| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website http://www.thejh.org |
Original Article
Volume 1, Number 1, February 2012, pages 1-14
Intravenous Administration of Glutamate Ionotropic Antagonists in Healthy Rats, Induces Lymphopenia and Affects Their Hematological Profile
Aliki Xochellia, Dorothea Kapoukranidoua, Maria Kritsepi-Konstantinoub, Vassilia Garipidouc, Anastastios Kourkoutelisd, Konstantinos Kallarasa, Maria Albania, e
aDepartment of Physiology & Pharmacology, Medical School, Aristotle University, Greece
bDiagnostic Laboratory, Faculty of Veterinary Medicine, Aristotle University of Thessaloniki, Greece
cHematology Section of Second Propedeutic Department of Internal Medicine, Aristotle University of Thessaloniki, Hippokration Hospital, Greece
dDiagnostics Division Thessaloniki, Greece
eCorresponding author: Maria Albani, Laboratory of Physiology, Medical School, Aristotle University of Thessaloniki, Thessaloniki, Greece
Manuscript accepted for publication October 31, 2011
Short title: Glutamate Ionotropic Antagonists
doi: https://doi.org/10.4021/jh101e
| Abstract | ▴Top |
Background: Glutamate is one of the primary endogenous amino acids of the Central Nervous System (CNS). Research over the last years has proven the existence of glutamate subunit receptors in additional non-neuronal tissues outside CNS such as the hematopoietic system.The purpose of this paper is to define the possible in vivo effect of glutamate ionotropic antagonists MK-801and NBQX in the hematopoietic system as well as their possible use in the cure of hematopoietic diseases and on their use on patients with co-existing neurological and hematopoietic disorders.
Methods: Glutamate ionotropic antagonists MK-801 and NBQX were administrated intravenously in male wistar rats of 250 - 350 g weight. Animals treated with glutamate antagonists (n = 24) were compared to a control group (n = 10). The following parameters were evaluated 12 h and 24 h after drug administraton: Full blood count with differential, aggregation intensity, plus CD3, CD54 and CD49a expression on T-lymphocytes with flow cytometry. Bone marrow was assessed with bone marrow smears. The results were analyzed by unpaired t-test.
Results: Both ionotropic antagonists reduced white blood cells with early lymphopenia (P < 0.05), whereas NBQX also increased Ht and Hb values (P < 0.05), but reduced platelet aggregation (P < 0.05). T-cell surface activation markers, CD3, CD49a were reduced (P < 0.05), whereas CD54 was increased (P < 0.05) 12 h after both antagonists’ administration but 24 h after NBQX all markers were increased (P < 0.05). Bone marrow cells were influenced in a way that peripheral blood changes and segmented cells showed a possible expression of glutamate receptors.
Conclusions: It is concluded that glutamate ionotropic antagonists affect the hematological profile and bone marrow of healthy intact rats as well as blood elements functions. Further research is needed in order to define the in vivo effect of glutamate and glutamate receptor antagonists on hematopoietic system either as a toxic agent or as a therapeutical one.
Keywords: NMDA; Glutamate; Bone marrow; Blood; Lymphocytes; Megakaryocytes
| Introduction | ▴Top |
Glutamate is one of the main endogenous CNS amino acids along with aspartate. Glutamate and its receptors are widely known to play an important part in a series of major physiological and pathological procedures such as neurogeneretion and synaptogenesis [1], learning and memory [2], Alzheimer’s disease [3] and epilepsy [4].
Glutamate receptors can be divided into two categories based on their pharmacological and physiological properties:
1. Ionotropic receptors, named after their agonists, N-methyl-D-Asparate (NMDA) [5], A-amino-3-hydroxy-5-methyl-4-isoxazolopropionic acid (AMPA) and kainate acid.
2. Metabotropic receptors connected with intracellular messengers [6].
After the establishment of the importance of glutamate and its receptors for CNS function, scientists started exploring the presence of those receptors in peripheral non-neuronal tissues. RT-PCR, PCR, Northern and Western Blot analysis, immunohistochemistry and in situ hybridization were used with remarkable results: glutamate receptors are present in many peripheral tissues including adrenal medulla [7], peripheral nerves, myelinated and unmyelinated [8], bone [9], endocrine pancreas [10], esophagus [11], hepatocytes [12] heart [13, 14], taste buds [15, 16] , keratinocytes [14], lungs [17], pituitary [18], pineal gland [19], ileal longitudinal muscle [20], autonomic and sensory ganglia [7], kidney, spleen, ovaries [11] and stomach [21]. Last but not least, glutamate receptors have been found in peripheral blood elements and in bone marrow cells (as mentioned above). All of the above suggest that glutamate can act as a widespread cytokine that can affect cell function in various peripheral tissues outside CNS.
In the hematopoietic system, glutamate receptors have been identified in megakaryocytes, platelets and lymphocytes.
There are not many papers published concerning glutamate, its receptors and their relationship with megakaryocytes, while existing results point out to a gap in our knowledge concerning glutamate’s action outside CNS. Research proved the presence of NR1 and NR2D subunits in human and rat megakaryocytes as well as in MEG-01 cell line [22]. In human megakaryocytes, there are also NR2A subunits as well as Yotaio and PSD-95 helping proteins [23]. The absence of the rest of the proteins related to the CNS NMDA receptor underlies the fact that those receptors may be similar but are definately not identical. Platelets have been described to alter their function in the presence of NMDA and Glutamate. In 1996, Fanconi et al [24] proved that glutamate has an anti-aggregating activity on platelets primaly exposed to araxidonic acid or ADP or PAF. All of the above led to the conclusion that platelet receptors have a selective affinity to their agonists. Later experiments showed that NMDA receptor activation (either by NMDA or by glutamate with the first being 3 times more powerful than the later) antagonizes the aggregating activity of arachidonic acid in human rich platelet plasma while there is no aggregating action neither from NMDA nor from glutamate. NMDA had no effect in the u-46619 induced aggregation, which shows that glutamate action has nothing to do with TxA2/PGH2 receptors on platelets. Furthermore, platelets have glutamate transporters, and glutamate is released on their activation [25].
Konstayan et al (1997) [26] studies on immune system, proved the presence of glutamate binding sites on the surface of human lymphocytes. These studies were expanded in rodents. It has been found that there are subgroups I and II of metabotropic receptors in thymus, isolated thymus cells, Thymic stromal cell line [27]. Subgroup III of metabotropic receptors and NR1 subunit of NMDA ionotropic receptors can be found in recently isolated lymphocytes [28].
As far as humans are concerned, GluR3 subunits of AMPA ionotropic receptors have been found on Peripheral T-Cells, in Jurkot T leukaemic cell line and in a CD4 alloprimed T-helper clone [29]. Group I of metabotropic receptors has also been found in both active and non-active T-cells as well as in several lymphoid series [30]. Recently, Miglio et al (2005) [31], have suggested the expression of sudunits NR1 and NR2 genes in human cells. In fact the NMDA receptor in human lymphocytes was found to be similar to the CNS receptor. To date, it has been found that glutamate alone (without the presence of other stimulating molecules) in direct interaction with its AMPA receptors, triggers integrin-mediated adhesion to laminin and fibronectin, something only seen in activated cells. Moreover, glutamate was found to regulate the CXCR4-mediated T cell chemotactic migration toward the key chemokine CXCL12/stromal cell-derived factor-1 encountered both in CNS and in peripheral tissues and mediating this way, many immune and neural functions [29]. Recently, research showed that MK-801, a non-competitive NMDA antagonist, inhibited PHA-induced T cell proliferation of resting T-cells (and not that of T blasts) in a concentration dependent manner without modifying cell viability or cell death. The same antagonist inhibited mitogen induced cell proliferation suggesting that NMDA antagonists affect T-cells activation [31].
No specific glutamate receptors subtypes have been described for erythrocytes although they have been described to contain pools of glutamate.
All of the above point out that glutamic acid receptors play an important role on T-cells, platelets, and bone marrow megakaryocyte function. There are still questions pertaining to the possible use of the glutamate receptors and their antagonists in the cure of hematopoietic diseases and on their use on patients with co-existing neurological and hematopoietic disorders.
In this paper we tried to investigate the clinical importance of glutamate receptor antagonists on the hematopoietic system of healthy intact Wistar rats using routine lab tests.
| Materials and Methods | ▴Top |
Animals
40 male Wistar rats (weighting about 300 g each) were used. They were housed in separate cages, all under the same conditions of temperature (22 ± 2 °C and humidity). Rats were fed a standard diet and had access to tap water via water bottles ad libitum. Experiments have been conducted in accordance with the European Communities Council Directive of 24 November 1986 (86/609/EEC) and the “Principles of laboratory animal care” (NIH publication No. 85 - 23, revised 1985) and were approved by the Ethical Committee of the School of Medicine of Aristotle University of Thessaloniki.
Study design
Glutamate NMDA ionotropic antagonist MK801 [(+)-5-methyl-10, 11-dihydro-5H-dibenzo [a, d] cyclohepten-5, 10-imine maleate)], (MK801 group) and glutamate non-NMDA ionotropic antagonist, NBQX (2, 3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline-2, 3-dione), (NBQX group), were intravenously (iv) administrated to healthy intact rats.
Complete blood counts, aggregation, flow cytometry studies were performed on blood samples and bone marrow smears were studied all 12 and 24 hours after drug administration, MK801-12 h and NBQX-12 h group, MK801-24 h and NBQX-24 h group.
Drug administration
Drugs were administrated through the dorsal lateral vein. Animals were placed in a suitable restrainer and the tail was dipped in warm water for a few minutes so as to succeed vein dilation. A 21G needle was inserted into the vein and drug was administrated. Rats were administrated intravenously with MK801 1 mg/kg [32] (MK801 group), NBQX 3 mg/kg [33] (NBQX group) or were free of drug administration (control group). All drugs were purchased from Sigma-Aldrich Co®.
Blood samples
Prior to blood collection, rats were anesthetized with an intraperitoneal injection of chloral hydrate. Blood was obtained via cardiac puncture using a No 23 gauge needle attached to a plastic disposable syringe and placed into different tubes. Blood samples for blood analysis and flow cytometry studies were placed into EDTA bottles, whereas blood for aggregation studies was anticoagulated with 3.8% trisodium citrate in a ratio of 9 parts of blood to 1 part of anticoagulant [34]. Differential centrifugation was used in order to obtain platelet rich (PRP) and platelet poor plasma (PPP) [35].
Complete blood count
An analyzer ADVIA 120 Hematology System was used in order to perform CBC. The following parameters were measured or analyzed: White Blood Cells (WBC), Red Blood Cells (RBC), Hemoglobin (Hb), Hematocrit (Hct ), Mean Cell Volume (MCV), Mean Cell Hemoglobin (MCH), Mean Cell Hemoglobin Concentration (MCHC), Platelets (PLT), and differential results (neutrophils, lymphocytes, monocytes, eosinophils, basophils) in absolute numbers.
Aggregation studies
Platelet aggregation studies were performed with a PAP4 Platelet aggregation profiler equipped with a recorder. 0.05ml of an antiaggregating agent was added to an aliquot of 0.045ml of PRP equilibrated to 37 °C and each aggregation was recorded for 5 minutes. Aggregations were quantified as the maximum extent (intensity) of light transmittance in stimulated PRP. Aggregations were induced with adenosine diphosphate (ADP) and collagen [36].
Flow cytometry studies
Blood was divided into three 100 µl aliquots. The first aliquot was labeled and incubated with 10 µl of CD54-PE and 10 µl of CD3e-FITC monoclonal antibodies, the second with 10 µl of CD49a and the third with 10 µl of CD3. After incubation cell lysis took place. Labeled cells were analyzed by flow cytometry on an FC 500 (Beckman Coulter, FL, USA) analyzer. All antibodies were purchased from eBioscience, Inc®.
Bone marrow smears
Bone marrow smears were collected from the ferum. Rats were put to death and then the bone marrow cavity was exposed after incision of the ferum. The whole procedure did not last more than 3 minutes so as to avoid cell damage [37]. Up to 500 cells were studied for each smear by two individual investigators and results were compared.
Statistical analysis
Blood count values, platelet aggregation and CD3, CD54 and CD49a expression on T-lymphocytes, were normally distributed and analyzed with unpaired t-test. For all tests, the level of significance was set at 5% (P < 0.05). Results are expressed as mean ± SEM.
| Results | ▴Top |
Complete blood counts
A. MK801 administration
When performing the same blood counts 12 hours after MK801 administration, we observed that the only value that reached a statistical significance was that of lymphocytes absolute value, namely, there was a reduction in lymphocytes mean absolute value from 509 ± 134/µl in control group to 349.8 ± 92/µl in MK801-12 h group (P < 0.05).
Blood count tests performed 24 hours after MK801 administration (MK801-24 h group) showed a great reduction in neutrophil absolute count mean volume (from 438.66 ± 108 to 87.4 ± 19.29 /µl, P < 0.05) as well as in lymphocyte, monocyte and eosinophil absolute counts mean values (Fig. 1).
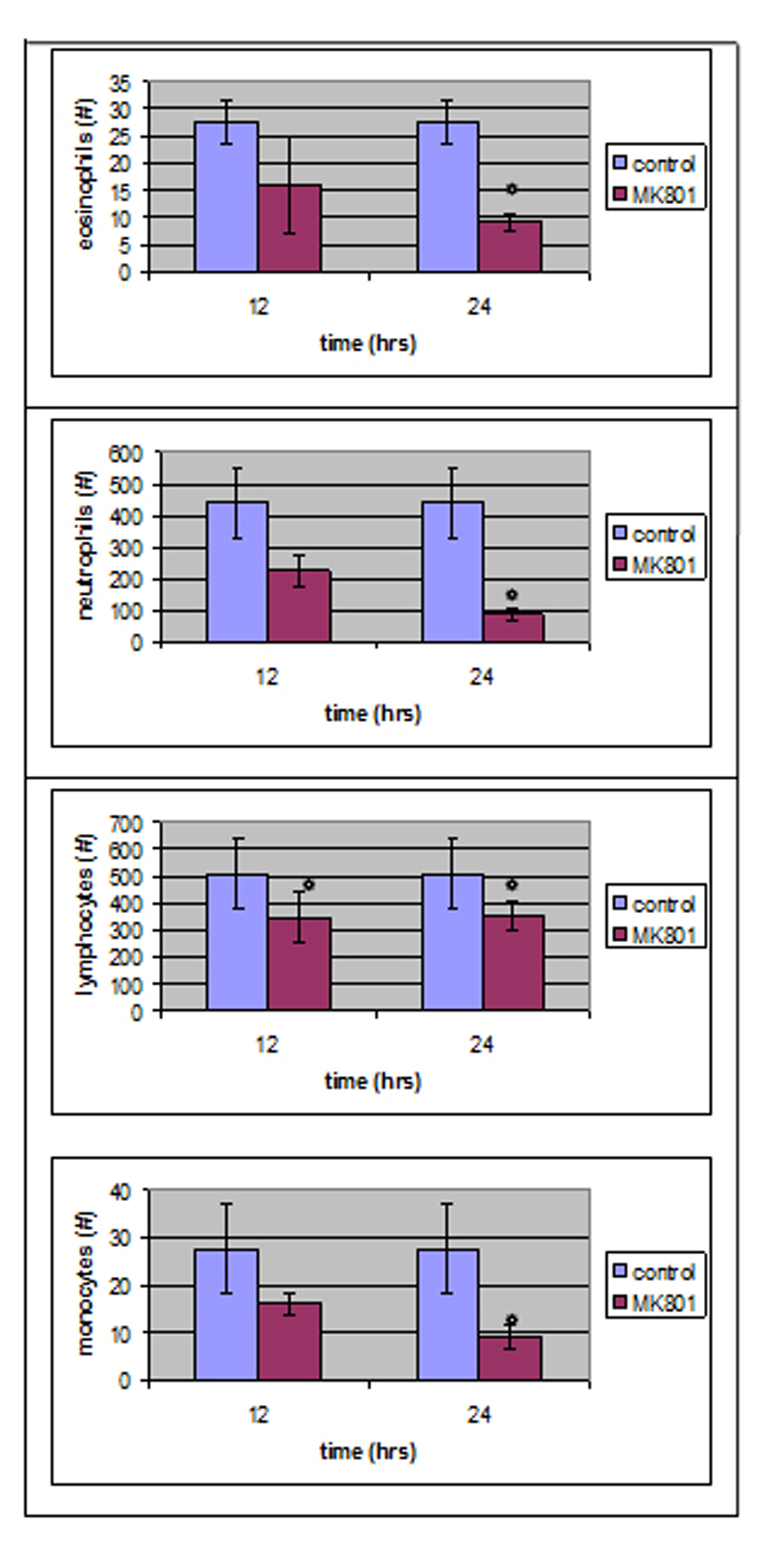 Click for large image | Figure 1. Twelve hours after MK801 administration, only lymphocytes absolute number was statistically different compared to control group whereas, four parameters reached a statistical significant level 24 hours after MK801 administration. |
B. NBQX Administration
The evaluation of blood count results 12 hours after ionotropic antagonist NBQX administration, group NBQX-12 h, showed that 7 parameters reached a statistical significance whereas in group NBQX-24 h, only 5.
In NBQX-12 h there was a decrease in neutrophil absolute counts (from 438.66 ± 108/µl in control group to 77.8 ± 17.82 /µl, P < 0.05), lymphocytes (from 509 ± 134 to 219.4 ± 22.16/µl, P < 0.05) as well as in monocytes (from 50 ± 9.5 to 15.8 ± 3.42/µl, P < 0.05) and in eosinophils (from 27.66 ± 3.84/µl to 12.2 ± 2.33/µl, P < 0.05).
An increase was observed in red blood cells mean value (from 8.18 ± 0.4×106/µl to 8.76 ± 0.16x106/µl, P < 0.05) whereas MCV and MCH were affected in a negative way with a decrease from 51.43 ± 0.71 fl to 50.38 ± 0.27 fl (P < 0.05) and 17.56 ± 0.38 pg to 16.46 ± 0.16 pg (P < 0.05) respectively.
In NBQX-24 group, there was a statistical significance in the mean values of neutrophils, lymphocytes, monocytes and eosinophils with an assessment to control group normal values and a statistical important increase in Hb.
Neutrophils absolute mean value count decreased to 170.4 ± 34.86/µl and lymphocytes decreased to 260.6 ± 37.11/µl (P < 0.05).
Monocytes reached at a level of 15.2 ± 2.2/µl whereas eosinophils also decreased (6.2 ± 2.31/µl, P < 0.05) in comparison to control group normal values. An increase was also noticed in Hb mean value (P < 0.05) (Fig. 2).
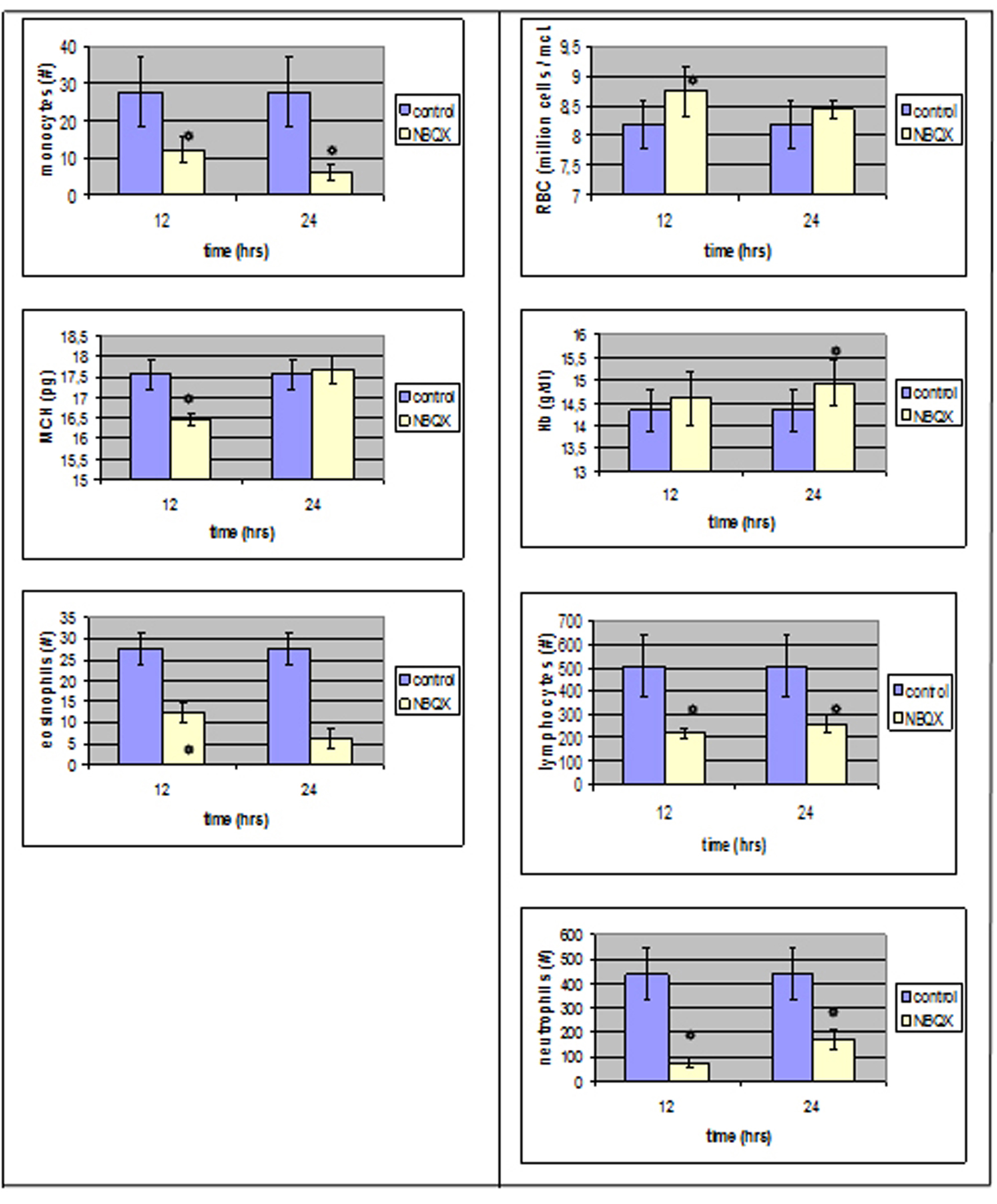 Click for large image | Figure 2. Parameters that reached a statistically significant level compared to the control group right after and 12 hours after NBQX administration. |
Platelet aggregation results
No statistical differences were observed in platelet aggregation between MK801-12 h, MK801-24 h and control group neither after ADP, nor after collagen addition to PRP.
The maximal intensity of platelet aggregation in response to collagen was significantly decreased (P < 0.05) in group NBQX-12 h (Fig. 3), whereas the addition of ADP induced similar intensity with control group.
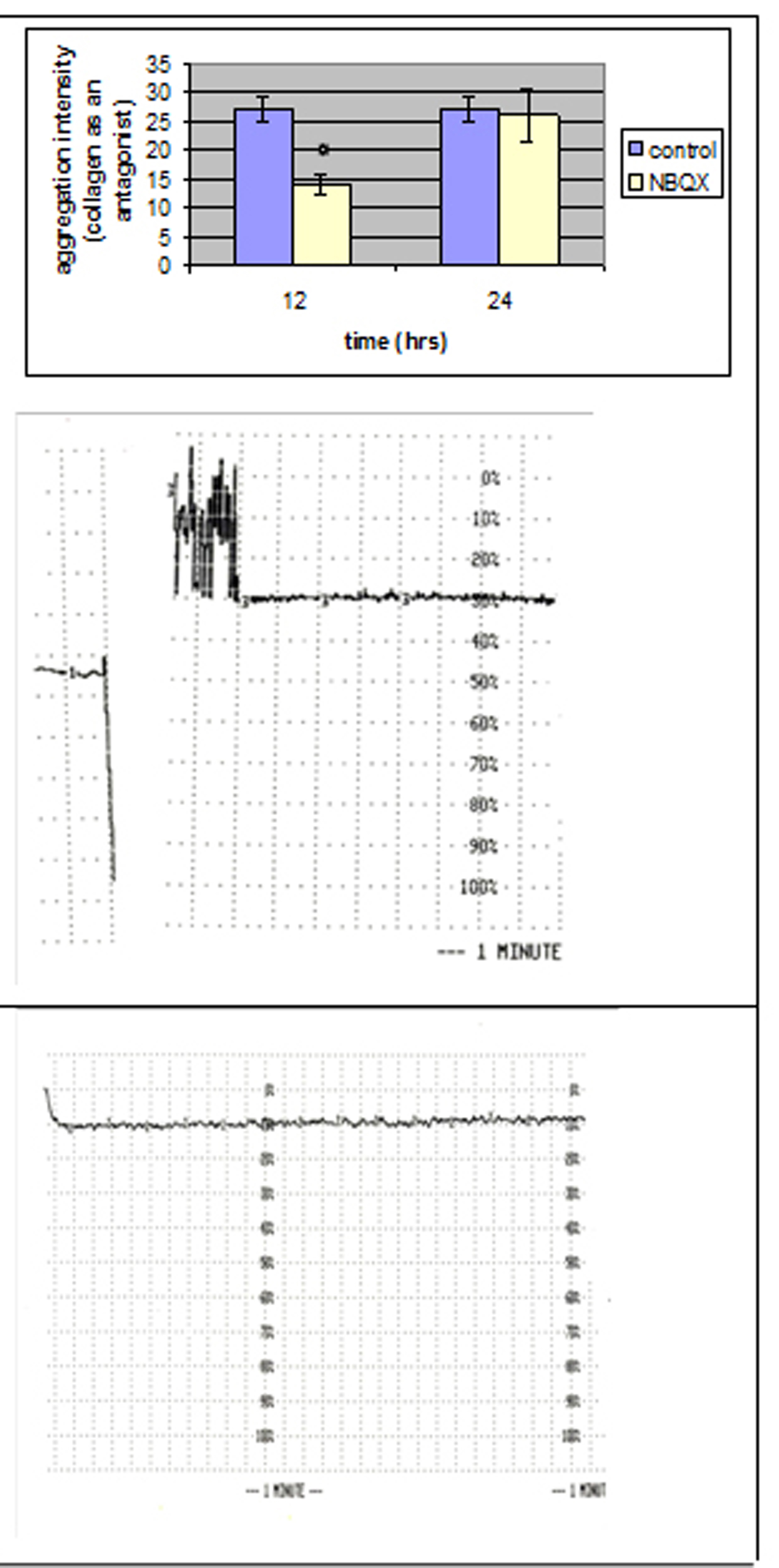 Click for large image | Figure 3. Comparison of the aggregation intensity that reached a statistically significant level between NBQX and control group with collagen as an antagonist. |
No statistical significant differences were observed 24 hours after NBQX administration.
Monoclonal antibodies expression
Monoclonal antibody expression in T lymphocytes was statistically different in MK801-12 h group whereas no statistical differences were observed in MK801-24 h group compared to control group.
To be more precise, CD3 and CD49a expression was decreased (P < 0.05) right after MK801’s addition whereas CD54 expression was increased (P < 0.05) compared to control group (Fig. 4).
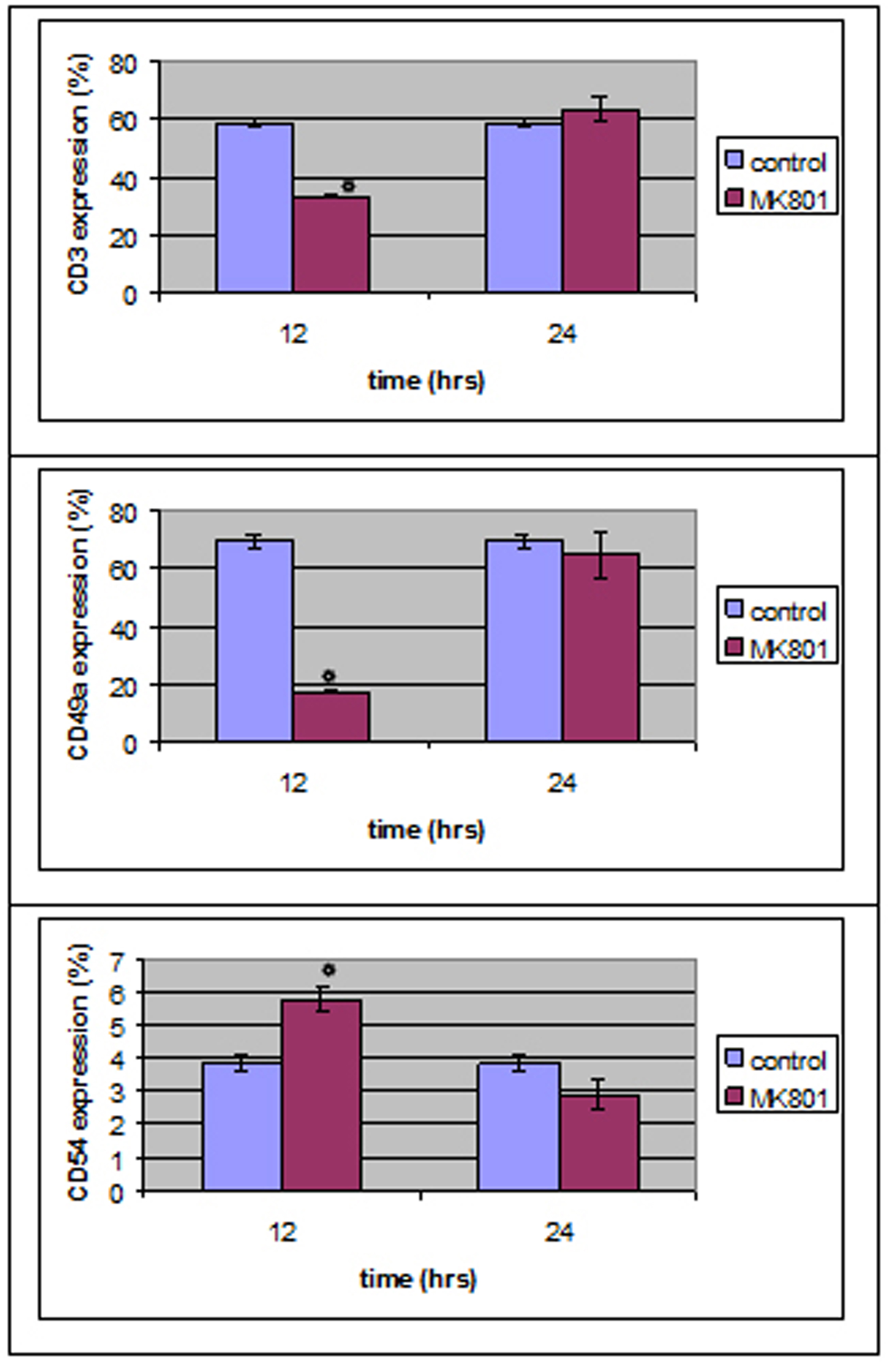 Click for large image | Figure 4. MK801 administration induced a statistically significant decrease in CD3 and CD49 expression and an increase in CD54 expression 12 hours after the administration, whereas no statistically significant differences were observed 24 hours after MK801 administration. |
CD3, CD49a and CD54 expression reached a statistically different level (P < 0.05) in both NBQX-12 h and NBQX-24 h group when compared to control group (Fig. 5). In NBQX-12 h group, CD3 and CD49a expression was decreased (P < 0.05) compared to control group whereas CD54 expression was increased (P < 0.05).
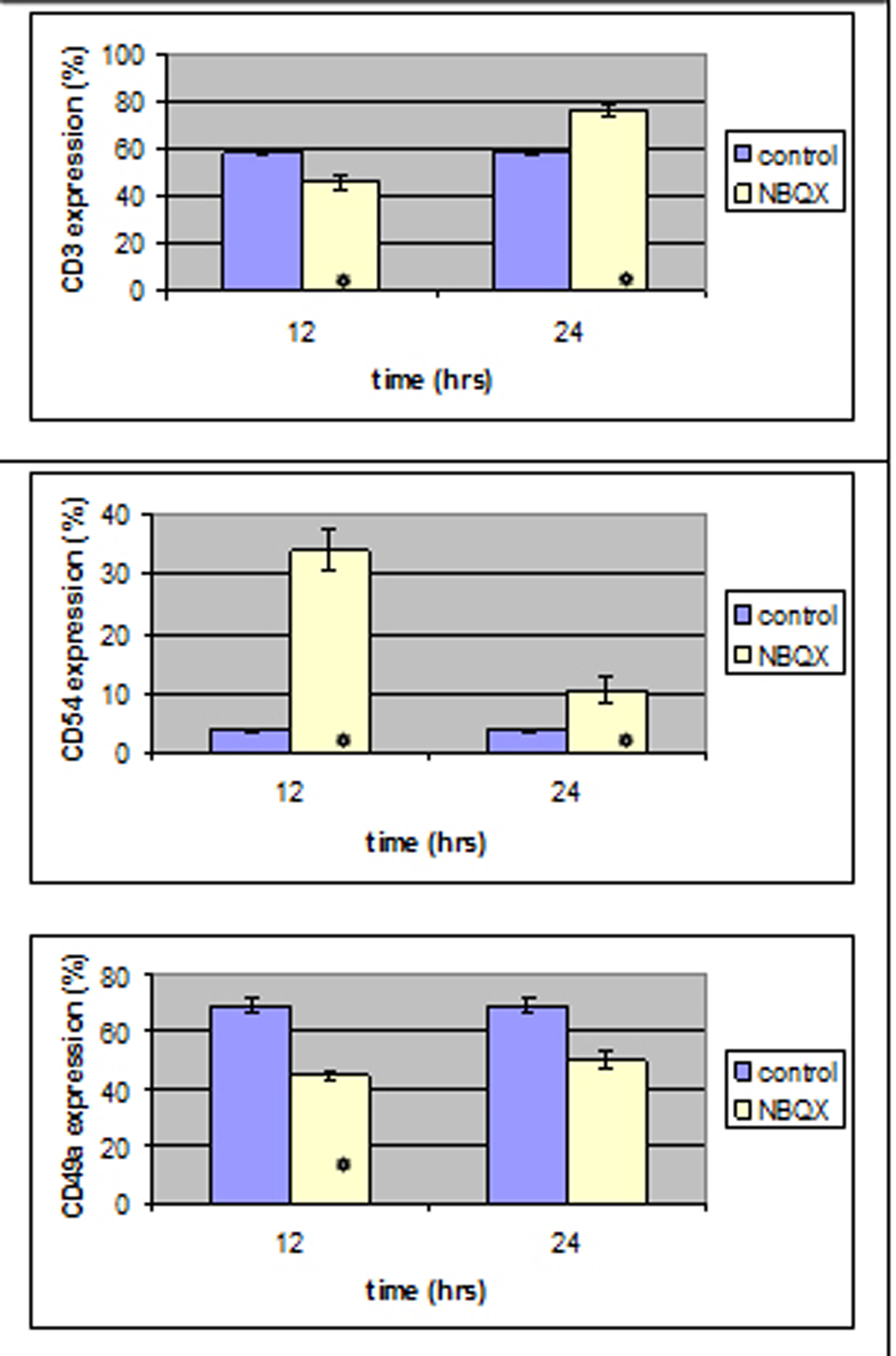 Click for large image | Figure 5. Twelve hours after NBQX administration a statistically significant decrease was induced in CD3 and CD49 expression and an increase in CD54 expression .12 hours after, all antibody expression was increased compared to the control group. |
In NBQX-24 h group CD3 and CD54 monoclonal antibody expression was statistically increased compared to the control group.
Bone marrow results
A. MK801 administration
No statistical important difference was noticed 12 hours after MK801 administration whereas 12 hours later band cells and eosinophils were increased from 4.6 ± 0.49(%) and 3.26 ± 0.4(%) in control group to 7.07 ± 0.87(%) and 5.19 ± 0.81(%) in MK801 group respectivelly.
Megakaryocytes were increased from 1.73 ± 0.86(%) to 2.9 ± 1.14(%).
B.NBQX administration
Twelve hours after NBQX administration all cell counts were normal compared to control group whereas 12 hours later segmented cells and lymphocytes (28.96 ± 1.56(%) and 6.56 ± 1(%)) were influenced (21.74 ± 3.02(%) and 12.75 ± 1.29(%)) in a statistical important way.
Megakaryocytes were decreased from 1.73 ± 0.86 to 0.96 ± 0.34.
In Figure 6 one can see the results of bone marrow study.
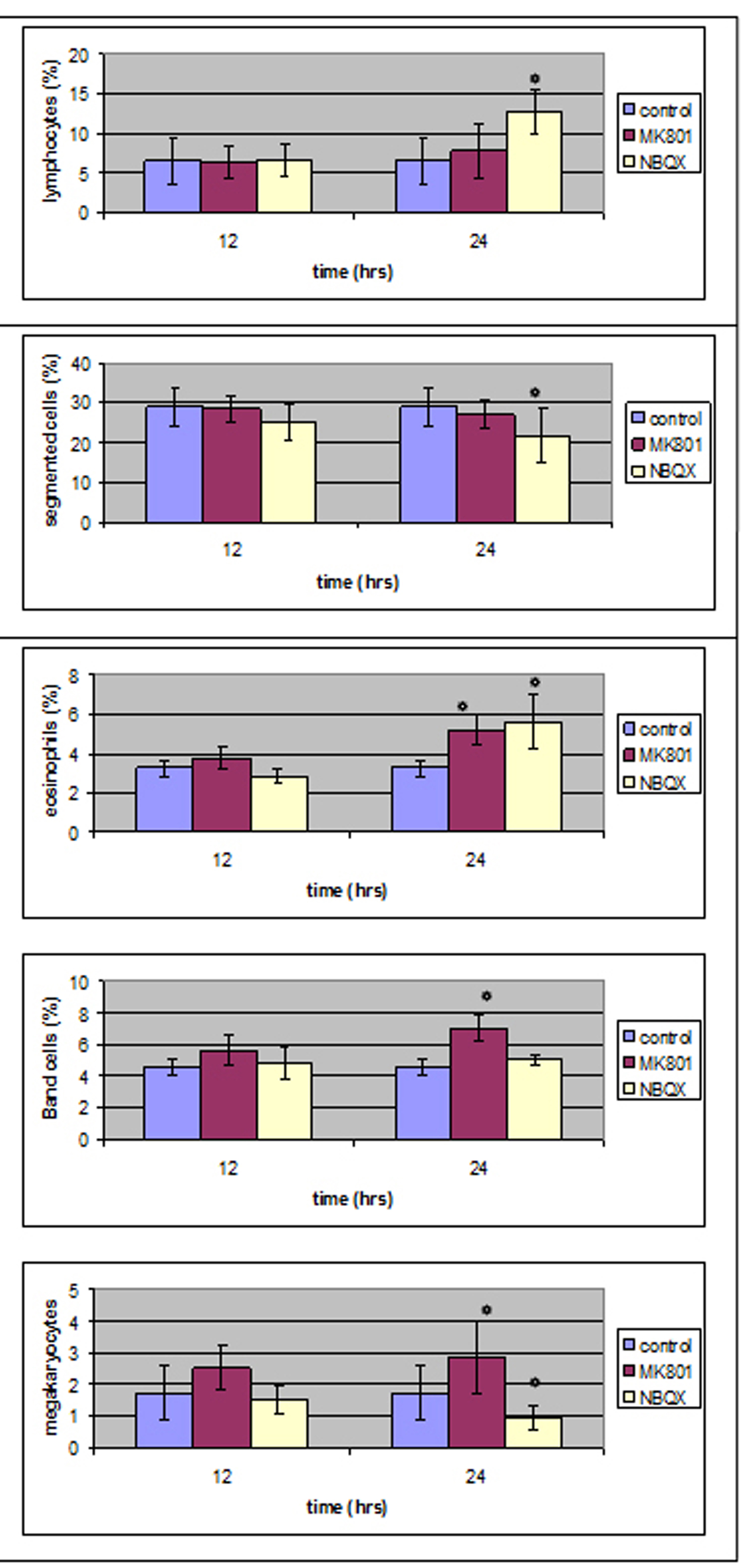 Click for large image | Figure 6. Bone marrow results in MK801 and NBQX group compared to control group. |
| Discussion | ▴Top |
Previous literature has shown that exogenous glutamate can affect elements of the hematopoietic system in many ways, and that those effects can be enhanced by glutamate ionotropic antagonists [31, 38]. Since glutamate is a natural plasma element and is also contained inside several cells, our study aimed to examine if and in what way glutamate ionotropic antagonists can affect the hematopoietic system without any previous glutamate administration.
Complete blood counts
When performing blood counts 12 hours after MK801 administration, only lymphocytes absolute count reached a statistical important level whereas 12 hours later, one can see a statistically significant reduction in neutrophil, lymphocyte, monocyte and eosinophil absolute count levels. When performing blood counts 12 hours after NBQX administration one can see a statistically significant reduction in neutrophil, lymphocyte, monocyte and eosinophil absolute counts. This reduction was also noticed 24 hours later, with eosinophils continuing their reduction. Judging from the above, one can notice an effect in white blood cell distribution. To be more precise, there was a reduction in lymphocyte’s absolute numbers both after MK801 and NBQX administration. Lymphocytes need a minimum level of glutamate (0.1 mM) or glutamine in order to keep proliferating, in a different environment, normal cell cycle progression is disturbed [39]. Ionotropic glutamate antagonist administration may cause such a disturbance by not allowing ion currents flow and this may lead to a reduction in lymphocyte number.
Neutrophils and monocytes are known to use glutamine for their needs [40] and to release glutamate [41], but there are no studies on glutamate receptors on those cells. Having in mind the possible absence of ionotropic glutamate receptors in these cells, one would expect that glutamate alone and glutamate ionotropic antagonists administration should not affect their absolute value. Such a thing did not occur. Both glutamate ionotropic antagonists reduced neutrophil and monocyte numbers, with NBQX having a stronger impact since the reduction started just 12 hours after administration. One possible explanation is that glutamate antagonists may act upon channels that release glutamate. Glutamate intracellular increase may induce cellular death or interfere with natural cell life progression by increasing intracellular free calcium or reactive oxygen species. Such an effect is noticed by NR1 activation in lymphocytes [35]. Could this reduction mean that all white blood cells have glutamate ionotropic receptors? Furthermore, based on the observation of Wen Jiang et al (2004) [42], that NMDA and non-NMDA antagonists inhibit granule cell precursors proliferation after seizures in adult rat brain, our results suggest a possible action of those antagonists on bone marrow precursors as well. More studies are needed in order to define the explanation for this reduction in white blood cell volumes focusing on bone marrow cell precursors.
As far as red blood cells are concerned, they are known to have intracellular glutamate concentrations more than twofold than that of plasma [43] but they do not have glutamate transporters and they cannot release or uptake glutamate [44]. Divino et al [45], showed that intracellular glutamate is affected by insulin levels and that insulin growth factor may drive glutamate in and out of cells [46].
In our study, MK801 administration did not affect red blood cell line, whereas NBQX negatively affected MCH value and induced an increase in RBC mean value 12 hours after its administration, 12 hours later, all values were back to normal and Hb was increased. MCH represents mean cell hemoglobin and is expressed with the average Hb/RBC. As a result, an increase in RBC with a smaller increase in Hb can explain the reduced MCH mean volumes.
Previous studies [47] have shown that intravenous administration of MK-801 (1mg/kg) to cats, causes apneusis and reduces phrenic neurogram and inspiratory-synchronous amplitude activity by 38% and 84% respectively without affecting tonic synchronous activity in cervical sympathetic neurogram, whereas NBQX only affected phrenic neurogram and inspiratory synchronous amplitude activity in a negative way by 54 and 60% respectively. Such an effect may result in less oxygen release in tissues and act as a signal for more Hb production. Pierrefiche et al in 1994 [48] have shown that although both ionotropic antagonist receptors play an important role in sustaining breathing, non-NMDA receptors play a larger role in the transmission of respiratory signals. Such an observation comes in terms with our findings that only NBQX administration affected RBC and Hb values. As far as kidneys are concerned, it was found that metabotropic glutamate receptors Ia and Ib are expressed in neonatal hamster kidney [49] and that NMDA binding agents modulate cell death in kidney cells [50]. Our effect concerns both NMDA and AMPA receptors. Could this effect be a result of additional kidney AMPA receptor expression, and if so how does glutamate affect erythropoietin production?
Platelet aggregation
Platelet aggregation was not affected neither 12 hours after MK801 administration nor 24 hours later. Aggregation was affected in NBQX-12 h group with collagen as an antagonist, 24 hours after NBQX administration aggregation was at the same levels with control group.
Platelets express AMPAR subunits GluR1-4, with GluR1 subunit on their surface [38]. They can also store and release glutamate with a transport system [25]. MK801 induced no changes in aggregation, whereas NBQX reduced aggregation intensity with collagen as an antagonist. Another non-NMDA antagonist had a similar effect in previous studies (glutamate mediates platelet activation through the AMPA receptor). The possible explanation for this behaviour may be the same supporting the hypothesis of an autocrine role glutamate may have in platelet activation.
Flow cytometry studies
CD3 is a molecule expressed on every T-cell and is involved in cell activation and co-interactions among T-lymphocytes and target cells. CD54 is a transmembrane protein ligand for intergrin, associated with the cell-cell interaction stabilization and leukocyte transmigration. CD49a an intergrin alpha subunit is involved in cellular adhesion to laminin and collagen when associated with CD29. As mentioned above, NMDA antagonists affect T-cell activation and regulate their intergrin-mediated adhesion to laminin and fibronectin. Ganor et al in 2003 [29], proved that glutamate induced T-cell adhesion to laminin and fibronectin via AMPA receptor subtype activation. In addition, they observed that glutamate increases normal human T-cell migration towards the potent chemokine CXCL12/SDF-1 in a concentration manner. They speculated that AMPA receptor antagonists ameliorate experimental autoimmune encephalomyelitis by preventing the in vivo activation of autoaggressive T-cells induced by glutamate derived from nerve endings by blocking those receptors. Since T-lymphocytes are now known to also express NMDA receptor subtypes, one should expect that all activation surface markers should be reduced after glutamate ionotropic antagonist administration. Such a thing did not occur. In our study, there was an effect on T-cell activation and proliferation markers both by NMDA and non-NMDA antagonists, with NBQX (non-NMDA) having more of an impact than MK-801 (NMDA antagonist). In particular, 12 hours after MK801 administration, there was a decrease in CD3 and CD49a expression and an increase in CD54 expression which lasted less than 12 hours, 12 hours after NBQX administration, there was a decrease in CD3 and CD49a expression and an increase in CD54 expression as in MK801-0h group but 12 hours later, all antibody expression was increased compared to the control group. The fact that ICAM-1 (CD54) was increased both after MK-801 and NBQX administration suggests a different role for glutamate antagonists in cell-cell interactions. Furthermore, we observed that 24 hours after NBQX administration, all antibody expression was increased compared to the control group. Could this mean that AMPA receptor antagonists have a time dependent action in T-cells and if so what is the underlying mechanism?
Bone marrow smears
A. MK801 administration
Twenty-four hours after MK801 administration, band and eosinophils were increased whereas at the same time in peripheral blood counts, eosinophils and segmented were decreased,12 hours after MK801 administration in peripheral blood counts there was a statistical important decrease in lymphocyte number and 12 hours later all values were back to normal control values. One should expect a different count in bone marrow as well. An increase was noticed but not in a statistical important way. It could be that there was an increase during12 - 24 hours that was back to normal when our count was performed. Band and eosinophil elevated bone marrow counts are considered as due to neutrophil and eosinophil decrease in peripheral blood.
Genever et al in 1999 [22] suggested the presence of receptors in bone marrow megakaryocytes. In later studies [23] MK801 was found to enhance proplatelet formation without affecting cell proliferation or apoptosis. In our study, MK801 induced an increase in megakaryocyte number 24 hours after its administration, without affecting proplatelet count in peripheral blood. So, why does MK801 has such a different effect in bone marrow megakaryocytes? Previous studies were performed with in vitro continuous MK801 administration for up to 14 days. In our study MK801 effect was examined after a single in vivo dose administration. Could it be that there is a difference in MK801 action when studied in vivo and in vitro?
B. NBQX administration
Twenty-four hours after NBQX administration there was statistical important reduction of bone marrow segmented cells count and a statistical important increase of lymphocytes and eosinophils. At the same time, in peripheral blood there was a statistical important reduction of segmented, eosinophil cells and lymphocytes.
Judging from the above, we can come to the following suggestions.
1) Lymphocytes and eosinophils were increased as a result of periphery decrease; 2) Segmented cells decrease in bone marrow as well as in peripheral blood suggests the presence of a receptor in those cells that inhibits their proliferation and acts both in bone marrow and peripheral blood.
Up to date, there is no proof of non-NMDA receptor presence in bone marrow magakaryocytes but there are reports of those receptors presence in platelets [51]. The effect of NBQX administration in megakayocyte number shows the presence of non-NMDA receptors in megakaryocytes that act in a different way than NMDA receptors -NMDA receptors induced a decrease in megakaryocyte number and non-NMDA receptors induced an increase.
Conclusions
The above data begin to address the gap in our present knowledge on glutamate action outside the CNS. Further studies are needed in order to define the in vivo effects of glutamate and it receptors on the hematopoietic system either as a toxic agent or as a therapeutical one.
Conflict of Interest
The authors declared no conflict of interest.
| References | ▴Top |
- Collingridge GL, Lester RA. Excitatory amino acid receptors in the vertebrate central nervous system. Pharmacol Rev. 1989;41(2):143-210.
pubmed - Van Harreveld A, Fifkova E. Involvement of glutamate in memory formation. Brain Res. 1974;81(3):455-467.
pubmed doi - Gazulla J, Cavero-Nagore M. [Glutamate and Alzheimer's disease]. Rev Neurol. 2006;42(7):427-432.
pubmed - Meldrum BS. The role of glutamate in epilepsy and other CNS disorders. Neurology. 1994;44(11 Suppl 8):S14-23.
pubmed - Armstrong N, Sun Y, Chen GQ, Gouaux E. Structure of a glutamate-receptor ligand-binding core in complex with kainate. Nature. 1998;395(6705):913-917.
pubmed doi - Nakanishi S. Molecular diversity of glutamate receptors and implications for brain function. Science. 1992;258(5082):597-603.
pubmed doi - Watanabe M, Mishina M, Inoue Y. Distinct gene expression of the N-methyl-D-aspartate receptor channel subunit in peripheral neurons of the mouse sensory ganglia and adrenal gland. Neurosci Lett. 1994;165(1-2):183-186.
pubmed doi - Aas P, Tanso R, Fonnum F. Stimulation of peripheral cholinergic nerves by glutamate indicates a new peripheral glutamate receptor. Eur J Pharmacol. 1989;164(1):93-102.
pubmed doi - Chenu C, Serre CM, Raynal C, Burt-Pichat B, Delmas PD. Glutamate receptors are expressed by bone cells and are involved in bone resorption. Bone. 1998;22(4):295-299.
pubmed doi - Inagaki N, Kuromi H, Gonoi T, Okamoto Y, Ishida H, Seino Y, Kaneko T, et al. Expression and role of ionotropic glutamate receptors in pancreatic islet cells. FASEB J. 1995;9(8):686-691.
pubmed - Gill SS, Pulido OM. Glutamate receptors in peripheral tissues: current knowledge, future research, and implications for toxicology. Toxicol Pathol. 2001;29(2):208-223.
pubmed doi - Sureda F, Copani A, Bruno V, Knopfel T, Meltzger G, Nicoletti F. Metabotropic glutamate receptor agonists stimulate polyphosphoinositide hydrolysis in primary cultures of rat hepatocytes. Eur J Pharmacol. 1997;338(2):R1-2.
pubmed doi - Gill SS, Pulido OM, Mueller RW, McGuire PF. Immunochemical localization of the metabotropic glutamate receptors in the rat heart. Brain Res Bull. 1999;48(2):143-146.
pubmed doi - Morhenn VB, Waleh NS, Mansbridge JN, Unson D, Zolotorev A, Cline P, Toll L. Evidence for an NMDA receptor subunit in human keratinocytes and rat cardiocytes. Eur J Pharmacol. 1994;268(3):409-414.
pubmed doi - Chaudhari N, Yang H, Lamp C, Delay E, Cartford C, Than T, Roper S. The taste of monosodium glutamate: membrane receptors in taste buds. J Neurosci. 1996;16(12):3817-3826.
pubmed - Lin W, Kinnamon SC. Physiological evidence for ionotropic and metabotropic glutamate receptors in rat taste cells. J Neurophysiol. 1999;82(5):2061-2069.
pubmed - Haxhiu MA, Erokwu B, Dreshaj IA. The role of excitatory amino acids in airway reflex responses in anesthetized dogs. J Auton Nerv Syst. 1997;67(3):192-199.
pubmed doi - Kiyama H, Sato K, Tohyama M. Characteristic localization of non-NMDA type glutamate receptor subunits in the rat pituitary gland. Brain Res Mol Brain Res. 1993;19(3):262-268.
pubmed doi - Mick G. Non-N-methyl-D-aspartate glutamate receptors in glial cells and neurons of the pineal gland in a higher primate. Neuroendocrinology. 1995;61(3):256-264.
pubmed doi - Moroni F, Luzzi S, Franchi-Micheli S, Zilletti L. The presence of N-methyl-D-aspartate-type receptors for glutamic acid in the guinea pig myenteric plexus. Neurosci Lett. 1986;68(1):57-62.
pubmed doi - Tsai LH, Lee YJ, Wu J. Effect of excitatory amino acid neurotransmitters on acid secretion in the rat stomach. J Biomed Sci. 1999;6(1):36-44.
pubmed doi - Genever PG, Wilkinson DJ, Patton AJ, Peet NM, Hong Y, Mathur A, Erusalimsky JD, et al. Expression of a functional N-methyl-D-aspartate-type glutamate receptor by bone marrow megakaryocytes. Blood. 1999;93(9):2876-2883.
pubmed - Hitchcock IS, Skerry TM, Howard MR, Genever PG. NMDA receptor-mediated regulation of human megakaryocytopoiesis. Blood. 2003;102(4):1254-1259.
pubmed doi - Franconi F, Miceli M, De Montis MG, Crisafi EL, Bennardini F, Tagliamonte A. NMDA receptors play an anti-aggregating role in human platelets. Thromb Haemost. 1996;76(1):84-87.
pubmed - Zoia C, Cogliati T, Tagliabue E, Cavaletti G, Sala G, Galimberti G, Rivolta I, et al. Glutamate transporters in platelets: EAAT1 decrease in aging and in Alzheimer's disease. Neurobiol Aging. 2004;25(2):149-157.
pubmed doi - Kostanyan IA, Merkulova MI, Navolotskaya EV, Nurieva RI. Study of interaction between L-glutamate and human blood lymphocytes. Immunol Lett. 1997;58(3):177-180.
pubmed doi - Storto M, de Grazia U, Battaglia G, Felli MP, Maroder M, Gulino A, Ragona G, et al. Expression of metabotropic glutamate receptors in murine thymocytes and thymic stromal cells. J Neuroimmunol. 2000;109(2):112-120.
pubmed doi - Boldyrev AA, Kazey VI, Leinsoo TA, Mashkina AP, Tyulina OV, Johnson P, Tuneva JO, et al. Rodent lymphocytes express functionally active glutamate receptors. Biochem Biophys Res Commun. 2004;324(1):133-139.
pubmed doi - Ganor Y, Besser M, Ben-Zakay N, Unger T, Levite M. Human T cells express a functional ionotropic glutamate receptor GluR3, and glutamate by itself triggers integrin-mediated adhesion to laminin and fibronectin and chemotactic migration. J Immunol. 2003;170(8):4362-4372.
pubmed - Pacheco R, Ciruela F, Casado V, Mallol J, Gallart T, Lluis C, Franco R. Group I metabotropic glutamate receptors mediate a dual role of glutamate in T cell activation. J Biol Chem. 2004;279(32):33352-33358.
pubmed doi - Miglio G, Varsaldi F, Lombardi G. Human T lymphocytes express N-methyl-D-aspartate receptors functionally active in controlling T cell activation. Biochem Biophys Res Commun. 2005;338(4):1875-1883.
pubmed doi - Kocaeli H, Korfali E, Ozturk H, Kahveci N, Yilmazlar S. MK-801 improves neurological and histological outcomes after spinal cord ischemia induced by transient aortic cross-clipping in rats. Surg Neurol. 2005;64 Suppl 2:S22-26; discussion S27.
pubmed - McManigle JE, Gillis RA, Dretchen KL, Taveira Da Silva AM, Hernandez YM. Respiratory depression produced by intravenously administered NBQX. Pharmacology. 1998;56(6):285-290.
pubmed doi - Dunbar JC, Reinholt L, Henry RL, Mammen E. Platelet aggregation and disaggregation in the streptozotocin induced diabetic rat: the effect of sympathetic inhibition. Diabetes Res Clin Pract. 1990;9(3):265-272.
pubmed doi - Paul W, Queen LR, Page CP, Ferro A. Increased platelet aggregation in vivo in the Zucker Diabetic Fatty rat: differences from the streptozotocin diabetic rat. Br J Pharmacol. 2007;150(1):105-111.
pubmed doi - Kermarrec N, Zunic P, Beloucif S, Benessiano J, Drouet L, Payen D. Impact of inhaled nitric oxide on platelet aggregation and fibrinolysis in rats with endotoxic lung injury. Role of cyclic guanosine 5'-monophosphate. Am J Respir Crit Care Med. 1998;158(3):833-839.
pubmed - Vali VG, Velleneuve DC, Reed B et al. Evaluation of blood and bone marrow rat in :Jones TC, Ward JM, Mhr U, Hunt RD, Eds Hematopoietic system 1990:9-26.
- Morrell CN, Sun H, Ikeda M, Beique JC, Swaim AM, Mason E, Martin TV, et al. Glutamate mediates platelet activation through the AMPA receptor. J Exp Med. 2008;205(3):575-584.
pubmed doi - Lombardi G, Dianzani C, Miglio G, Canonico PL, Fantozzi R. Characterization of ionotropic glutamate receptors in human lymphocytes. Br J Pharmacol. 2001;133(6):936-944.
pubmed doi - Newsholme P, Lima MM, Procopio J, Pithon-Curi TC, Doi SQ, Bazotte RB, Curi R. Glutamine and glutamate as vital metabolites. Braz J Med Biol Res. 2003;36(2):153-163.
pubmed doi - Pithon-Curi TC, De Melo MP, Curi R. Glucose and glutamine utilization by rat lymphocytes, monocytes and neutrophils in culture: a comparative study. Cell Biochem Funct. 2004;22(5):321-326.
pubmed doi - Jiang W, Wolfe K, Xiao L, Zhang ZJ, Huang YG, Zhang X. Ionotropic glutamate receptor antagonists inhibit the proliferation of granule cell precursors in the adult brain after seizures induced by pentylenetrazol. Brain Res. 2004;1020(1-2):154-160.
pubmed doi - Hangenfeldt L & Arvdsson A. The distribution of amino acids between plasma and red blood cells in the dog. Fed Proc 1980; 25:854-861.
- Watford M. Net interorgan transport of L-glutamate in rats occurs via the plasma, not via erythrocytes. J Nutr. 2002;132(5):952-956.
pubmed - Divino Filho JC, Hazel SJ, Furst P, Bergstrom J, Hall K. Glutamate concentration in plasma, erythrocyte and muscle in relation to plasma levels of insulin-like growth factor (IGF)-I, IGF binding protein-1 and insulin in patients on haemodialysis. J Endocrinol. 1998;156(3):519-527.
pubmed doi - Bertrand G, Gross R, Puech R, Loubatieres-Mariani MM, Bockaert J. Evidence for a glutamate receptor of the AMPA subtype which mediates insulin release from rat perfused pancreas. Br J Pharmacol. 1992;106(2):354-359.
pubmed - Chae LO, Melton JE, Neubauer JA, Edelman NH. Phrenic and sympathetic nerve responses to glutamergic blockade during normoxia and hypoxia. J Appl Physiol. 1993;74(4):1954-1963.
pubmed - Pierrefiche O, Foutz AS, Champagnat J, Denavit-Saubie M. NMDA and non-NMDA receptors may play distinct roles in timing mechanisms and transmission in the feline respiratory network. J Physiol. 1994;474(3):509-523.
pubmed - Pickering DS, Thomsen C, Suzdak PD, Fletcher EJ, Robitaille R, Salter MW, MacDonald JF, et al. A comparison of two alternatively spliced forms of a metabotropic glutamate receptor coupled to phosphoinositide turnover. J Neurochem. 1993;61(1):85-92.
pubmed doi - Skerry TM, Genever PG. Glutamate signalling in non-neuronal tissues. Trends Pharmacol Sci. 2001;22(4):174-181.
pubmed doi - Foster AC, Wong EH. The novel anticonvulsant MK-801 binds to the activated state of the N-methyl-D-aspartate receptor in rat brain. Br J Pharmacol. 1987;91(2):403-409.
pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


