| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Case Report
Volume 12, Number 2, April 2023, pages 92-99
Treatment and Response Evaluation Challenges in a Pregnant Woman With B-Cell Lymphoblastic Leukemia and Li-Fraumeni Syndrome
Bjarte Skoe Eriksteina, g, Aymen Bushra Ahmedb, Rakel Brendsdal Forthunc, Friedemann Lehd, Bjørn Tore Gjertsenb, e, Håkon Reikvamb, f
aDepartment of Immunology and Transfusion Medicine, Haukeland University Hospital, Bergen, Norway
bDepartment of Medicine, Hematology Section, Haukeland University Hospital, Bergen, Norway
cSection for Tumor Genomics, Haukeland University Hospital, Bergen, Norway
dDepartment of Pathology, Haukeland University Hospital, Bergen, Norway
eCentre for Cancer Biomarkers, Department of Clinical Science, Precision Oncology Research Group, University of Bergen, Bergen, Norway
fInstitute of Clinical Medicine, Faculty of Medicine, University of Oslo, Norway
gCorresponding Author: Bjarte Skoe Erikstein, Department of Immunology and Transfusion Medicine, Haukeland University Hospital, N-5021 Bergen, Norway
Manuscript submitted February 25, 2023, accepted April 19, 2023, published online April 30, 2023
Short title: Treatment and Response in B-ALL and LFS
doi: https://doi.org/10.14740/jh1107
| Abstract | ▴Top |
Li-Fraumeni syndrome (LFS) is a cancer predisposing syndrome caused by pathogenic germline TP53 gene mutations with important therapeutic and prognostic implications for many types of cancer. A small proportion of LFS patients develop B-cell lymphoblastic leukemia (B-ALL) in adult years. Standard treatment often proves inadequate, but immunotherapy has provided new treatment options. The current case report presents a pregnant woman with LFS and newly diagnosed B-ALL with hypodiploidy developed after treatment for early-onset breast cancer. We describe the treatment course, treatment-related complications and provide laboratory data crucial for evaluating and modifying treatment for this difficult clinical case. Our findings support the need for close collaboration between clinicians and experts on immunophenotyping. Through our report, we show that immunotherapy is feasible in patients with LFS and B-ALL, despite a poor initial response to induction therapy.
Keywords: Heritable TP53-related cancer; Li-Fraumeni syndrome; B-cell lymphoblastic leukemia; Pregnancy; Immunotherapy; Anti-CD22; Anti-CD20; Bispecific anti-CD19/anti-CD3
| Introduction | ▴Top |
Li-Fraumeni syndrome (LFS) is a prototypical cancer predisposing syndrome caused by pathogenic germline TP53 gene mutations. The syndrome was originally described in 1969 by Drs. Li and Fraumeni [1]. LFS predisposes development of a wide spectrum of primary childhood and adult on-set cancers, including breast cancer and hematological malignancies like myeloid and lymphoid leukemia [2]. The incidence of leukemia in LFS is approximately 4% [3]; however, the lifetime risk of cancer for persons with LFS is approximately 75% for men and 100% for women. At 40 years of age, the risk for men and women is approximately 35% and 80%, respectively [4, 5]. The perception of cancer related to germline alterations of TP53 has changed over the last 50 years after initial recognition of the syndrome. The LFS concept is now expanded to a wider cancer predisposition syndrome designated heritable TP53-related cancer (hTP53rc) [6].
There is no reported incidence of B-cell lymphoblastic leukemia (B-ALL) in pregnancy in patients with LFS, but this condition is assumed extremely rare. The incidence of acute leukemia in pregnancy is reported to be 1 in 100,000, and most of these are of myeloid origin [7-9]. However, a small proportion of leukemia are of lymphoid origin arising from T- or B-cell progenitor cells. B-ALL in adults generally has a dismal prognosis with overall cure rates < 40% [10]. Survival outcome in pregnant women after therapy approximates that of non-pregnant women [7, 9]. In situations of leukemia in a pregnant woman, clinical assessments must carefully balance between the need for treatment for the mother and the health of the fetus [7, 8].
Risks associated with glucocorticoid treatment during pregnancy include a mild increased risk of orofacial cleft, gestational hypertension, diabetes, osteoporosis, intrauterine growth retardation, and preterm delivery [11]. Cytotoxic chemotherapy during pregnancy is not considered to increase incidence of major malformations of the child when the therapy is given during the second or third trimester [8, 12, 13]. High-dose methotrexate is not advised, neither are biological agents like anti-CD20 or anti-CD22 due to the ability of the drugs to cross the placental barrier [8, 13, 14].
The knowledge base for optimal treatment of patients with B-ALL and LFS is limited, and thus this also applies to pregnant women with this disease. Standard chemotherapy has been reported to have limited effects in B-ALL patients with LFS [15], and other approaches, including immunotherapy and allogenic hematopoietic stem cell transplantation (allo-HSCT) should be considered as treatment options [16, 17]. In the present report, we describe a pregnant woman with LFS and newly diagnosed B-ALL. We describe the treatment course, illustrated with laboratory data, supporting treatment responses and evaluations, for this difficult clinical case.
| Case Report | ▴Top |
The patient was a 33-year-old woman, diagnosed with LFS at the age of 28 years. Her mother was successfully treated for bilateral breast cancer at 33 and 41 years of age, but died of cholangiocarcinoma at 50 years of age. At the age of 28, the patient was diagnosed with early-onset breast cancer with local lymph node metastasis to the right axilla, and was successfully treated with neoadjuvant cyclophosphamide, ablation of the right breast and axilla dissection, followed by adjuvant docetaxel and goserelin. As the patient was under the age of 31 years when she got the breast cancer diagnosis, she fulfilled one of the revised Chompret criteria for germline TP53 mutational testing (Table 1) [3, 4, 6]. Sanger sequencing of TP53 revealed a heterozygote (variant allele frequency (VAF) about 50%) germline missense mutation at codon 273, c.818G>A, resulting in p.(Arg273His) (NM_000546). The variant was compatible with dominantly inheritable LFS. Germline multiplex ligation-dependent probe amplification (MLPA) copy number analysis and Sanger sequencing of BRCA1, BRCA2, and CHEK2 c.1100 showed normal findings, as well as normal findings for TP53 by MLPA. Following treatment of the breast cancer, the left breast was prophylactically removed. She went to regular standard monitoring and follow-up for breast cancer in the following years but no additional clinical monitoring with regard to her LFS was performed.
 Click to view | Table 1. The Chompret Criteria for Germline TP53 Mutational Testing From 2015 |
Five years after diagnosis of the breast cancer, the patient was admitted to hospital with pain in the upper right quadrant of abdomen, dyspnea, and fatigue, gradually worsening over the last week prior to admission. The patient was pregnant at gestational week 26 + 4. Laboratory tests revealed anemia and thrombocytopenia, and elevated lactate dehydrogenase (LDH). The spleen was slightly enlarged. Ultrasound investigation of the abdomen showed that the fetus had normal biophysical profile and growth. Based on the history with LFS, breast cancer and current findings of cytopenia, inflammation and splenomegaly, a bone marrow aspiration was performed. Flow cytometry detected a monoclonal expansion of cells amounting to 25% of viable white cells expressing CD45weak, CD19pos, cyCD79bpos, TdTpos and CD34pos, while the cells were negative for myeloid and T-cell markers (Fig. 1a-d). The B-cell expansion was staged according to the Associazone Italiana Ematologia Oncologia Pediatrica and Berlin-Frankfurt-Munster group (AIEOP-BFM) staging system [18]. Approximately 90% of the immature B cells lacked CD10 and corresponded to the AIEOP-BFM B-I type. Importantly, most of these cells had a weak expression of the more mature B-cell markers CD22 and CD20. In the remaining 10% immature B cells, there was a positive CD10 expression that correlated to stronger CD20 expression, indicating that these cells were more similar to AIEOP-BFM B-II. G-banding and copy number analyses showed low-hypodiploid karyotype with 37 chromosomes and monosomies for chromosome 3, 4, 5, 7, 9, 15, 16, 17 and 20 in addition to del(2)(q11.2) (Fig. 2b), resulting in high-risk genetics according to the ALLTogether protocol (ClinicalTrials.gov identifier: NCT03911128). Due to monosomy for chromosomes 5, 7 and 9, the patient had functional loss of one copy of the EBF1, IKZF1, CDKN2A, CDKN2B, and PAX5 genes, associated with unfavorable copy number alteration profile. Fluorescence in situ hybridization (FISH) analyses (probes for ABL2, BCR-ABL1, PDGFRb and KMT2A) confirmed results from the G-banding, and RNA-based translocation analysis showed normal findings. In summary, the findings were diagnostic for Philadelphia chromosome negative B-ALL with low-hypodiploid karyotype (Fig. 2b). Bone marrow biopsy confirmed the diagnosis of B-ALL, and immunohistochemical investigation determined the malignant cells to be PAX5pos, TdTpos and CD34pos (Fig. 2c).
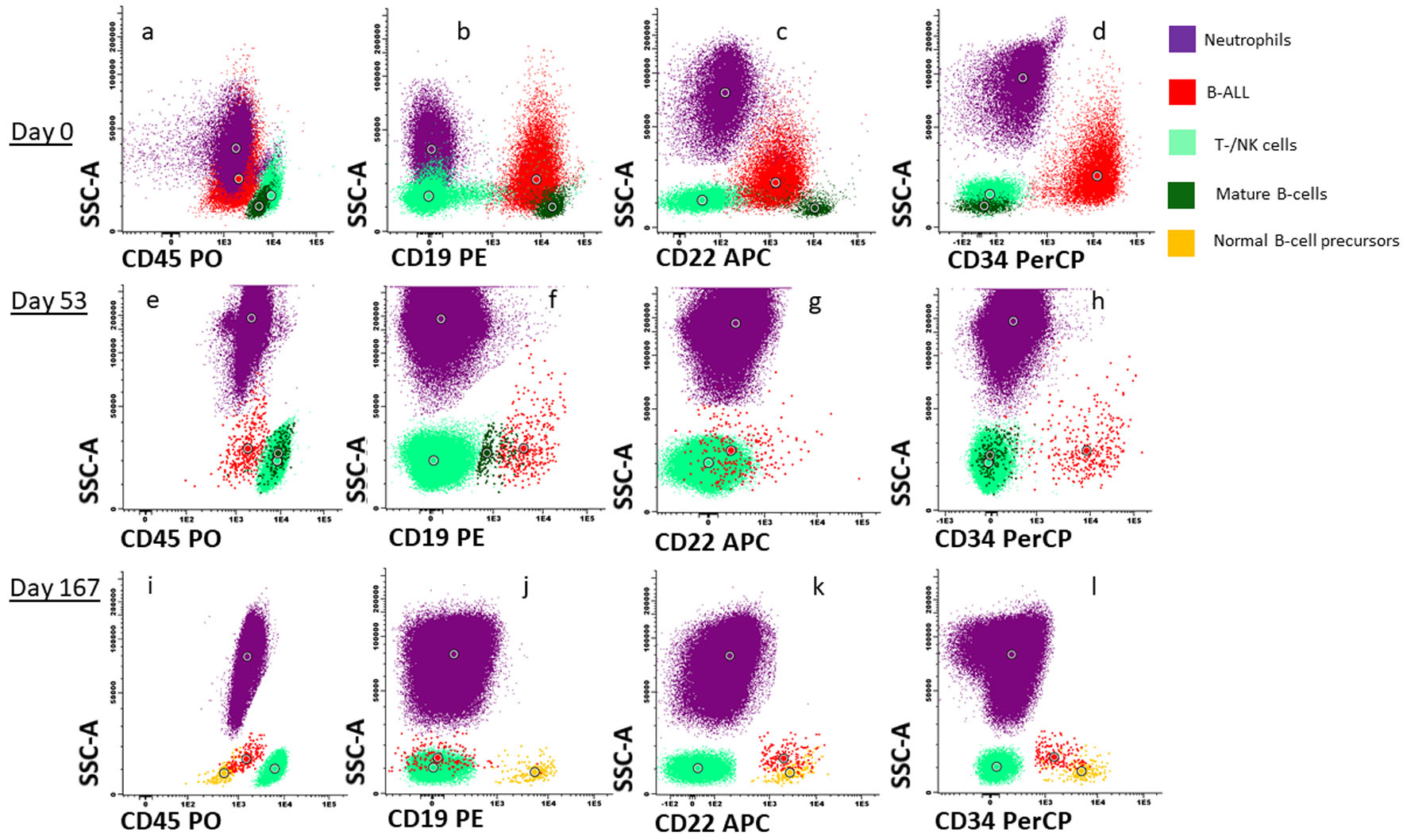 Click for large image | Figure 1. Flowcytometric characterization and response after treatment. Dot plot (a) to (d) represents CD45, CD19, CD22 and CD34 expression respectively on neutrophils (purple), B-ALL cells (red), T-/NK cells (light green), mature B cells (dark green) and normal B-cell progenitors (orange) at time of diagnosis. Dot plot (e) to (h) represents CD45, CD19, CD22 and CD34 expression respectively in the same cell subsets at day 53 after first induction treatment and dot plot (i) to (l) represents cell subsets at day 167. B-ALL: B-cell lymphoblastic leukemia. |
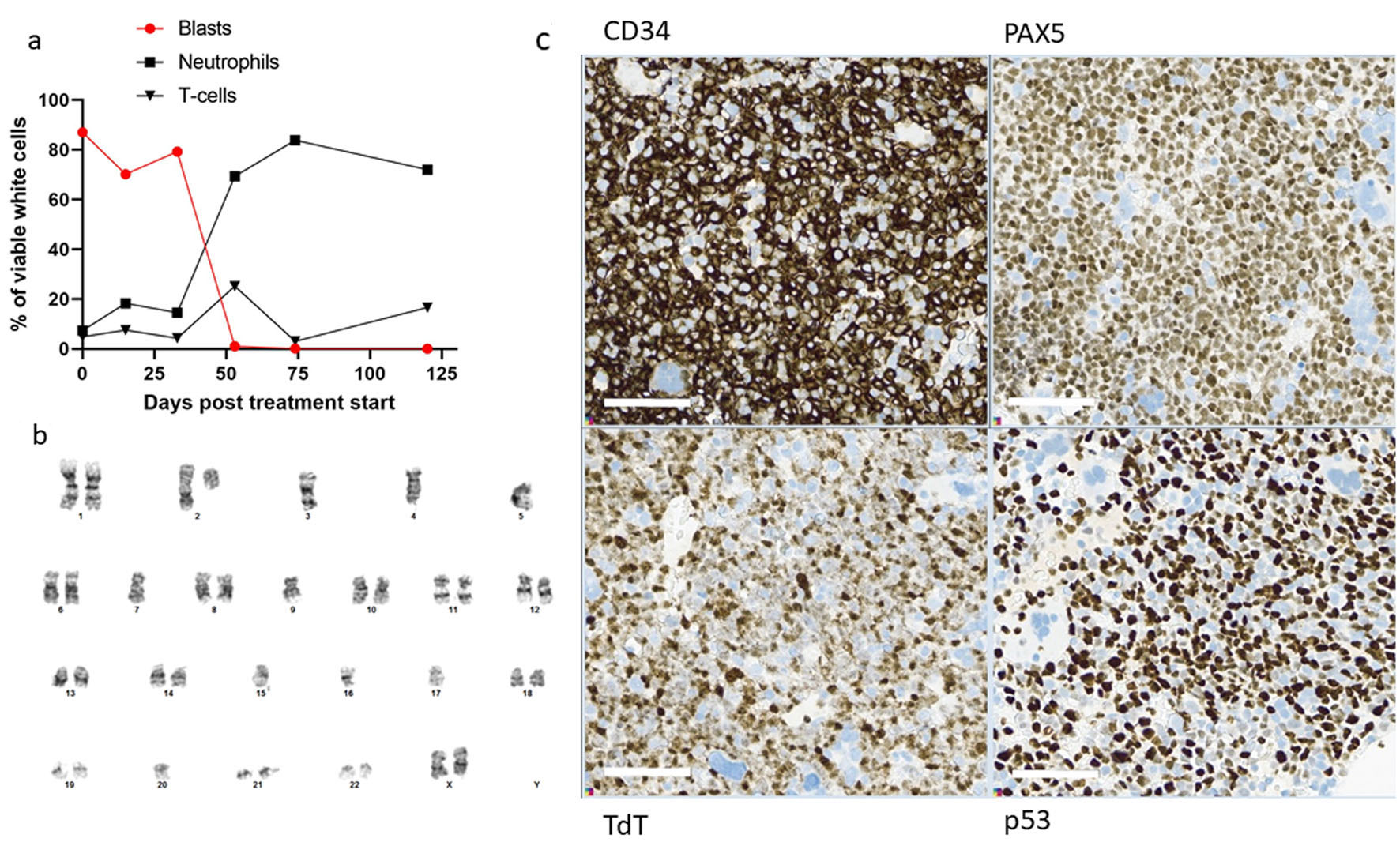 Click for large image | Figure 2. Treatment response. (a) Flowcytometric assessment of percentage B-ALL cells (circle), neutrophils (square) and T cells (triangle), at diagnosis, day 15, day 33, day 53, day 74 and day 120 after induction treatment. (b) G-banding of 17 metaphases after unstimulated cultivation showed low-hypodiploid phenotype with the karyotype 37,XX,del(2)(q11.2),-3,-4,-5,-7,-9,-15,-16,-17,-20[cp6]/46,XX[11]. Copy number array analysis supported the findings. (c) Immunohistochemical stains of bone marrow biopsy, showing many blast cells positive for CD34, PAX5, TdT and p53. In wild-type p53 patients, staining for p53 is varying weak to moderate positive. B-ALL: B-cell lymphoblastic leukemia. |
As the patient now was in gestational week 27 and in relatively good general health, delivery and induction treatment were postponed for 4 weeks to avoid an early delivery that can potentially give immaturity-related health problems for the child. However, during the next days, the patient experienced increasing pain in the right lower costae and increasing dyspnea. Blood samples showed decreased hemoglobin (8.4 g/dL) and thrombocytes (51 × 109/L) while lactate increased to 6.9 mmol/L. Due to lactic acidosis and increasing cancer-related pain, it was regarded crucial to start anti-leukemic treatment. B-ALL treatment within the NOHPO ALL2008 protocol was initiated [19], including administration of vincristine, doxorubicin, and dexamethasone. Lactate increased from 6.9 to 12.2 mmol/L the day after initiation of induction therapy, but gradually decreased over the next 3 days. The patient’s general condition gradually improved, especially during the second week of chemotherapy. Flowcytometric analysis of bone marrow aspirate on day 15, however, showed 77% B-ALL cells (Fig. 2a) with a stronger CD20 expression than before treatment. Due to the poor treatment response and increased fetal maturation, induced vaginal delivery was prepared. Chemotherapy was discontinued, and peripheral white blood cells (WBCs) increased to acceptable levels prior to birth. An uncomplicated vaginal delivery took place in gestational week 31 + 4, 26 days after initiation of induction therapy, with the birth of a healthy child.
Based on the clinical situation, coexisting LFS and lack of initial response to conventional therapy, a treatment course with the mini-hyper-CVD plus Ara-C/mini-MTX regimen with addition of inotuzumab (anti-CD22), rituximab (anti-CD20) and blinatumomab (bi-specific anti-CD3 and anti-CD19), was initiated (Fig. 3) [16, 20-22].
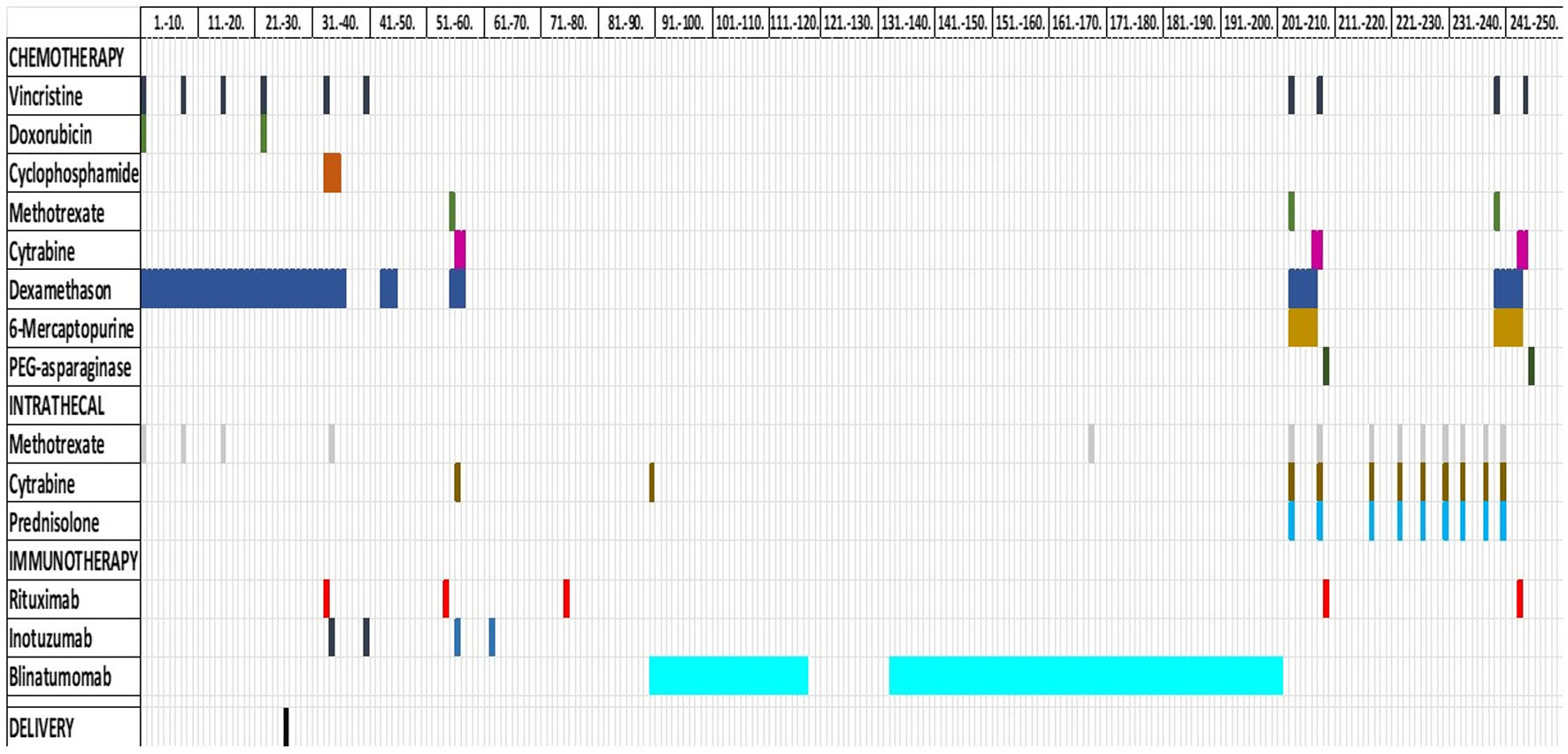 Click for large image | Figure 3. Treatment. Overview of the systemic chemo-immunotherapy that the patient received, both intravenously and intrathecally. Time of delivery is also marked. |
Three days postpartum, the patient complained of gradually decreased vision, and optical coherence tomography revealed a central serous chorioretinopathy. Hence, steroid treatment was terminated and the vision improved after this. In order to rule out extraocular reasons for the loss of vision, a magnetic resonance imaging (MRI) of caput was performed. This exhibited a cortical vein thrombosis in the convex part of the left hemisphere and the patient was anticoagulated with low molecular heparin.
Flow cytometry analysis of bone marrow aspirate at day 53 after initiation of induction treatment, 20 days after initiation of the mini-hyper-CVD with inotuzumab and rituximab, detected 1% remaining B-ALL cells (Figs. 1e-h and 2a). These cells were negative for CD20 expression and CD22 was also weaker than at diagnosis. At day 73, the patient had received two doses of rituximab and four doses of inotuzumab. Flow cytometry analysis of bone marrow aspirate indicated 0.14% remaining B-ALL cells. At day 90, blinatumomab treatment was initiated. The patient experienced mild cytokine release syndrome from the first day, with elevated core temperature, fatigue, vomiting, arthralgia, and myalgia [23]. The symptoms resolved within 3 days, but some arthralgia remained during the first 28 days of treatment.
Flow cytometry analysis of bone marrow aspirate at day 120 detected a residual clone of blast cells of 0.08%. To discern whether these cells were normal progenitor cells or malignant cells was challenging. The important B-cell markers CD19, CD20, and CD22 were downregulated after anti-CD19, anti-CD20 and anti-CD22 treatment; however, some of the cells were still CD22pos and CD34pos. We assumed that these cells represented residual B-ALL as progenitor B cells would probably have CD19 expression.
After the second course of blinatumomab, flow cytometry detected 0.11% CD19neg, CD22pos, CD34pos blast cells. At this time, a small proportion of normal B-cell progenitor cells positive for CD19, CD10 and CD34, was also evident (Fig. 1i-l). However, 1 month later, following two rounds of blinatumomab, there were no longer flowcytometric evidence of residual B-ALL cells in the bone marrow.
During the third round of blinatumomab, while the patient awaited allo-hematopoietic stem cell transplant (HSCT), she experienced apraxia, aphasia and a reduced visual field. MRI of caput exhibited a 2.5 cm expansive process in occipital region of the brain at the left side (Fig. 4). The lesion was surgically removed and histological examination revealed it to be a relapse of B-ALL. It was assumed that leukemic cells could be dispersed in the brain at other places than in the expanded lesion. As the patient had LFS, whole brain radiation was excluded as a possibility. Instead, ALLTogether protocol block B was initiated with methotrexate, cytarabine, vincristine, dexamethasone, 6-mercaptopurine and pegylated asparaginase. Due to the central nervous system (CNS) involvement, the patient also received the intrathecal protocol treatment with methotrexate, cytarabine and a steroid twice per week (Fig. 3). Post-treatment MRI of CNS revealed remission with some remaining contrast along the resection margin, which could be due to reactive changes or persistent tumor cells. A second ALLTogether protocol block B was initiated as the patient awaited allogeneic stem cell transplantation. Due to severe therapy-related lower extremity and bladder paresthesia, allo-HSCT was delayed. Six months after the second block B treatment, toxic effects were mostly resolved and allo-HSCT could commence. MRI and flow minimal residual disease (MRD) assessment of bone marrow and spinal fluid did not show signs of ALL cells at this time.
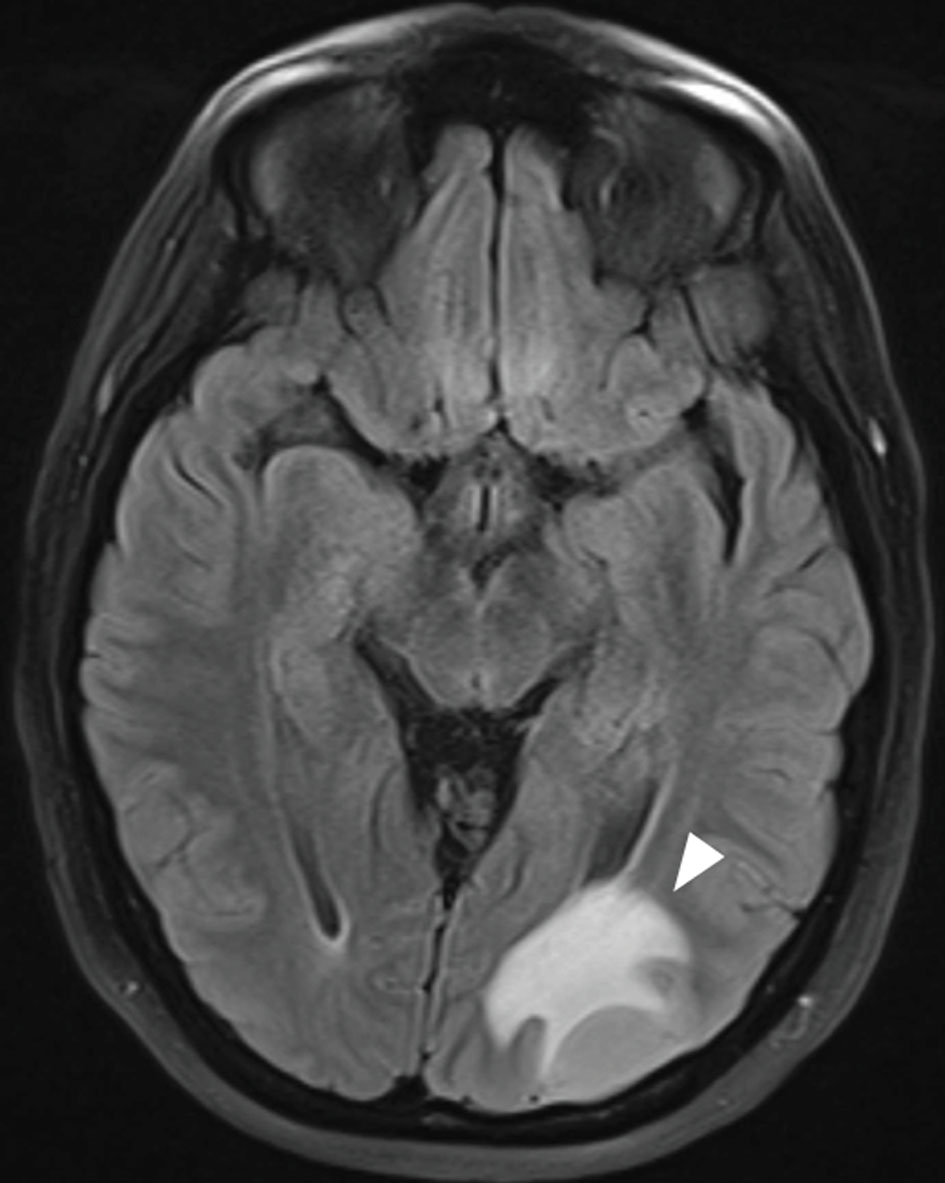 Click for large image | Figure 4. MRI of caput of B-ALL relapse in CNS demonstrating widespread leukemic tumor infiltration in the left occipital hemisphere. The white arrow indicates the B-ALL relapse. B-ALL: B-cell lymphoblastic leukemia; CNS: central nervous system; MRI: magnetic resonance imaging. |
On day 53 after initiation of CNS relapse treatment, she was consolidated with allo-HSCT with a 10/10 human leukocyte antigen (HLA)-matched unrelated donor after receiving reduced intensity conditioning (RIC) with thiotepa, fludarabine and busulfan. The donated cells engrafted after 15 days, but dermatological acute graft versus host disease (GVHD) followed, requiring systemic corticosteroid treatment.
At day 30 after transplantation, the patient experienced diarrhea and nausea. Colonoscopy of the sigmoid colon revealed an adenocarcinoma (T2N0M0). Immunosuppressive medication was subsequently gradually discontinued, before a robot-assisted sigmoid resection was performed. At day 105 after allo-HSCT, MRD was < 0.1%, and 5 months after control, no signs of GVHD had further developed.
| Discussion | ▴Top |
This case presentation exhibits several uncommon coexisting features: 1) B-ALL in a pregnant woman; 2) LFS-related malignancy; 3) therapeutic side effects amplified by pregnancy; and 4) individualized antigen-dependent immune-mediated medicine. The main challenge was to treat the mother’s malignancy and at the same time preserve the fetus’ health.
LFS is related to germline loss of function of TP53, associated with cancer at young age, especially soft-tissue sarcomas, adrenocortical carcinomas, CNS tumors, leukemias or early-onset breast cancers in females, and clinical guidelines for follow-up of these patients are developed [6]. Even though there is little knowledge about optimal treatment of LFS-related B-ALL, Pepper et al demonstrated that lymphoblastic cells exhibited intrinsic resistance to conventional chemotherapeutics like chlorambucil and fludarabine [24]. The TP53 mutation is furthermore likely to result in a loss of the p53-mediated DNA damage response and thus infer resistance to some of the conventional anti-leukemic agents [16, 25]. This might explain the poor response during the initial induction of the patient. Obstetric physicians wanted to postpone delivery as long as possible in order to let the fetus mature more. However, due to the poor treatment effect, a more intensive and effective anti-leukemic treatment was warranted. This new regiment could affect the fetus adversely and delivery had to be achieved before this treatment could be initiated. One of the main changes in the chemotherapeutic regiment was inclusion of cyclophosphamide instead of doxorubicin. Interestingly, her breast cancer treated 5 years previously was considered to have a partial response to cyclophosphamide. The drug is further reported to induce a higher rate of response in subtypes of TP53-mutated breast carcinomas, possibly due to lack of cell cycle arrest and instead induction of aberrant mitoses and subsequent cell death [26]. Whether this was the reason for the increased effect of the new therapeutic regiment is unknown. The tubulin inhibitor vincristine, in addition to dexamethasone, was continued. Inclusion of immunotherapy has been a promising and advised new development in therapy against refractory B-ALL [16]. However, this type of therapy could not start until the fetus was delivered, due to the possibility of fetal B-cell depletion. Blinatumomab, rituximab, and inotuzumab do all add anti-leukemic effect to already existing chemotherapeutic regiments [20-22], and the first two also have a favorable non-genotoxic profile in patients with TP53 mutations [6]. Inotuzumab, however, is a conjugate between anti-CD22 and calicheamicin, a DNA-alkylating agent [21]. Whether this substance might increase the risk of new primary cancers in TP53 mutated B-ALL patients is unknown.
Allo-HSCT treatment includes chemotherapeutics with mutagenic potential. This treatment regimen is, however, preferred for B-ALL patients with poor prognosis.
Glucocorticoids like dexamethasone have remained a cornerstone in treatment of B-ALL for at least 70 years [27]. These substances are anti-leukemic through binding to the glucocorticoid receptor, changing genetic transcription and subsequent increasing apoptosis and cell cycle arrest. Functional p53 seems to be involved in dexamethasone’s toxic effects [28], although probably only partially as also other intracellular pathways are involved [27]. The patient reported vision disturbances during the last 3 weeks of the pregnancy, but the symptom became stronger and more pronounced after dexamethasone treatment. Both pregnancy [29] and systemic corticoid therapy [30] can induce central serous chorioretinopathy. The effects are probably due to both endogenous and exogenous cortisol stimulation. The prognosis is good as the natural course of the condition often is self-limiting [31]. This was also the case for our patient as the symptoms resolved after discontinuation of dexamethasone. When she was administered dexamethasone later in the treatment, she once more experienced transitional blurred vision.
Flow cytometry is an important method for determination of residual disease after anti-leukemic treatment [19, 32, 33]. The expression of antigens on B-ALL cells can change after standard chemotherapy but with the introduction of immunotherapy, flow cytometric detection of B-ALL cells has become even more difficult [32, 33]. Some of the B-ALL cells might be negative for the antigens that the immunotherapy is directed against, and these cells will survive. The reported B-ALL cells immunophenotypic profile was complex at diagnosis, but the more mature CD10pos/CD20weak AIEOP-BFM B-II subtype seemed to disappear after immunotherapy. As CD19 expression of B-ALL cells after blinatumomab treatment decreased, we had to focus on other B-cell markers [32] to monitor therapy response. Rituximab treatment effectively eradicated CD20pos cells, but still left 0.08% cells that could represent residual B-ALL as they were CD22pos and CD34pos, but without CD19 expression like normal B-cell precursor cells often have (Fig. 1). CD19neg, CD34pos and CD22pos normal B-cell progenitors have been reported [32], but the patient’s blast cells were slightly larger and more granulated than normal B-cell progenitor cells, according to our experience.
The prognosis of patients with B-ALL correlates to the presence of MRD after treatment. MRD indicates both a poorer event free survival (EVF), and decreased overall survival [34]. The patient in this case achieved MRD < 0.01% indicating longer EVF [34].
Learning points
This case report highlights the challenge of balancing a mother’s health with her fetus’ health when the mother has LFS and B-ALL, the importance of extended immunophenotypic characterization in order to reveal therapeutic options, and the difficulty of immunophenotypic monitoring of anti-leukemic effect after immune therapy. Close collaboration among clinicians, geneticists, pathologists and experts on immunophenotypic characterization is essential. The complex treatment also imposes considerable risk of side effects. Early recognition of these is important to avoid unnecessary morbidity and mortality. Through our report, we show that immunotherapy is feasible in patients with LFS and B-ALL, despite a poor initial response to induction therapy.
Acknowledgments
We thank Dr. Knut Liseth and the Department of Immunology and Transfusion Medicine for the assistance with analysis and interpretation of flow cytometry data. All health care workers involved in the treatment of the patient are acknowledged. We are also very grateful to the patient, who consented to share her medical history.
Financial Disclosure
The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest
The authors declare that they have no conflict of interest.
Informed Consent
The patient gave written, informed consent for publication of this case report.
Author Contributions
BSE: concept, design, data collection, flowcytometric analysis and initial draft of the manuscript. ABA: treating physician and revised the manuscript for important intellectual content. RBF: reviewed the genetic information and revised the draft for important intellectual content. FL: reviewed that pathology slides and revised the draft for important intellectual content. BTG: treating physician and revised the draft for important intellectual content. HR: treating physician, created figures, edited and revised the manuscript for important intellectual content.
Data Availability
The authors declare that data supporting the findings of this study are available within the article. Any questions regarding data availability should be directed to the corresponding author and will be made available upon reasonable request.
| References | ▴Top |
- Li FP, Fraumeni JF, Jr. Soft-tissue sarcomas, breast cancer, and other neoplasms. A familial syndrome? Ann Intern Med. 1969;71(4):747-752.
doi pubmed - Valdez JM, Nichols KE, Kesserwan C. Li-Fraumeni syndrome: a paradigm for the understanding of hereditary cancer predisposition. Br J Haematol. 2017;176(4):539-552.
doi pubmed - Bougeard G, Renaux-Petel M, Flaman JM, Charbonnier C, Fermey P, Belotti M, Gauthier-Villars M, et al. Revisiting Li-Fraumeni syndrome from TP53 mutation carriers. J Clin Oncol. 2015;33(21):2345-2352.
doi pubmed - Chompret A, Brugieres L, Ronsin M, Gardes M, Dessarps-Freichey F, Abel A, Hua D, et al. P53 germline mutations in childhood cancers and cancer risk for carrier individuals. Br J Cancer. 2000;82(12):1932-1937.
doi pubmed pmc - Hwang SJ, Lozano G, Amos CI, Strong LC. Germline p53 mutations in a cohort with childhood sarcoma: sex differences in cancer risk. Am J Hum Genet. 2003;72(4):975-983.
doi pubmed pmc - Frebourg T, Bajalica Lagercrantz S, Oliveira C, Magenheim R, Evans DG, European Reference Network Group. Guidelines for the Li-Fraumeni and heritable TP53-related cancer syndromes. Eur J Hum Genet. 2020;28(10):1379-1386.
doi pubmed pmc - McGregor AK, Das-Gupta E. Acute myeloid leukaemia in pregnancy. Br J Haematol. 2015;170(4):441-442.
doi pubmed - Zhu D, Tang D, Chai X, Zhang G, Wang Y. Acute leukemia in pregnancy: a single institutional experience with 21 cases at 10 years and a review of the literature. Ann Med. 2021;53(1):567-575.
doi pubmed pmc - Ticku J, Oberoi S, Friend S, Busowski J, Langenstroer M, Baidas S. Acute lymphoblastic leukemia in pregnancy: a case report with literature review. Ther Adv Hematol. 2013;4(5):313-319.
doi pubmed pmc - Paul S, Kantarjian H, Jabbour EJ. Adult acute lymphoblastic leukemia. Mayo Clin Proc. 2016;91(11):1645-1666.
doi pubmed - Gerosa M, Meroni PL, Cimaz R. Safety considerations when prescribing immunosuppression medication to pregnant women. Expert Opin Drug Saf. 2014;13(12):1591-1599.
doi pubmed - Hepner A, Negrini D, Hase EA, Exman P, Testa L, Trinconi AF, Filassi JR, et al. Cancer during pregnancy: the oncologist overview. World J Oncol. 2019;10(1):28-34.
doi pubmed pmc - Milojkovic D, Apperley JF. How I treat leukemia during pregnancy. Blood. 2014;123(7):974-984.
doi pubmed - Pavanello F, Zucca E, Ghielmini M. Rituximab: 13 open questions after 20years of clinical use. Cancer Treat Rev. 2017;53:38-46.
doi pubmed - Kappel S, Janschek E, Wolf B, Rudas M, Teleky B, Jakesz R, Kandioler D. TP53 germline mutation may affect response to anticancer treatments: analysis of an intensively treated Li-Fraumeni family. Breast Cancer Res Treat. 2015;151(3):671-678.
doi pubmed - Li B, Brady SW, Ma X, Shen S, Zhang Y, Li Y, Szlachta K, et al. Therapy-induced mutations drive the genomic landscape of relapsed acute lymphoblastic leukemia. Blood. 2020;135(1):41-55.
doi pubmed pmc - Winter G, Kirschner-Schwabe R, Groeneveld-Krentz S, Escherich G, Moricke A, von Stackelberg A, Stanulla M, et al. Clinical and genetic characteristics of children with acute lymphoblastic leukemia and Li-Fraumeni syndrome. Leukemia. 2021;35(5):1475-1479.
doi pubmed pmc - Dworzak MN, Buldini B, Gaipa G, Ratei R, Hrusak O, Luria D, Rosenthal E, et al. AIEOP-BFM consensus guidelines 2016 for flow cytometric immunophenotyping of Pediatric acute lymphoblastic leukemia. Cytometry B Clin Cytom. 2018;94(1):82-93.
doi pubmed - Toft N, Birgens H, Abrahamsson J, Bernell P, Griskevicius L, Hallbook H, Heyman M, et al. Risk group assignment differs for children and adults 1-45 yr with acute lymphoblastic leukemia treated by the NOPHO ALL-2008 protocol. Eur J Haematol. 2013;90(5):404-412.
doi pubmed - Thomas DA, O'Brien S, Faderl S, Garcia-Manero G, Ferrajoli A, Wierda W, Ravandi F, et al. Chemoimmunotherapy with a modified hyper-CVAD and rituximab regimen improves outcome in de novo Philadelphia chromosome-negative precursor B-lineage acute lymphoblastic leukemia. J Clin Oncol. 2010;28(24):3880-3889.
doi pubmed pmc - Abuasab T, Rowe J, Tvito A. Emerging monoclonal antibody therapy for the treatment of acute lymphoblastic leukemia. Biologics. 2021;15:419-431.
doi pubmed pmc - Jabbour E, Sasaki K, Ravandi F, Huang X, Short NJ, Khouri M, Kebriaei P, et al. Chemoimmunotherapy with inotuzumab ozogamicin combined with mini-hyper-CVD, with or without blinatumomab, is highly effective in patients with Philadelphia chromosome-negative acute lymphoblastic leukemia in first salvage. Cancer. 2018;124(20):4044-4055.
doi pubmed pmc - Tvedt THA, Vo AK, Bruserud O, Reikvam H. Cytokine release syndrome in the immunotherapy of hematological malignancies: the biology behind and possible clinical consequences. J Clin Med. 2021;10(21):5190.
doi pubmed pmc - Pepper C, Thomas A, Hoy T, Tighe J, Culligan D, Fegan C, Bentley P. Leukemic and non-leukemic lymphocytes from patients with Li Fraumeni syndrome demonstrate loss of p53 function, Bcl-2 family dysregulation and intrinsic resistance to conventional chemotherapeutic drugs but not flavopiridol. Cell Cycle. 2003;2(1):53-58.
pubmed - Irving JA, Enshaei A, Parker CA, Sutton R, Kuiper RP, Erhorn A, Minto L, et al. Integration of genetic and clinical risk factors improves prognostication in relapsed childhood B-cell precursor acute lymphoblastic leukemia. Blood. 2016;128(7):911-922.
doi pubmed pmc - Bertheau P, Lehmann-Che J, Varna M, Dumay A, Poirot B, Porcher R, Turpin E, et al. p53 in breast cancer subtypes and new insights into response to chemotherapy. Breast. 2013;22(Suppl 2):S27-S29.
doi pubmed - Inaba H, Pui CH. Glucocorticoid use in acute lymphoblastic leukaemia. Lancet Oncol. 2010;11(11):1096-1106.
doi pubmed pmc - Olivas-Aguirre M, Torres-Lopez L, Pottosin I, Dobrovinskaya O. Overcoming glucocorticoid resistance in acute lymphoblastic leukemia: repurposed drugs can improve the protocol. Front Oncol. 2021;11:617937.
doi pubmed pmc - Sunness JS, Haller JA, Fine SL. Central serous chorioretinopathy and pregnancy. Arch Ophthalmol. 1993;111(3):360-364.
doi pubmed - Wakakura M, Song E, Ishikawa S. Corticosteroid-induced central serous chorioretinopathy. Jpn J Ophthalmol. 1997;41(3):180-185.
doi pubmed - Semeraro F, Morescalchi F, Russo A, Gambicorti E, Pilotto A, Parmeggiani F, Bartollino S, et al. Central serous chorioretinopathy: pathogenesis and management. Clin Ophthalmol. 2019;13:2341-2352.
doi pubmed pmc - Cherian S, Miller V, McCullouch V, Dougherty K, Fromm JR, Wood BL. A novel flow cytometric assay for detection of residual disease in patients with B-lymphoblastic leukemia/lymphoma post anti-CD19 therapy. Cytometry B Clin Cytom. 2018;94(1):112-120.
doi pubmed - Liu Z, Li Y, Shi C. Monitoring minimal/measurable residual disease in B-cell acute lymphoblastic leukemia by flow cytometry during targeted therapy. Int J Hematol. 2021;113(3):337-343.
doi pubmed - Berry DA, Zhou S, Higley H, Mukundan L, Fu S, Reaman GH, Wood BL, et al. Association of minimal residual disease with clinical outcome in pediatric and adult acute lymphoblastic leukemia: a meta-analysis. JAMA Oncol. 2017;3(7):e170580.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


