgroups was comparable. Patients with a diagnosis of MCN had the lowest eGFR, hemoglobin and platelets. The total plasma proteins and the percentage of plasma cells in the bone marrow were higher in patients with MCN.
 Click to view | Table 3. Clinical and Laboratory Features According to the Renal Pathologic Diagnosis |
Biopsied patients with MCN were more likely to have presented with AKI than the LCDD and AL-amyloid groups (69.2% vs. 18% and 5.1%, respectively, P = 0.0001). The percentage of tubular atrophy and interstitial fibrosis did not significantly differ between MCN, LCDD and AL-amyloidosis (data not shown).
Outcomes
RRT requirement
During the study period, 113 patients (39.3%) reached end-stage kidney disease (ESKD, eGFR < 15 mL/min/1.73 m2). RRT was initiated in 56 of these patients (49.6%). The main indications for commencing RRT were anuria and fluid overload, severe electrolyte abnormalities, and uremic symptoms. UK patients received RRT either in the form of hemodialysis (70.4%) or peritoneal dialysis (29.6%), whereas Italian uremic patients only received hemodialysis as acute peritoneal dialysis was not an option. Of these patients, 88.5% belonged to the AKI group, whereas 11.5% belonged to the NRI group and needed RRT during the course of the disease. Of patients, 80% who required RRT needed it within 7 days from presentation (with a mean for acute necessity of 4.3 ± 5.8 days). In univariate analysis, the variables associated with the need for RRT in the UK cohort were previous MGUS (OR: 6, 95% CI: 1.4 - 26.1, P = 0.017), lower levels of albumin (OR: 0.6, 95% CI: 0.5 - 0.8, P = 0.0001), lower levels of hemoglobin (OR: 0.7, 95% CI: 0.7 - 0.8, P < 0.001) and a higher white blood cell count (OR: 2.6, 95% CI: 1.5 - 4.4, P = 0.0006). Another associated factor with the requirement for RRT was the need for a kidney biopsy (OR: 12.3, 95% CI: 5.1 - 9.6, P < 0.001).
Of the 41 patients with paraprotein-related biopsy findings according to the renal pathological diagnosis (MCN, LCDD, and AL-amyloidosis), there was no significant requirement for RRT or difference in mortality in any particular cohort (Fig. 1).
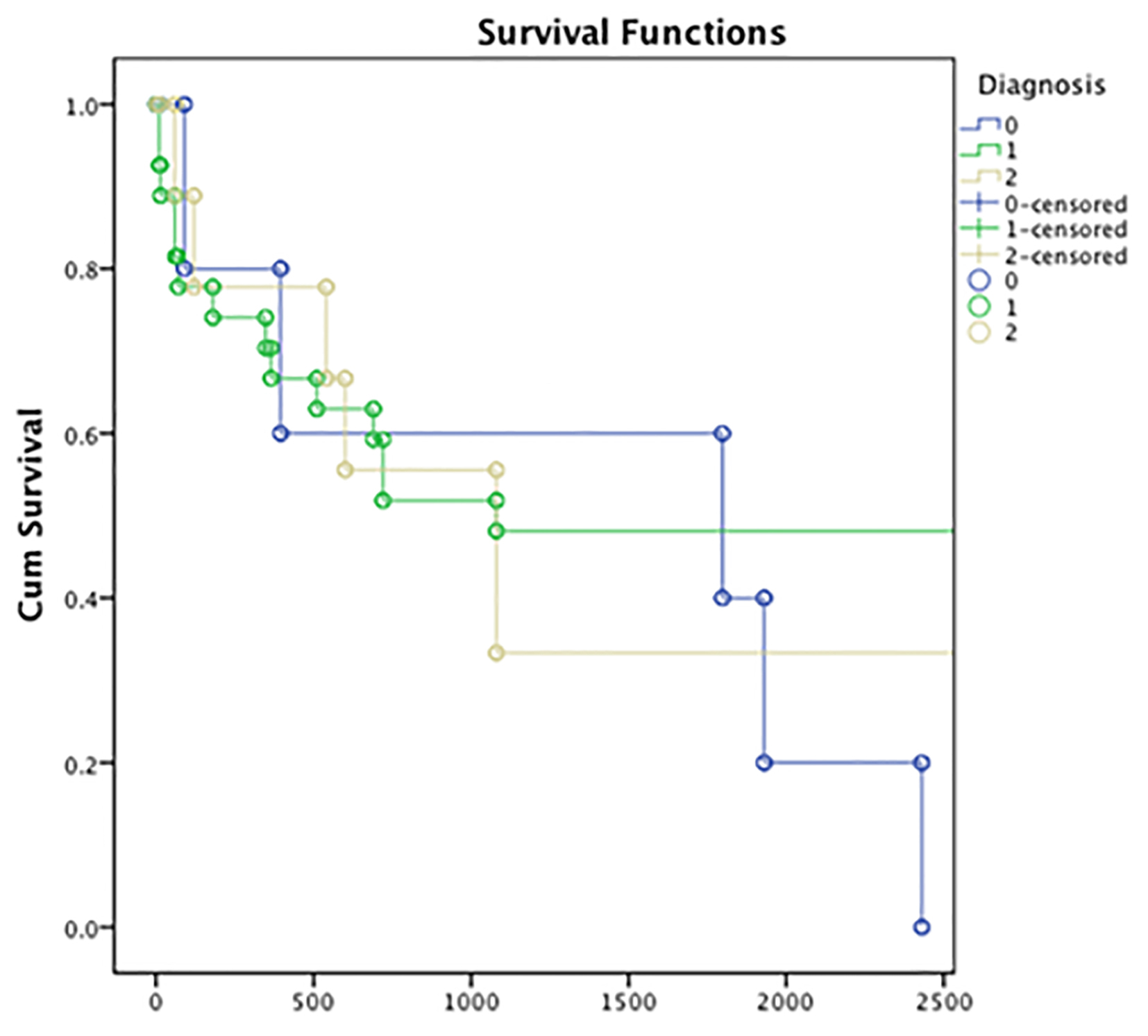 Click for large image | Figure 1. Survival rate in the 41 patients with para-protein related biopsy findings according to the renal pathological diagnosis (MCN, LCDD, and AL-amyloidosis). Y axis: cumulative survival; x-axis: length of follow-up in days. Group 0: AL-amyloid (blue); group 1: MCN (green); group 2: LCDD (yellow). MCN: myeloma cast nephropathy; LCDD: light chain deposition disease. |
These data were not replicated in the Italian patients as only five of them received hemodialysis. The only variable associated with a recovery from RRT was a BM transplant (OR: 18.7, 95% CI: 2 - 172, P = 0.01). Thirty-nine patients received a BM transplant, three of whom required two stem cell transplants.
Overall survival
During the entire follow-up period, 73 patients (25.4%) died, of whom 26 (36%) were in the AKI group. The most common causes of death (Table 4) were infection (76.9%), uremia (8.2%, only in the conservatively managed group), bleeding (4.1%), and myocardial infarction (4.1%).
 Click to view | Table 4. Most common causes of death in the entire population |
Of the patients who reached ESKD, there was no statistically significant difference in the proportion of patients who died in the RRT versus non-RRT group (39.5% vs. 21.1%, P = 0.245); there was also no difference in the proportion of patients with infective death (93.8% vs. 86.7%, P = 0.417).
In Cox analysis (Table 5) of the UK patients (backward and forward regression), variables associated with an increased risk for death were a higher percentage of plasma cells on BM biopsy (HR: 1.06, 95% CI: 1.01 - 1.12, P = 0.01), higher levels of serum paraprotein (HR: 1.05, 95% CI: 1 - 1.11, P = 0.037) and lower levels of proteinuria (HR: 0.28, 95% CI: 0.09 - 0.93, P = 0.037). The need for RRT did not associate with an increased risk for death.
 Click to view | Table 5. Backwards and Forwards Cox’s Model for Death in the UK Subgroup |
The 12-month mortality was higher in the AKI than in NRI group (28% vs. 11%, P = 0.0032; Fig. 2). We also found a higher 12-month survival in all the patients receiving chemotherapy vs. the untreated (84% vs. 74%, P = 0.022; Fig. 3).
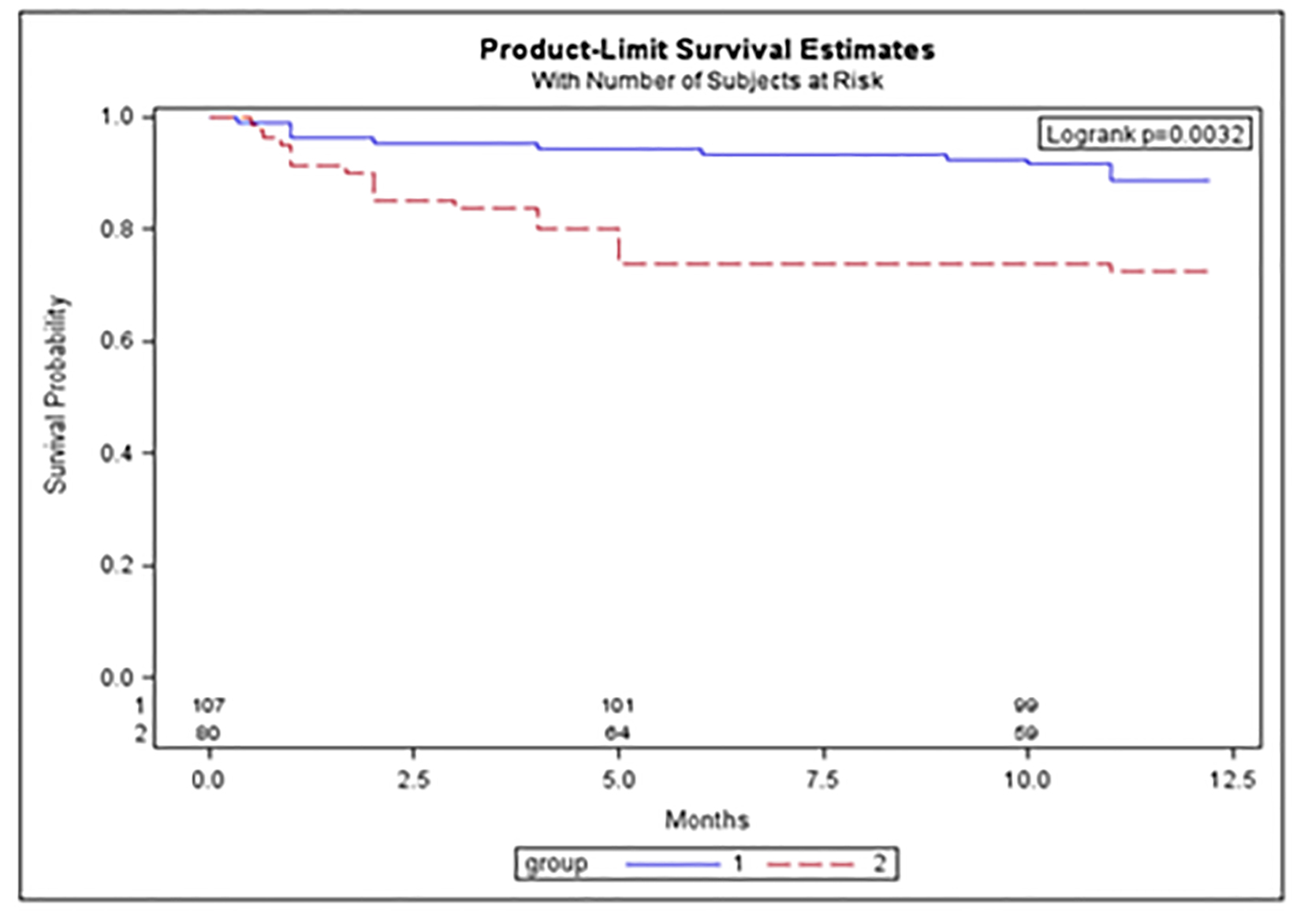 Click for large image | Figure 2. The 12-month survival in NRI (no renal impairment) and AKI (acute kidney injury) in UK patients. Group 1 (blue): NRI patients; group 2 (red): AKI patients. Y-axis: survival probability; x-axis: length of follow-up in months. |
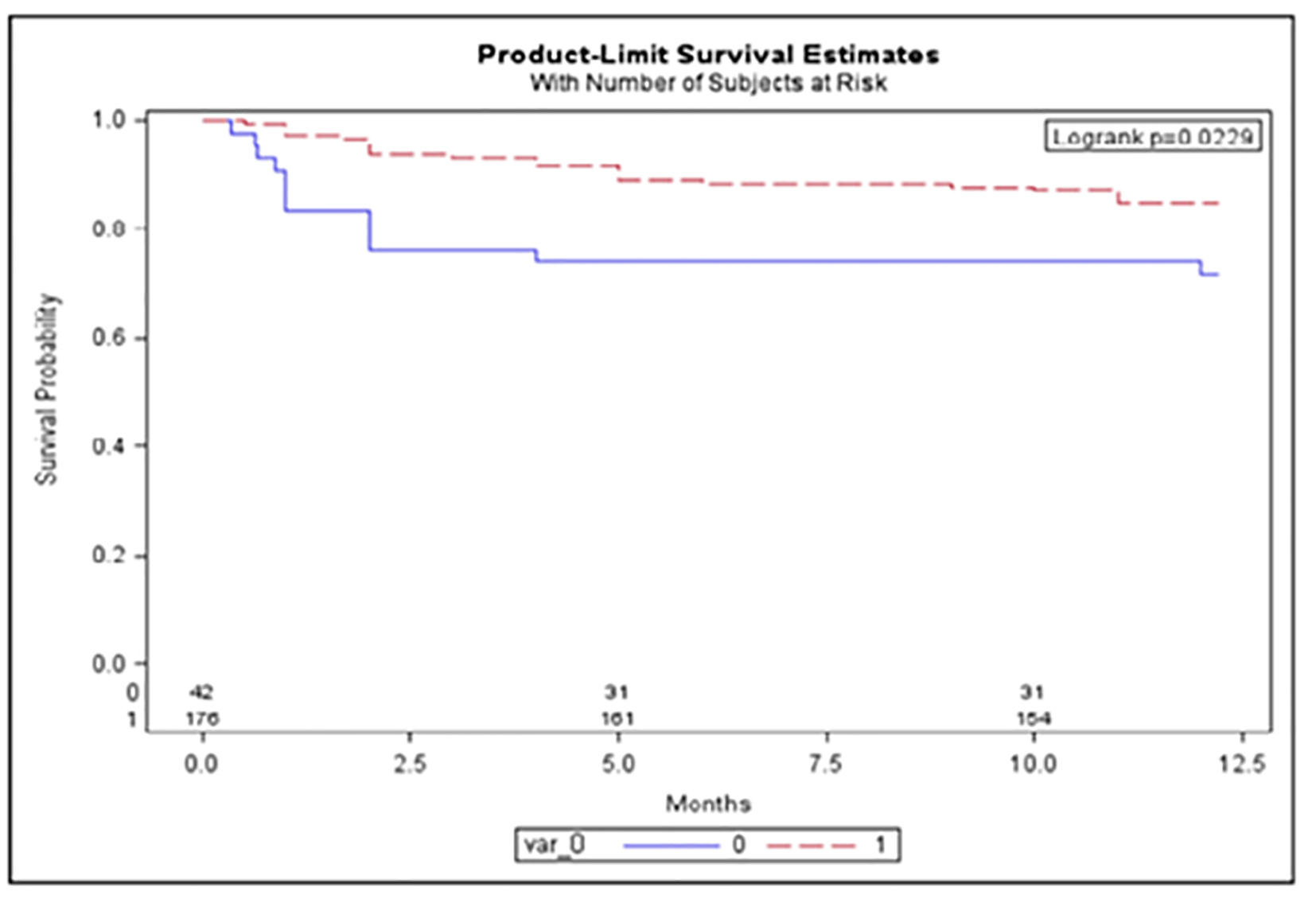 Click for large image | Figure 3. The 12-month survival between chemotherapy treated vs. untreated UK patients. Red: chemotherapy treated; blue: untreated cohort. Y-axis: survival probability; x-axis: length of follow-up in months. |
| Discussion | ▴Top |
The first goal of our study was to assess clinical and renal-hematologic characteristics at the diagnosis of myeloma.
Historically, renal failure has been a common and serious complication of MM. In the literature, the estimates of its incidence vary broadly, and depend upon the definition of renal failure used in particular studies. In a cohort of 1,353 incident cases of MM, Knudsen et al found that 31% of the patients had renal failure (defined by a creatinine > 130 μmol/L), but this incidence increased to 46% if renal failure was defined according to eGFR instead [12]. Further discrepancies derive from how the myeloma was defined. Some diagnose “myeloma” as patients with a full-blown disease, including osteolytic lesions. Others extend this definition to a wider range of monoclonal gammopathies even when the site of malignant cell proliferation has not been localized [17, 18].
The results of our case series showed that MM, as presenting to these two renal centers, was more frequent in the male and elderly population. Overall, renal impairment was a relatively common finding in the course of the disease, and was present in 55.4% of subjects. The most common form of MM-related renal impairment at presentation was AKI (36.6%) which we staged and defined according to the most robust definitions of the disease. Our study highlighted that MM with AKI represents a more aggressive disease, being associated with worse hematologic and renal features, such as lower hemoglobin, higher percentage of PC in bone marrow, higher κ/λ ratio, higher calcium and phosphate levels and greater proteinuria. This is in line with what the scientific literature has reported thus far. In fact, patients with MM-related renal failure tend to have larger tumor loads. This concurs with the description of the International Scoring System for MM that identified most patients with renal failure (creatinine > 2 mg/dL) to have stage 3 disease [19].
We wished to determine possible associations between the development of AKI and any previous clinical condition that might have predisposed to it. A history of MGUS was significantly associated with the presentation with AKI at the diagnosis of MM, but only in the UK group.
The seemingly higher frequency of known MGUS in the Italian cohort is most likely explained by the fact that all subjects were referred exclusively from a cancer department and are likely to have been checked for MGUS development on serum electrophoresis/immunofixation previously.
This finding is of particular interest, because Leung et al recently proposed a novel and helpful term, monoclonal gammopathy of renal significance (MGRS), indicating a causal relationship between the monoclonal gammopathy and renal damage [20]. They suggested that the term MGUS should be limited to those cases where no connection to organ damage can be demonstrated. Meanwhile, MGRS should be used when the monoclonal protein is playing a direct role in the kidney disease. MGRS-caused kidney diseases are the result of toxic monoclonal protein produced by “dangerous” small B clones [ 21]. These disorders do not require treatment from a “tumoral” viewpoint, but treatment is often mandatory to prevent renal deterioration. MGRS-related kidney injury could predispose to the development of a more severe AKI in the context of other pathologic injuries that frequently happen during the course of MM (hypercalcemia, dehydration, and the use of NSAIDs). Recovery of renal function is possible with adequate hematologic response to chemotherapy.
The second objective of our study was to analyze the spectrum of kidney biopsy findings in patients with MM. In our case series, renal biopsy was performed only in 54 UK patients presenting with AKI, who represented 19% of the overall cohort and 51% of the AKI cohort. The reasons for not performing a renal biopsy were a change in the clinical management would be unlikely to occur, different local practice, or because the patients were too unwell to undergo the procedure. It is known that the pattern of renal parenchymal disease in MM is determined by the particular characteristics of the immunoglobulin molecule or subunit [9]. However, we have not analyzed this within this study. Future research in this field is required to define an accurate renal phenotype, perhaps even without the need for renal biopsy.
Similar to previous kidney biopsy and autopsy series [22, 23], MCN was the most common finding overall, seen in 51% of patients undergoing biopsy. In our series, LCDD was more common than AL-amyloidosis. In contrast, the kidney biopsy incidence of AL-amyloidosis in the general population (i.e., with or without MM) is approximately three-fold higher than LCDD [10]. Of course, not all cases of MCN, amyloidosis, and LCDD are mutually exclusive, although we did not find a coexistence of these diseases in our population.
In the small subset of patients with MM who developed a nephrotic syndrome (5.7%), amyloidosis accounted for 70% of cases, whereas LCDD, cryoglobulinemia, and focal segmental glomerulosclerosis (FSGS) accounted for the remainder. The pathologic findings in our study were less heterogeneous than those reported in previous kidney biopsy series of MM [9]. This could be due to the small number of patients, different ethnicity of the sample or different local practice in performing kidney biopsy. Non-paraprotein-associated lesions were found in 23% of patients in our study. These included lesions that were related indirectly to MM: a case of FSGS secondary to pamidronate therapy (a well-recognized association [24]), and a case of acute interstitial nephritis due to lenalidomide.
In our cohort, the diagnosis of MCN was associated with lowest eGFR, hemoglobin and platelets levels when compared with the other two histological types. Also, the levels of total protein and the BM percentage of plasma cells were higher in patients with MCN.
There was a higher rate of renal functional recovery in this group and this relationship merits further analysis in future studies. Although the histological dataset was limited in number, there was no significant difference with regard to the need for RRT in the three subgroups, and mortality was also similar, a finding replicated by others [22].
The analysis of the requirement for a dialytic treatment was another aim of this study. Historically, severely impaired renal function and the need for dialysis were considered as markers of poor prognosis [25]. This is no longer the case, especially if adjustment is made for tumor burden [26]. In our population at diagnosis, MM was associated with ESKD (eGFR < 15 mL/min/1.73 m2) in 38% of subjects, and RRT was needed by 23% of this group. Furthermore, MM-related ESKD was often acute, and required RRT within a few days (less than 7 days in 80% of the cohort) from its development. It is important to underline that, in our cohort, RRT requirement did not represent a cause for increased overall or infective death (which is the leading cause of death in MM patients). Although some studies have suggested that recovery from renal function (hence avoidance of maintenance dialysis) is associated with improved survival, these have included few patients, and our opinion is that patients should promptly receive RRT if indicated, as they may benefit from this therapy with a good quality of life. Dialysis is now safer, more efficient and new techniques have been developed [27].
Chemotherapy and RRT, especially high cut-off hemodialysis (HCO-HDx), are currently the best treatment for patients with MM and ESKD [19]. We found that BM transplant was the only factor that was associated with the recovery from RRT. The reasons for this are complex and will include patient fitness in terms of selection for BM transplant. Further studies are needed to establish the potential beneficial role of new chemotherapy strategies, combined with the novel BM transplant schemes, on renal functional outcome.
We recognize that this study is limited by its retrospective design, which was necessary to collect a relevant number of cases for analysis, as the incidence of MM-related renal disorders is relatively uncommon. There were confounding factors due to case mix, with potential spurious associations, and the multiple statistical analyses were not preplanned and may have led to false-positive results.
Conclusion
In summary, our study showed that 1) MM patients referred to a renal center are more likely to be elderly and male; 2) when renal impairment is present, the most common form at presentation is AKI; 3) MGUS may predispose to renal damage and may increase likelihood of AKI in the context of MM; 4) kidney biopsy should be performed whenever indicated and feasible as it establishes diagnosis, prognosis and the most appropriate therapeutic strategies; 5) of the AKI patients who require RRT, 80% need it at presentation; 6) the RRT treated cohort does not have a higher risk of death than those not requiring RRT; 7) MM with AKI represents a more aggressive disease, being associated with worse hematologic features and increased risk for short-term death; 8) MM-related renal failure is a medical emergency with the need for rapid diagnosis and prompt supportive care, RRT and MM-directed therapy.
Conflict of Interest
We the authors declare that we have no conflict of interests related to this manuscript.
| References | ▴Top |
- Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364(11):1046-1060.
doi pubmed - Bergsagel PL, Kuehl WM. Molecular pathogenesis and a consequent classification of multiple myeloma. J Clin Oncol. 2005;23(26):6333-6338.
doi pubmed - Weiss BM, Abadie J, Verma P, Howard RS, Kuehl WM. A monoclonal gammopathy precedes multiple myeloma in most patients. Blood. 2009;113(22):5418-5422.
doi pubmed - Zingone A, Kuehl WM. Pathogenesis of monoclonal gammopathy of undetermined significance and progression to multiple myeloma. Semin Hematol. 2011;48(1):4-12.
doi pubmed - Leung N, Rajkumar SV. Renal manifestations of plasma cell disorders. Am J Kidney Dis. 2007;50(1):155-165.
doi pubmed - Sanders PW, Booker BB. Pathobiology of cast nephropathy from human Bence Jones proteins. J Clin Invest. 1992;89(2):630-639.
doi pubmed - Hutchison CA, Batuman V, Behrens J, Bridoux F, Sirac C, Dispenzieri A, Herrera GA, et al. The pathogenesis and diagnosis of acute kidney injury in multiple myeloma. Nat Rev Nephrol. 2012;8(1):43-51.
doi pubmed - Keeling J, Teng J, Herrera GA. AL-amyloidosis and light-chain deposition disease light chains induce divergent phenotypic transformations of human mesangial cells. Lab Invest. 2004;84(10):1322-1338.
doi pubmed - Nasr SH, Valeri AM, Sethi S, Fidler ME, Cornell LD, Gertz MA, Lacy M, et al. Clinicopathologic correlations in multiple myeloma: a case series of 190 patients with kidney biopsies. Am J Kidney Dis. 2012;59(6):786-794.
doi pubmed - Furness P. Paraproteinaemia and renal disease. Current Diagnostic Pathology. 2004;10,52-60.
doi - Lam AQ, Humphreys BD. Onco-nephrology: AKI in the cancer patient. Clin J Am Soc Nephrol. 2012;7(10):1692-1700.
doi pubmed - Knudsen LM, Hjorth M, Hippe E. Renal failure in multiple myeloma: reversibility and impact on the prognosis. Nordic Myeloma Study Group. Eur J Haematol. 2000;65(3):175-181.
doi pubmed - Katagiri D, Noiri E, Hinoshita F. Multiple myeloma and kidney disease. ScientificWorldJournal. 2013;2013:487285.
doi pubmed - KDIGO Board Members. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney international Supplements. 2012;2:124-138.
- Dimopoulos MA, Roussou M, Gavriatopoulou M, Zagouri F, Migkou M, Matsouka C, Barbarousi D, et al. Reversibility of renal impairment in patients with multiple myeloma treated with bortezomib-based regimens: identification of predictive factors. Clin Lymphoma Myeloma. 2009;9(4):302-306.
doi pubmed - Durie BG, Kyle RA, Belch A, Bensinger W, Blade J, Boccadoro M, Child JA, et al. Myeloma management guidelines: a consensus report from the Scientific Advisors of the International Myeloma Foundation. Hematol J. 2003;4(6):379-398.
doi pubmed - Landgren O, Kyle RA, Rajkumar SV. From myeloma precursor disease to multiple myeloma: new diagnostic concepts and opportunities for early intervention. Clin Cancer Res. 2011;17(6):1243-1252.
doi pubmed - Alexanian R, Barlogie B, Dixon D. Renal failure in multiple myeloma. Pathogenesis and prognostic implications. Arch Intern Med. 1990;150(8):1693-1695.
doi pubmed - Hutchison CA, Blade J, Cockwell P, Cook M, Drayson M, Fermand JP, Kastritis E, et al. Novel approaches for reducing free light chains in patients with myeloma kidney. Nat Rev Nephrol. 2012;8(4):234-243.
doi pubmed - Leung N, Bridoux F, Hutchison CA, Nasr SH, Cockwell P, Fermand JP, Dispenzieri A, et al. Monoclonal gammopathy of renal significance: when MGUS is no longer undetermined or insignificant. Blood. 2012;120(22):4292-4295.
doi pubmed - Merlini G, Stone MJ. Dangerous small B-cell clones. Blood. 2006;108(8):2520-2530.
doi pubmed - Montseny JJ, Kleinknecht D, Meyrier A, Vanhille P, Simon P, Pruna A, Eladari D. Long-term outcome according to renal histological lesions in 118 patients with monoclonal gammopathies. Nephrol Dial Transplant. 1998;13(6):1438-1445.
doi pubmed - Herrera GA, Joseph L, Gu X, Hough A, Barlogie B. Renal pathologic spectrum in an autopsy series of patients with plasma cell dyscrasia. Arch Pathol Lab Med. 2004;128(8):875-879.
pubmed - Markowitz GS, Appel GB, Fine PL, Fenves AZ, Loon NR, Jagannath S, Kuhn JA, et al. Collapsing focal segmental glomerulosclerosis following treatment with high-dose pamidronate. J Am Soc Nephrol. 2001;12(6):1164-1172.
pubmed - Augustson BM, Begum G, Dunn JA, Barth NJ, Davies F, Morgan G, Behrens J, et al. Early mortality after diagnosis of multiple myeloma: analysis of patients entered onto the United kingdom Medical Research Council trials between 1980 and 2002--Medical Research Council Adult Leukaemia Working Party. J Clin Oncol. 2005;23(36):9219-9226.
doi pubmed - Eleutherakis-Papaiakovou V, Bamias A, Gika D, Simeonidis A, Pouli A, Anagnostopoulos A, Michali E, et al. Renal failure in multiple myeloma: incidence, correlations, and prognostic significance. Leuk Lymphoma. 2007;48(2):337-341.
doi pubmed - Hutchison CA, Cook M, Heyne N, Weisel K, Billingham L, Bradwell A, Cockwell P. European trial of free light chain removal by extended haemodialysis in cast nephropathy (EuLITE): a randomised control trial. Trials. 2008;9:55.
doi pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


