| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website http://www.thejh.org |
Case Report
Volume 3, Number 2, June 2014, pages 50-53
Spontaneous Remission of Paroxysmal Nocturnal Hemoglobinuria During Eculizumab Treatment
Fahri Sahina, d, Asu Fergun Yilmaza, Melda Comerta, Fusun Ozdemirkiranb, Nihal Mete Gokmenc, Guray Saydama
aDepartment of Hematology, Ege University, Izmir, Turkey
bDepartment of Hematology, Izmir Katipcelebi University, Izmir, Turkey
cDepartment of Immunology, Ege University, Izmir, Turkey
dCorresponding author: Fahri Sahin, Department of Hematology, Ege University School of Medicine, Bornova, Izmir 35100, Turkey
Manuscript accepted for publication February 25, 2014
Short title: Paroxysmal Nocturnal Hemoglobinuria
doi: https://doi.org/10.14740/jh120w
| Abstract | ▴Top |
Paroxysmal nocturnal hemoglobinuria (PNH) is a very rare acquired disease which is characterized by increased sensitivity to complement proteins. The only curative treatment option for PNH has been still allogeneic stem cell transplantation from HLA-matched donor. Eculizumab, which is a monoclonal antibody, has been established as the effective approach not only for prevention of hemolysis, but also for prevention of potential organ damages such as thromboses and failure of target organs. But eculizumab does not have any impact on, or may increase, clone status and nature of the disease. We, hereby, report a case whose disease undergone spontaneous remission under treatment of eculizumab treatment.
Keywords: Paroxysmal nocturnal hemoglobinuria; Spontaneous remission; Eculizumab
| Introduction | ▴Top |
Paroxysmal nocturnal hemoglobinuria (PNH) is an acquired clonal disorder of the hematopoietic stem cells caused by a mutation in X-linked phosphatidylinositol glycan class A (PIG-A) gene. It results in partial or complete absence of certain GPI-linked proteins. As a result, increased sensitivity of red blood cells to complement system, intravascular hemolysis and generation of inflammatory mediators occur. Especially the absence of CD55 (decay accelerating factor) and CD59 (membrane inhibitory of reactive lysis) proteins which are linked to the membrane by GPI anchor proteins leads to intravascular hemolysis, generation of inflammatory mediators and the other symptoms of the disease [1, 2].
The clinical manifestations and presenting symptoms are diverse at the presentation and during the course of the disease due to the effected organs and systems. Hemolysis, bone marrow failure with cytopenias and life-threatening thrombotic episodes are the most common clinical manifestations. Myelodysplastic syndrome and acute leukemias can also be seen in the course of the disease. One of the most common causes of mortality and morbidity in PNH patients is thromboembolism. Other causes of death are hemorrhage, renal and cardiac problems which can be related to thrombosis and intravascular hemolysis, infections, transformation to myelodysplastic syndrome or aplastic anemia [3-5]. During the course of the disease, spontaneous remission is a rare entity. A retrospective analysis consisted of 80 patients, and 12 patients had spontaneous clinical remission [6].
Eculizumab, a complement inhibitor, is a monoclonal antibody which decreases risk of thrombosis and hemolysis in patients with PNH by inhibiting complement system [7-9]. In the era of eculizumab, although the complications such as hemolysis and thrombosis can be taken under control, cure and the remission of the disease is stil a rare entity.
This report describes a case of spontaneous PNH remission during eculizumab treatment.
| Case Report | ▴Top |
A 27-year-old male was admitted to hospital due to bilateral bruising in the arm. The physical examination was normal except ecchymoses. The complete blood count revealed low platelet (13,000/mm3) and hemoglobin/hematocrit (Hb/Hct: 68g/L/19.8%) with normal leukocyte (WBC: 4,600/mm3) levels. Initial diagnosis was idiopathic thrombocytopenic purpura and the patient received intravenous immunoglobulin plus corticosteroids. Following an increase in the platelet count, the therapy was maintained with cyclosporine. Six months later, the patient has presented with thrombocytopenia once again and transferred to Ege University Hospital after an initial red blood cell and platelet transfusions. On admission, the platelet count was 20,600/mm3, Hb/Hct 95 g/L/28.7%. Biochemistry tests were normal but high lactate dehydrogenase (LDH: 629 IU/L) level was remarkable. His vitamin B12, folic acid and serum iron parameters were all normal. Hemolysis was prominent with low haptogloblin, high LDH and reticulocyte levels. Both direct and indirect combs tests were negative. His medical history was insignificant except for the prior thrombocytopenia/bruising episode. He was diagnosed as non-immune hemolytic anemia and thrombocytopenia. Bone marrow aspiration and biopsy was reported as normocellular with dyserythropoiesis. PNH was suspected due to cytopenias with hemolysis. Flow cytometry showed a PNH clone of granulocytes 25% with CD55, CD59 and the clone size was increased up to 69% next month. The eculizumab treatment was initiated. Eculizumab rapidly improved platelet count, Hb values and normalized LDH levels of the patient (Fig. 1, 2). Approximately 15 months after eculizumab therapy, flow cytometry results showed a granulocyte clone of 6.2%. Considering a PNH clone < 10% suggested spontaneous remission and we have discontinued eculizumab therapy. The patient has been closely monitored after eculizumab discontinuation, most recently at 4 months following the last dose. During the follow-up period LDH and Hb levels were stable and normal. Flow cytometry revealed a granulocyte PNH clone of 6.65% at December 2012. No thrombosis or other complications have occurred. Platelets, Hb and LDH results before, during and after the end of eculizumab treatment are shown in Figures 1-3.
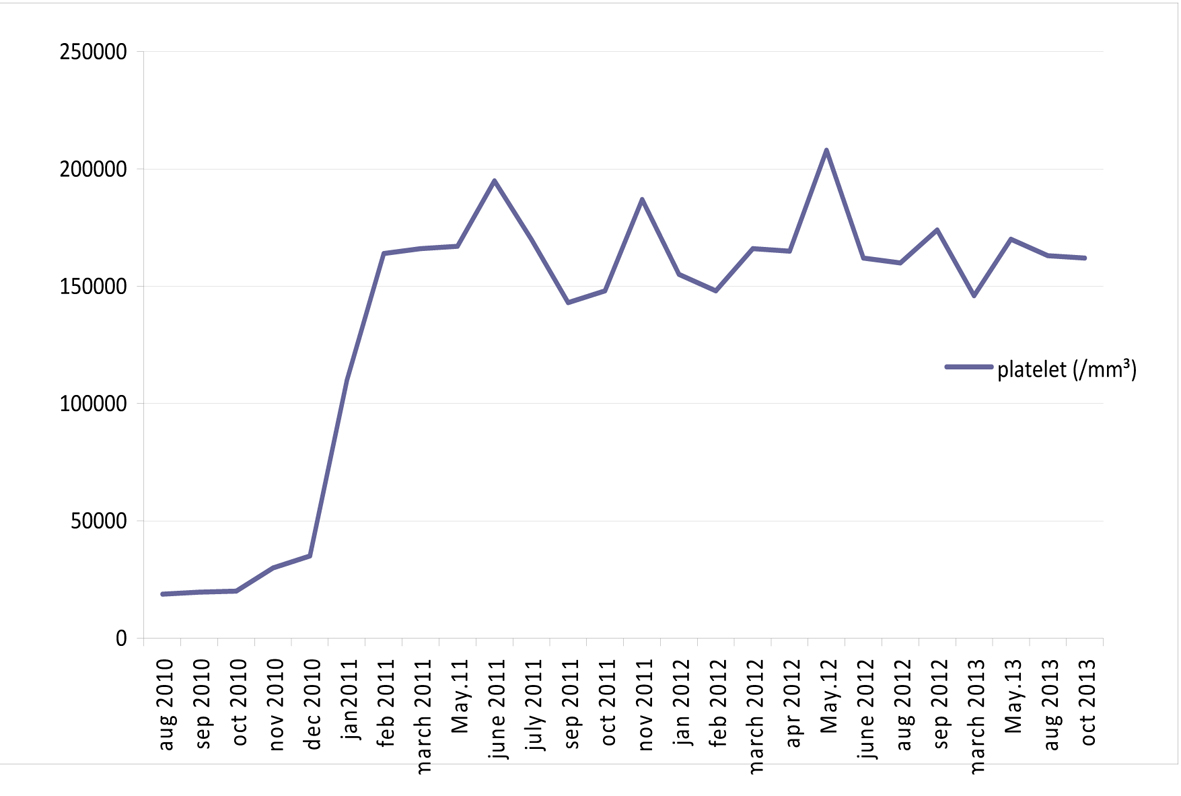 Click for large image | Figure 1. Platelet results during and after the end of eculizumab treatment. |
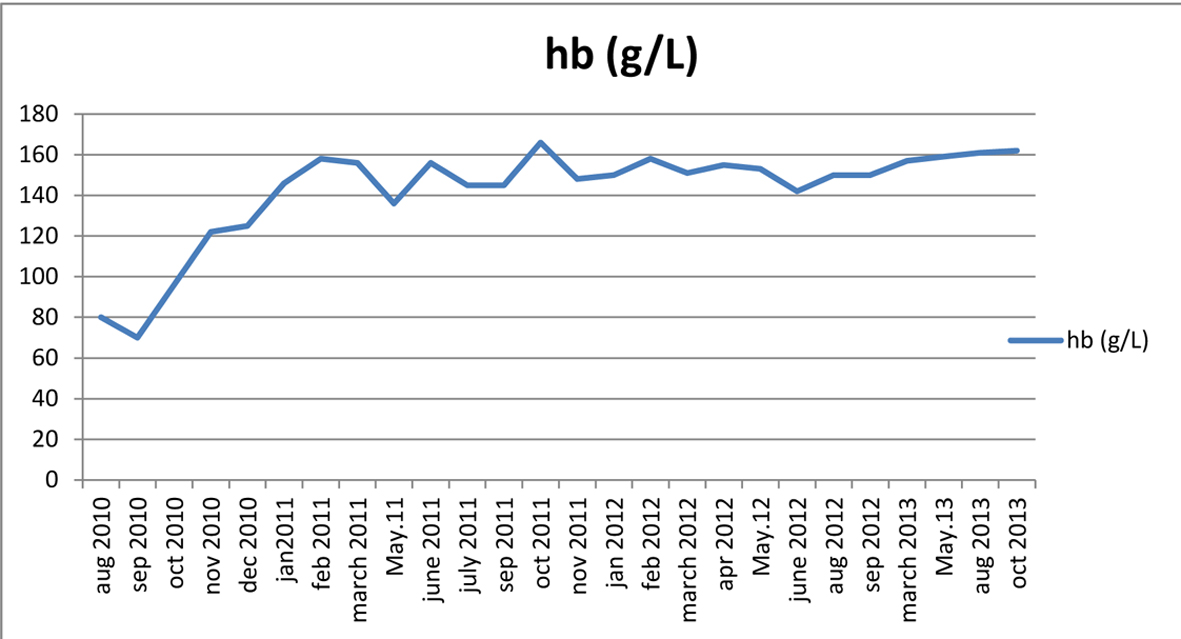 Click for large image | Figure 2. Hemoglobin results during and after the end of eculizumab treatment. |
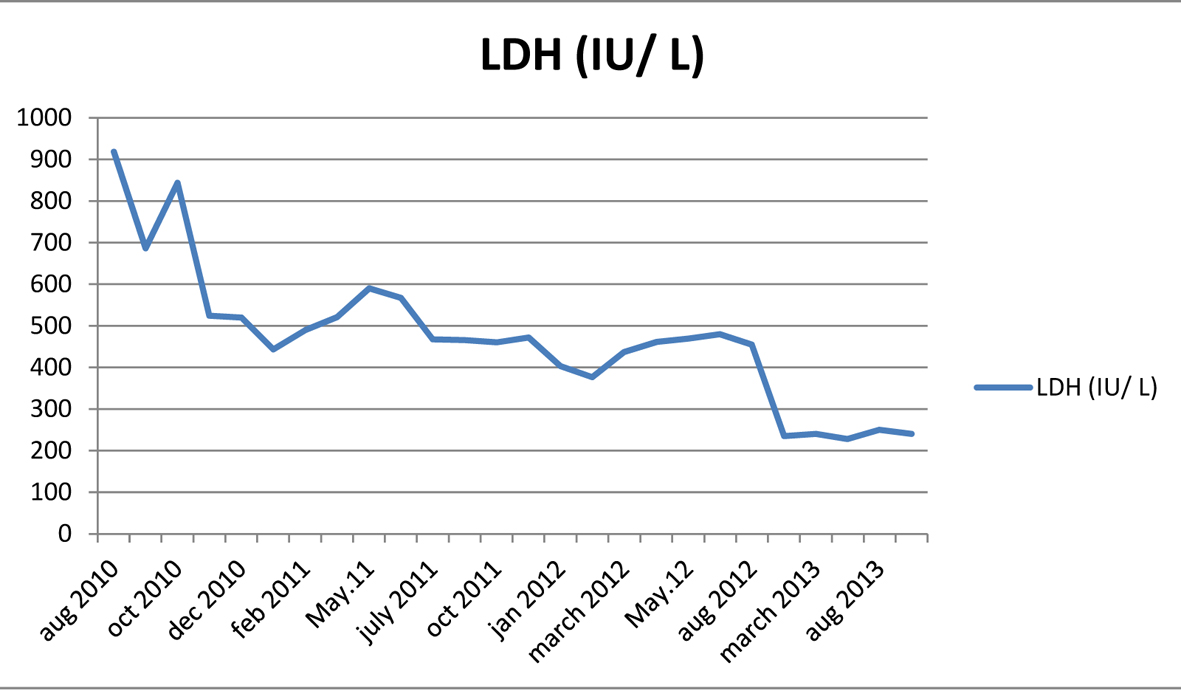 Click for large image | Figure 3. Lactate dehydrogenase results during and after the end of eculizumab treatment. |
He is still followed up with normal Hb, platelet and leucocyte counts in remission without any complication.
Conclusion
PNH is a clonal hematopoietic stem cell disease which is characterized by hemolysis, thrombosis and cytopenias. Before the treatment with eculizumab, a monoclonal antibody which inhibits the terminal complements activation [10], there is not much treatment option for PNH patients. Supportive cares like platelet and red blood cell transfusions, folic acid and vitamin B12 supplementations, treatment of thrombosis with warfarin and low molecular weight heparin and immunosuppressive therapies like cyclophosphomide are the only treatment options before the era of eculizumab [5].
Treatment with eculizumab decreases mortality and morbidity and improves the quality of life by diminishing the rate of hemolysis, need for transfusions and thrombosis without any evidence of cumulative toxicity and serious side effects. But it does not cure the disease [10-15], since this agent does not cure the disease and does not affect the clonal stem cells in the marrow; the size of the clone is not affected or even can expand during the treatment with eculizumab [13]. But in our case, the size of the PNH clones decreased during the treatment less than 10% and the treatment with eculizumab was stopped.
Allogenic stem cell transplantation is the only curative treatment option in PNH patients but it is associated with a substantial risk of graft-versus-host disease, mortality and morbidity [16].
Spontaneous remissions are rare in PNH patients. It is documented that in a retrospective study consisting of 80 patients only 12 PNH patients achieved clinical remission. In nine of these patients, the remission was documented with historical Hams test. Normal levels of GPI-linked proteins can be demonstrated in only five of these patients and only four of these patients had no neutrophils of PNH clone. In this historical series, spontaneous remission was achieved between 10 and 20 years after the diagnosis [6]. Socie et al also demonstrated spontaneous remission in 11 patients among 220 PNH patients in France [17]. A spontaneous remission was reported in a case report of a Japanese girl. She achieved clinical and hematological remission without decreasing the clonal size of PNH cells in the first years which indicates that clonal regression is not a rule for normalization of complete blood count levels [18]. Another case report from China also demonstrates the same hypothesis that it is not essential to eliminate all abnormal clones to get a clinical remission with decreasing the level pancytopenia [19]. In these case reports, the clonal size regression did not accompany the clinical remission. By contrast with this in our case, the size of the PNH clone also diminished with recovering of hemolysis and pancytopenia. We could not find a case of remission under the treatment of eculizumab.
Allogenic stem cell transplantation, the only curative treatment of PNH, should be reserved for imminently life-threatening cytopenias since even spontaneous remissions can be achieved during the course of the disease. Eculizumab should be considered before SCT to support improved quality of life, survival and allow for potential remission.
Conflict of Interest
The authors declare no competing financial interests.
| References | ▴Top |
- Rosse WF. Paroxysmal nocturnal hemoglobinuria as a molecular disease. Medicine (Baltimore). 1997;76(2):63-93.
doi - Takeda J, Miyata T, Kawagoe K, Iida Y, Endo Y, Fujita T, Takahashi M, et al. Deficiency of the GPI anchor caused by a somatic mutation of the PIG-A gene in paroxysmal nocturnal hemoglobinuria. Cell. 1993;73(4):703-711.
doi - Hill A, Kelly RJ, Hillmen P. Thrombosis in paroxysmal nocturnal hemoglobinuria. Blood. 2013;121(25):4985-4996; quiz 5105.
doi pubmed - Bessler M, Hiken J. The pathophysiology of disease in patients with paroxysmal nocturnal hemoglobinuria. Hematology Am Soc Hematol Educ Program. 2008:104-110.
doi pubmed - Risitano AM, Rotoli B. Paroxysmal nocturnal hemoglobinuria: pathophysiology, natural history and treatment options in the era of biological agents. Biologics. 2008;2(2):205-222.
pubmed - Hillmen P, Lewis SM, Bessler M, Luzzatto L, Dacie JV. Natural history of paroxysmal nocturnal hemoglobinuria. N Engl J Med. 1995;333(19):1253-1258.
doi pubmed - Hillmen P, Muus P, Duhrsen U, Risitano AM, Schubert J, Luzzatto L, Schrezenmeier H, et al. Effect of the complement inhibitor eculizumab on thromboembolism in patients with paroxysmal nocturnal hemoglobinuria. Blood. 2007;110(12):4123-4128.
doi pubmed - Kelly RJ, Hill A, Arnold LM, Brooksbank GL, Richards SJ, Cullen M, Mitchell LD, et al. Long-term treatment with eculizumab in paroxysmal nocturnal hemoglobinuria: sustained efficacy and improved survival. Blood. 2011;117(25):6786-6792.
doi pubmed - Luzzatto L, Gianfaldoni G, Notaro R. Management of paroxysmal nocturnal haemoglobinuria: a personal view. Br J Haematol. 2011;153(6):709-720.
doi pubmed - Parker C. Eculizumab for paroxysmal nocturnal haemoglobinuria. Lancet. 2009;373(9665):759-767.
doi - Hillmen P, Young NS, Schubert J, Brodsky RA, Socie G, Muus P, Roth A, et al. The complement inhibitor eculizumab in paroxysmal nocturnal hemoglobinuria. N Engl J Med. 2006;355(12):1233-1243.
doi pubmed - Hill A, Rother RP, Wang X, Morris SM, Jr., Quinn-Senger K, Kelly R, Richards SJ, et al. Effect of eculizumab on haemolysis-associated nitric oxide depletion, dyspnoea, and measures of pulmonary hypertension in patients with paroxysmal nocturnal haemoglobinuria. Br J Haematol. 2010;149(3):414-425.
doi pubmed - Brodsky RA, Young NS, Antonioli E, Risitano AM, Schrezenmeier H, Schubert J, Gaya A, et al. Multicenter phase 3 study of the complement inhibitor eculizumab for the treatment of patients with paroxysmal nocturnal hemoglobinuria. Blood. 2008;111(4):1840-1847.
doi pubmed - Schubert J, Hillmen P, Roth A, Young NS, Elebute MO, Szer J, Gianfaldoni G, et al. Eculizumab, a terminal complement inhibitor, improves anaemia in patients with paroxysmal nocturnal haemoglobinuria. Br J Haematol. 2008;142(2):263-272.
doi pubmed - Hillmen P, Muus P, Roth A, Elebute MO, Risitano AM, Schrezenmeier H, Szer J, et al. Long-term safety and efficacy of sustained eculizumab treatment in patients with paroxysmal nocturnal haemoglobinuria. Br J Haematol. 2013;162(1):62-73.
doi pubmed - Saso R, Marsh J, Cevreska L, Szer J, Gale RP, Rowlings PA, Passweg JR, et al. Bone marrow transplants for paroxysmal nocturnal haemoglobinuria. Br J Haematol. 1999;104(2):392-396.
doi pubmed - Socie G, Mary JY, de Gramont A, Rio B, Leporrier M, Rose C, Heudier P, et al. Paroxysmal nocturnal haemoglobinuria: long-term follow-up and prognostic factors. French Society of Haematology. Lancet. 1996;348(9027):573-577.
doi - Inukai T, Sugita K, Goto M, Nakamura M, Tezuka T, Goi K, Iijima K, et al. [Spontaneous recovery from pancytopenia in a young female patient with paroxysmal nocturnal hemoglobinuria(PNH): changes in the GPI-anchor expression on peripheral blood cells]. Rinsho Ketsueki. 2004;45(3):238-242.
pubmed - Li Q, Zhang Z, Lu Z. [Remnant abnormal clonal hematopoiesis in patients with paroxysmal nocturnal hemoglobinuria of clinical remission]. Zhonghua Xue Ye Xue Za Zhi. 1999;20(4):183-186.
pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


