| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Original Article
Volume 12, Number 1, February 2023, pages 16-26
Clofarabine in Pediatric Acute Relapsed or Refractory Leukemia: Where Do We Stand on the Bridge to Hematopoietic Stem Cell Transplantation?
Sarah Ramiza, Osama Elhaja, Khawar Siddiquia, Saadiya Khana, Hawazen AlSaedia, Awatif AlAnazia, Ali Al-Ahmaria, Abdullah Al-Jefria, Oudai Sahvanb, Mouhab Ayasa, Ibrahim Ghemlasa, c
aDepartment of Pediatric Hematology/Oncology, King Faisal Specialist Hospital and Research Center, Riyadh 11211, Saudi Arabia
bCollege of Medicine, Alfaisal University, Riyadh 11533, Saudi Arabia
cCorresponding Author: Ibrahim Ghemlas, Department of Pediatric Hematology/Oncology, MBC 53, King Faisal Specialist Hospital and Research Center, PO Box 3354, Riyadh 11211, Saudi Arabia
Manuscript submitted October 6, 2022, accepted January 12, 2023, published online February 25, 2023
Short title: Bridging Chemotherapy to HSCT in Pediatric Leukemia
doi: https://doi.org/10.14740/jh1065
| Abstract | ▴Top |
Background: Despite pronounced improvement in overall survival (OS) in pediatric leukemia, a proportion of patients continue to suffer from lack of response or relapse, and the management of such patients is exceedingly difficult. Immunotherapy and engineered chimeric antigen receptor (CAR) T-cell therapy have shown promising results in the course of relapsed or refractory acute lymphoblastic leukemia (ALL). However, conventional chemotherapy continues to be utilized for re-induction purposes whether independently or in combination with immunotherapy.
Methods: Forty-three pediatric leukemia patients (age < 14 years at diagnosis) consecutively diagnosed at our institution and got treated with clofarabine based regimen at a single tertiary care hospital between January 2005 and December 2019 were enrolled in this study. ALL comprised of 30 (69.8%) patients of the cohort while the remaining 13 (30.2%) were with acute myeloid leukemia (AML).
Results: Post-clofarabine bone marrow (BM) was negative in 18 (45.0%) cases. Overall clofarabine failure rate was 58.1% (n = 25) with 60.0% (n = 18) in ALL and 53.8% (n = 7) in AML (P = 0.747). Eighteen (41.9%) patients eventually underwent hematopoietic stem cell transplantation (HSCT); 11 (61.1%) were from ALL group and remaining seven (38.9%) were AML (P = 0.332). Three- and 5-year OS of our patients was 37.7±7.6% and 32.7±7.3%. There was a trend of better OS for ALL patients compared to AML (40.9±9.3% vs. 15.4±10.0%, P = 0.492). Cumulative probability of 5-year OS was significantly better in transplanted patients (48.1±12.1% vs. 21.4±8.4%, P = 0.024).
Conclusions: Though almost 90% of our patients proceeded to HSCT with complete response post-clofarabine treatment, yet clofarabine-based regimens are associated with the significant burden of infectious complications and sepsis-related deaths.
Keywords: Clofarabine; Stem cell transplantation; Bridging chemotherapy
| Introduction | ▴Top |
The collaborative research efforts over the past 60 years have resulted in dramatic improvement in outcomes for children with acute leukemia, particularly for B lymphoblastic leukemia where the overall survival (OS) is approaching 90% utilizing state of the art protocols [1]. Meanwhile, the OS for myelocytic and T lymphoblastic leukemia has also improved and is currently reported at around 70-80% respectively [2, 3]. Despite this pronounced improvement, a proportion of patients continue to suffer from lack of response or relapse, and the management of such patients is exceedingly difficult. There have been many promising advances with the incorporation of immunotherapy and engineered chimeric antigen receptor (CAR) T-cell therapy in the course of relapsed or refractory acute lymphoblastic leukemia (ALL). However, conventional chemotherapy continues to be utilized for re-induction purposes whether independently or in combination with immunotherapy [4].
Different chemotherapy protocols are used for re-induction with variable efficacy reported. One of the agents frequently utilized in salvage regimes is clofarabine (CLOF). CLOF is a second-generation purine nucleoside analogue, which was designed to overcome the limitations of its counterpart; fludarabine and cladribine with enhanced efficacy and decreased toxicity [5]. Initially, it showed promising results in inducing remission in refractory/relapsed leukemia as a single agent, prompting accelerated Food and Drug Administration (FDA) approval in the early 2000s for the treatment of refractory and relapsed leukemia in children ages 1 - 21 years [5-7]. Subsequent trials incorporated CLOF in multi-agent chemotherapy backbone with improved efficacy reported. Hijiya et al investigated the combination of CLOF, cyclophosphamide (CPM) and etoposide (ETOP) in the CLO218 phase I and II trials, which enrolled both relapsed ALL and acute myeloid leukemia (AML) [8]. The dose escalation part of the phase I trial recommended a phase II evaluation of CLOF 40 mg/m2/day, CPM 440 mg/m2/day and ETOP 100 mg/m2/day for 5 consecutive days. Subsequently, the phase II trial reported an overall response rate of 64%; noted in 20 ALL and five AML patients. The overall response rate for ALL patients was 55%. A very similar dose combination of CLOF 40 mg/m2/day, CPM 400 mg/m2/day and ETOP 150 mg/m2/day for 5 days was also studied in refractory and relapsed T-cell ALL and B-cell ALL in Italy, with a reported overall response rate of 56%. The response was found to be strikingly higher in the B-cell ALL group, where 13 out of 17 patients responded to treatment [9]. Among reported toxicities, the most significant adverse events are hepatic toxicity, risk of sinusoidal obstruction syndrome and capillary leak syndrome. Severe hepatic toxicity was more evident in patients post-hematopoietic stem cell transplantation (HSCT). Another CLOF-based regimen utilized in acute leukemia includes CLOF and cytarabine, which has been investigated in preclinical and clinical models. CLOF is a potent inhibitor of ribonucleotide reductase, which can increase the accumulation of the cytotoxic triphosphate form of cytarabine (triphosphate cytarabine (Ara-CTP)) in leukemia cells [10, 11]. The Children’s Oncology Group evaluated this regimen in their AAML0532 study including both dosing and efficacy phases. The study concluded with a recommended dosing regimen of CLOF 52 mg/m2 and cytarabine 1 g/m2 for 5 days with demonstrated acceptable toxicity profile. This combination was shown to be more efficacious in the AML group with an overall response of 48% compared to a significantly inferior response of 14% in the ALL group [12, 13]. As such, we report our experience with utilizing aforementioned CLOF-based regimens in 30 patients with relapse/refractory ALL and 13 patients of relapse/refractory AML at our institution.
| Materials and Methods | ▴Top |
Patients
Forty-three pediatric leukemia patients (age < 14 years at diagnosis) consecutively diagnosed at our institution and got CLOF during the course of their treatment at a single tertiary care hospital between January 2005 and December 2019 were enrolled in this study. Our first patient received the very first cycle of CLOF in February 2011. ALL comprised of 30 (69.8%) patients of the cohort while the remaining 13 (30.2%) were with AML. Clinical data including demographic characteristics, treatment and transplant outcomes were obtained through the review of paper and electronic medical records of the patients as well as downloaded from the prospectively maintained databases at our institution.
Definitions
Disease-related characteristics including classification of cytogenetics and risk group stratification were extrapolated from the Children’s Oncology Group criteria. Negative bone marrow (BM) result was defined as per institutional parameters for negative minimal residual disease (MRD) below 0.1-0.01% for lymphoblastic leukemia and M1 marrow for myelocytic leukemia. Complete response (CR) was defined as the attainment of negative BM after treatment with complete or partial hematological recovery. Complete hematological recovery was defined as attainment of absolute neutrophil count ≥ 0.75 × 109 and platelet count ≥ 75 × 109. Partial hematological recovery was defined as not meeting absolute neutrophil count and/or platelet count recovery threshold. Adverse events were graded as per Common Terminology Criteria for Adverse Events (CTCAE) version 5.0.
OS was defined as survival time in months from the date of initial diagnosis to last follow-up or death. Death from any cause was considered as an event. The last follow-up on the patients was carried out in February 2022.
Treatment and transplant details
As per institutional guidelines, patients with relapsed ALL received CLOF 40 mg/m2/day, CPM 440 mg/m2/day and ETOP 100 mg/m2/day for 5 consecutive days and patients with relapsed AML received CLOF 52 mg/m2 and cytarabine 1 g/m2 for 5 days for one to two cycles.
Data management and statistical analyses
IBM-SPSS version 20.0 (IBM, Armonk, USA) was used to collect, manage and statistical analysis of the data.
Institutional Review Board approval
This clinical research study was approved by the Institutional Review Board (IRB) of the hospitals via approval number 2201279, which was to be conducted under the international guidelines for the enrollment of human subjects. The data from patients’ medical records were collected and maintained in accordance with institutional policy on data confidentiality, security, and safety. As the study was designed as a retrospective review, no consent/assent was taken from patients/parents. A waiver of informed consent/assent was sought from the IRB and was duly granted.
Ethical compliance with human/animal study
This study was conducted in compliance with the ethical standards of the institution on human subjects as well as with the Helsinki Declaration.
| Results | ▴Top |
Clinical presentation
Clinical characteristics of patients enrolled in this study are provided in Table 1 and Supplementary Material 1 (www.thejh.org). Median age at diagnosis was 5.4 years whereas majority of the patients were male (n = 31, 72.1%). Median time in months to receive the first cycle of CLOF was lower in AML as compared to ALL patients; however, it did not reach statistical significance (11.2 vs. 15.8, P = 0.428). Majority of our ALL patients were B cell (n = 25, 83.3%) and AML morphology was unknown for 76.9% (n = 10). In terms of cytogenetics, favorable cytogenetics were almost similar to unfavorable in ALL cases. However, our AML subgroup comprised of 76.9% of high-risk cytogenetics (10 out of 13). Majority of the cases were central nervous system (CNS)-1 for both the subgroups. Relapse of primary disease was the significant indication for CLOF, whereas 17 (68.0%) of these 25 relapsed patients had experienced second relapse before getting treated with CLOF. One of our patients had already been a failure of HSCT before receiving CLOF and then undergoing CAR T-cell therapy. This patient is alive and doing well at 51.3 months of follow-up. BM remained the most common site of relapse while median time to first cycle of CLOF was 13.4 months (1.1 - 77.8) from diagnosis. Around 60% of the cases in both the subgroups received only one cycle of CLOF (Table 1).
 Click to view | Table 1. Patient and Underlying Disease Characteristics (N = 43) |
CLOF bridging to HCT
Post-CLOF BM was negative in 18 (45.0%) cases. Overall CLOF failure rate was 58.1% (n = 25) with 60.0% (n = 18) in ALL and 53.8% (n = 7) in AML (P = 0.747) (Table 2). Number of CLOF cycles (one vs. two), pre-treatment disease status (relapse vs. refractory disease), initial risk assignment, cytology (favorable vs. unfavorable), CNS disease (CNS-1 vs. CNS-2 vs. CNS-3), site and number of pre-CLOF relapse of primary disease were not found to be significantly associated with CLOF treatment failure. Eighteen (41.9%) patients eventually underwent HSCT; 11 (61.1%) were from ALL group and remaining seven (38.9%) were AML (P = 0.332). Median time to HSCT from first cycle of CLOF was 2.7 months (range: 1.2 - 6.4) (Table 2).
 Click to view | Table 2. Treatment Response, Outcome and Clofarabine Regimen Related Toxicity |
Pre- and post-CLOF hematological parameters are provided in Table 1. Post-treatment white blood cell counts (WBC) and platelets were significantly lower in ALL subgroup (P < 0.001) compared to pre-treatment values as well as in AML cases (P = 0.001 and P = 0.001, respectively).
CLOF regimen-related toxicity
Toxicity profile of CLOF is provided in Table 3; febrile neutropenia followed by bacterial infections were the most common treatment-related toxicity. Intensive care unit (ICU) admissions were recorded for 19 (44.2%) patients due to septic shock (n = 13).
 Click to view | Table 3. Clofarabine Regimen-Related Toxicity |
Survival
With a median follow-up time of 26 months (range, 4.7 - 98.6) and an overall mortality rate of 81.4% (n = 35), 3- and 5-year OS of our patients was 37.7±7.6% and 32.7±7.3% for the whole cohort. There was a trend of better OS at 5-year for ALL patients compared to AML (40.9±9.3% vs. 15.4±10.0%, P = 0.492) (Table 2, Fig. 1). Cumulative probability of 5-year OS was significantly better in transplanted patients (48.1±2.1% vs. 21.4±8.4%, P = 0.024) (Fig. 2). Similarly, survival benefits were observed within ALL and AML subgroups in transplanted vs. non-transplanted patients (Tables 2, 4, Figs. 3, 4).
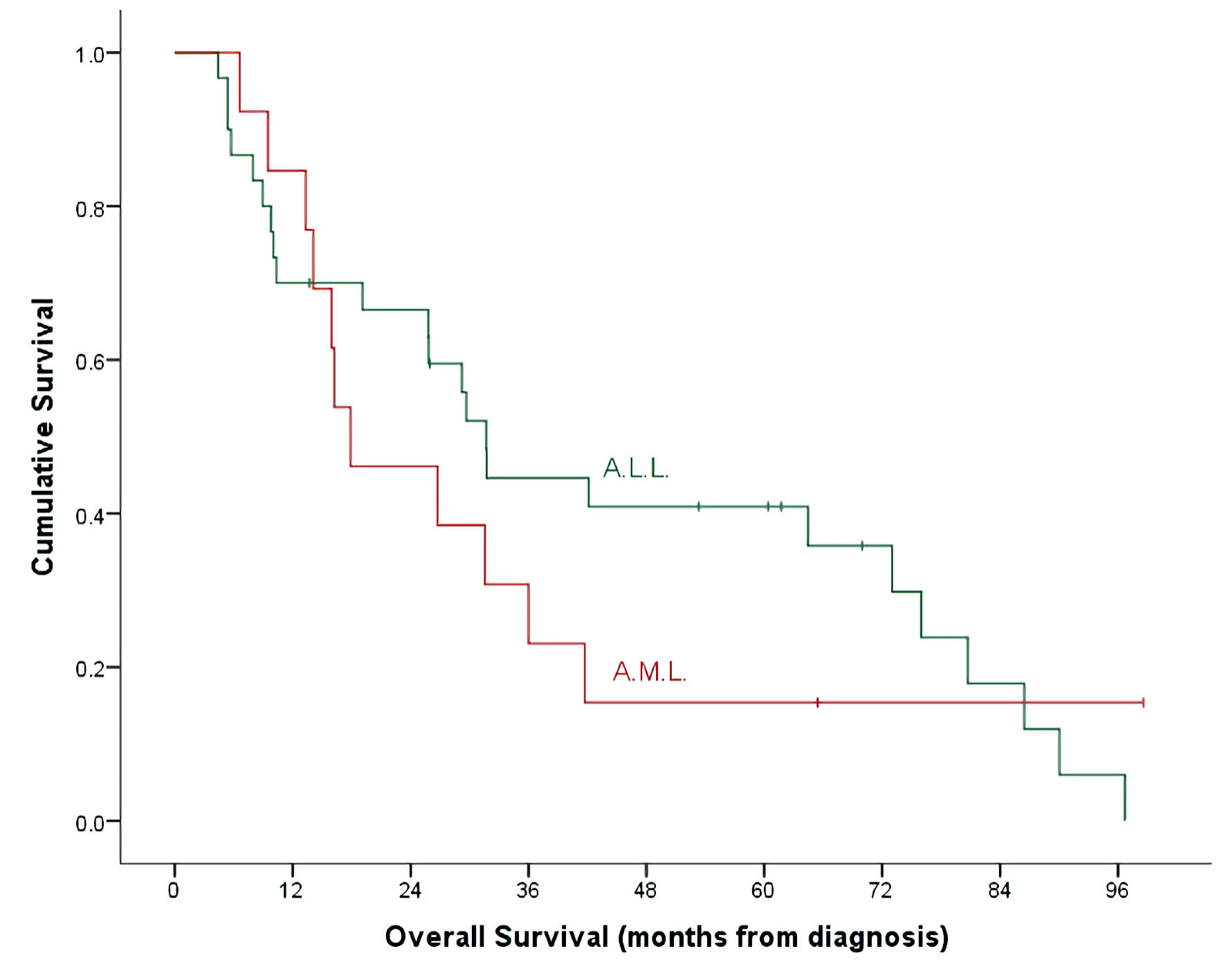 Click for large image | Figure 1. OS by leukemia subtypes. ALL: acute lymphoblastic leukemia; AML: acute myeloid leukemia; OS: overall survival. |
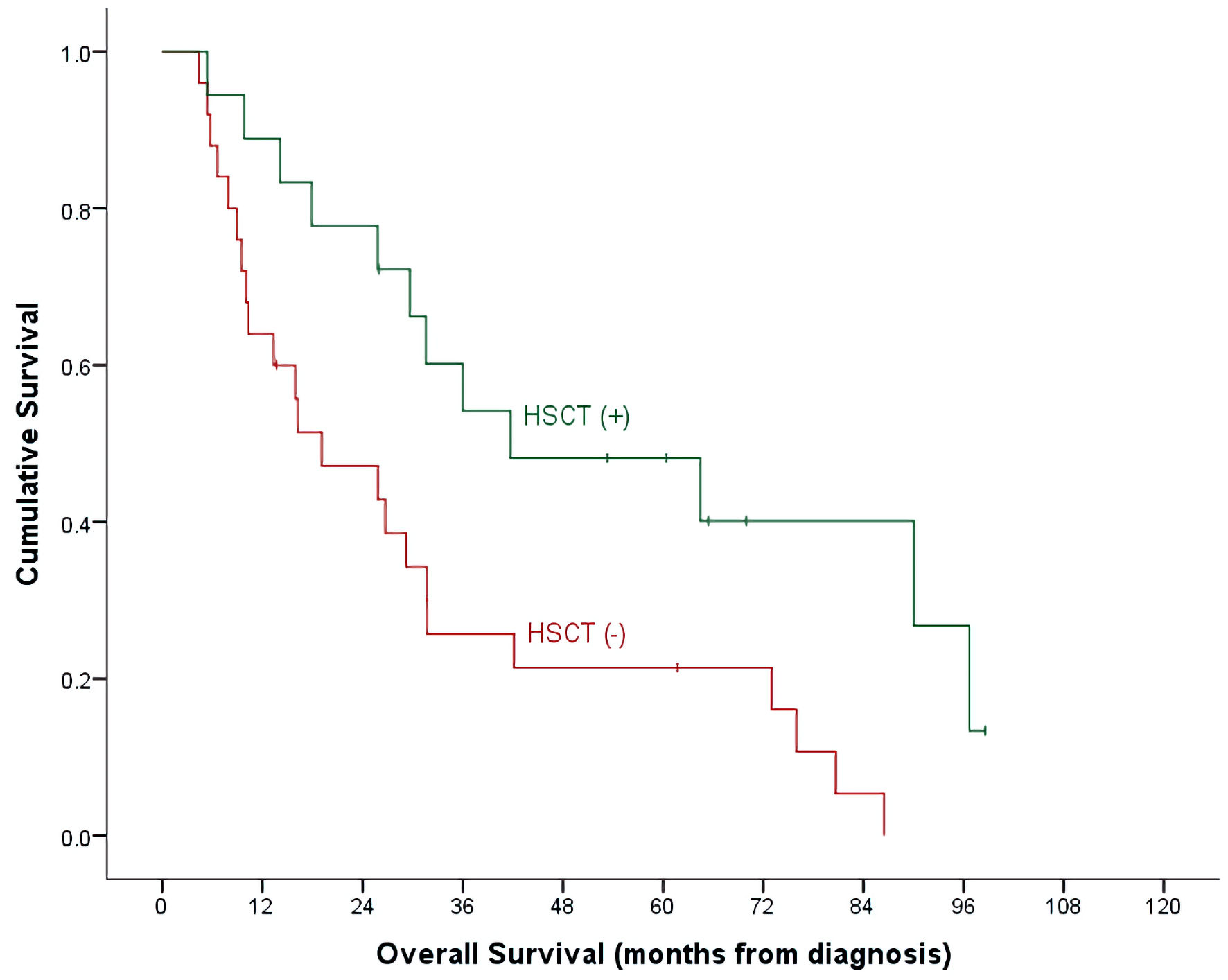 Click for large image | Figure 2. OS by HSCT and non-HSCT groups. OS: overall survival; HSCT: hematopoietic stem cell transplantation. |
 Click to view | Table 4. Hematopoietic Stem Cell Transplant-Related Parameters and Outcome |
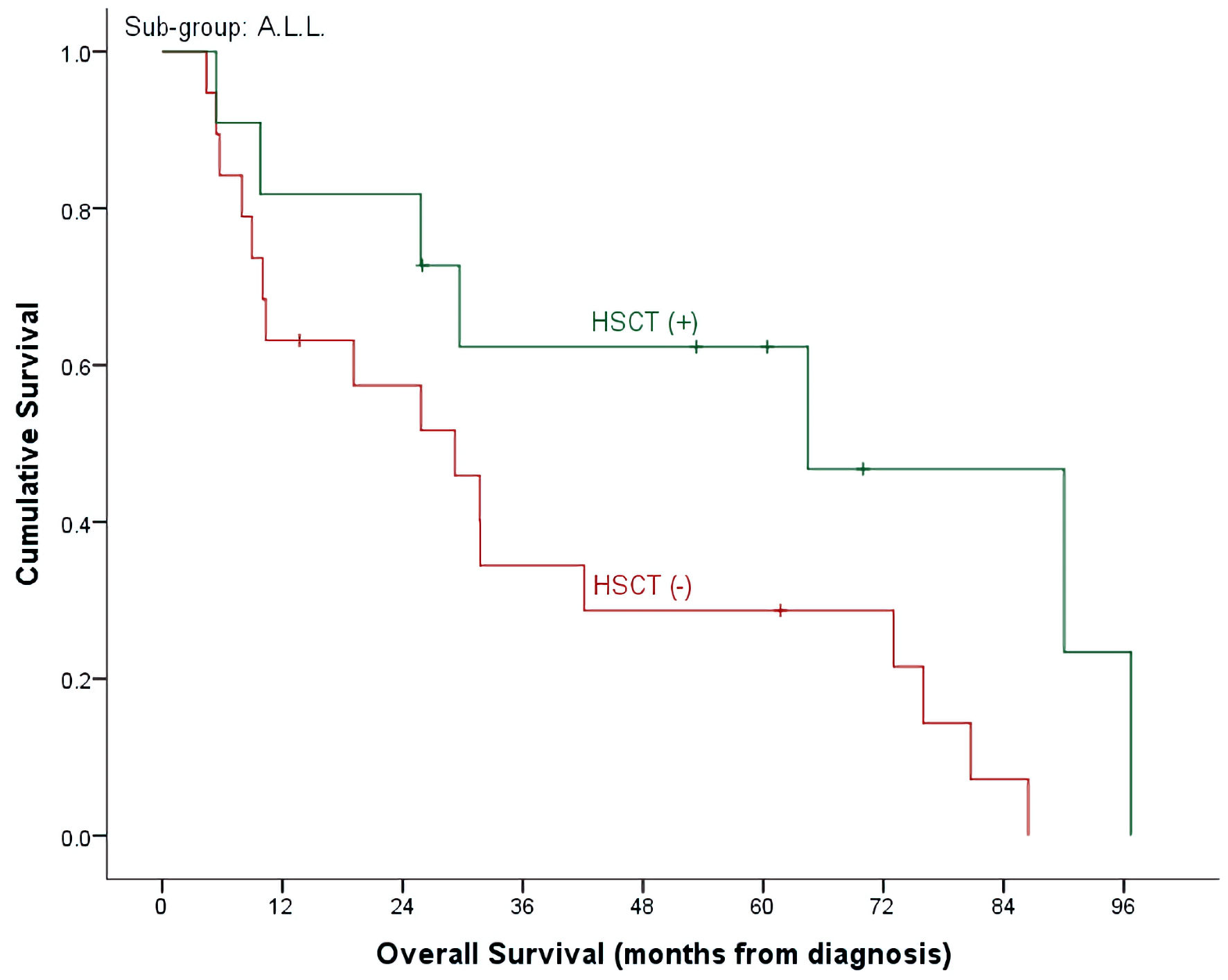 Click for large image | Figure 3. OS by HSCT and non-HSCT groups in ALL patients. OS: overall survival; HSCT: hematopoietic stem cell transplantation; ALL: acute lymphoblastic leukemia. |
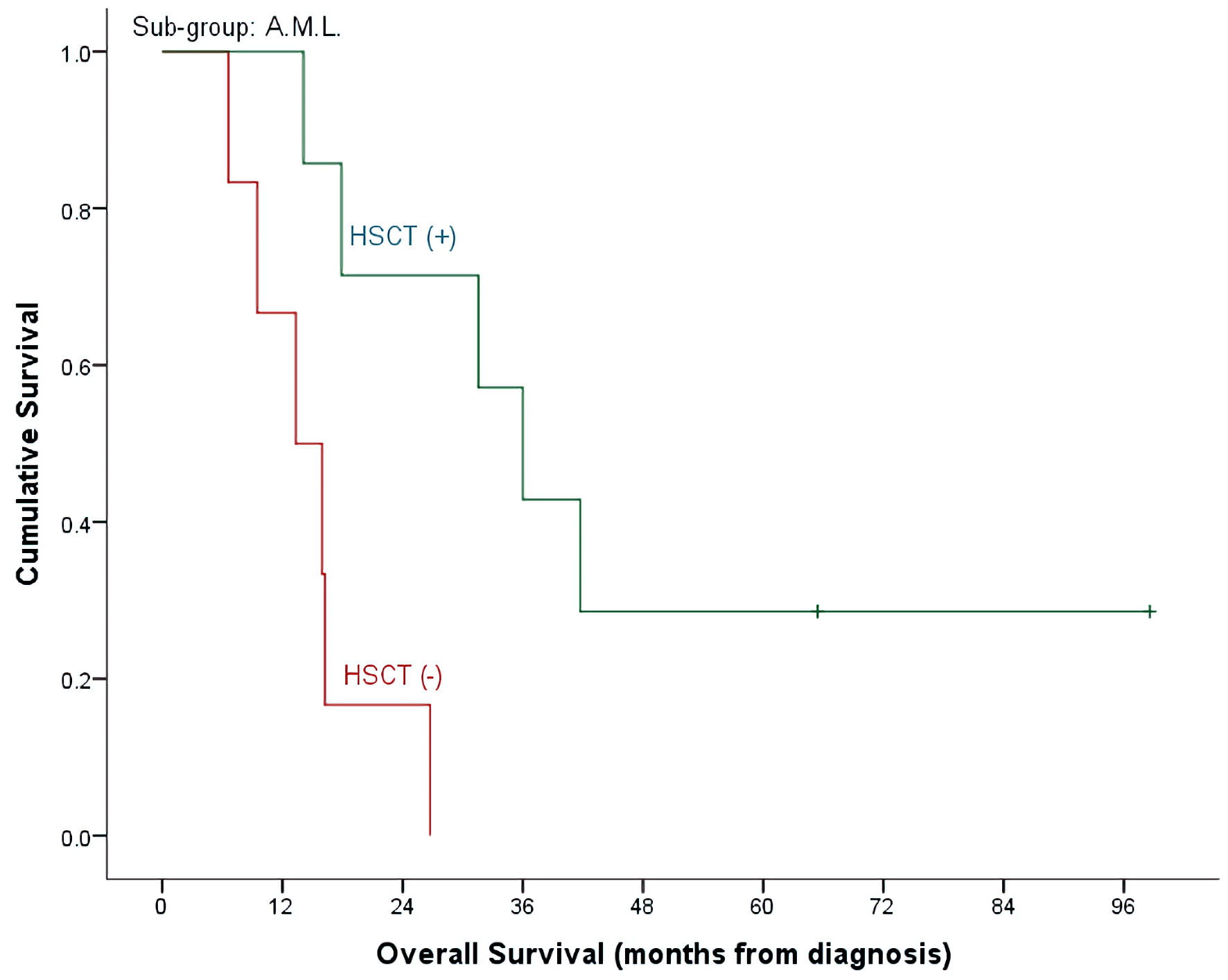 Click for large image | Figure 4. OS by HSCT and non-HSCT groups in AML patients. OS: overall survival; HSCT: hematopoietic stem cell transplantation; AML: acute myeloid leukemia. |
Disease progression was the primary cause of death in majority of the patients (n = 26, 74.3%), followed by transplant related (n = 6, 17.1%) and CLOF-associated toxicity in three (8.6%) patients. Twelve out of 18 patients who underwent HSCT died, making 34.3% of the overall mortality (n = 35) of our cohort.
| Discussion | ▴Top |
Our results are concordant with prior published retrospective and prospective reports, demonstrating 40% CR in the ALL group receiving CLOF/CYTOX/ETOP and 46% CR in the AML group receiving CLOF/cytosine arabinoside (Ara-C). Additionally, CLOF-based regimens demonstrated noteworthy response in our cohort of heavily treated patients in second relapse, where CR was achieved in eight out of 17 patients. CR was also achieved in two out of four patients with T-cell ALL, who were able to proceed to HSCT after but eventually, succumbed to disease progression. On the other hand, primary refractory AML had strikingly poor response, where none of the four patients achieved response. Durable remissions permitted all patients to proceed to definitive cellular therapy, where 90% of patients proceeded with HSCT and two patients who received CLOF post-HSCT proceeded to CAR T-cell therapy. In the HSCT group, 5-year OS was found to be 48.1%, with proportionally higher 5-year OS in the ALL group at 62% compared to 28% in the AML group. The rate of HSCT post-CLOF in our cohort is amongst the highest reported in the literature.
In our experience toxicity was common and significant (Table 3). Bacterial infections and ICU admissions were encountered at 62.8% and 44.2% consecutively. In contrast to reports of high-grade hepatotoxicity with CLOF regimens, hepatic failure in the context of multi-organ failure was encountered in three patients and grade II sinusoidal obstruction syndrome was encountered in one patient. Otherwise, transient transaminitis and hyperbilirubinemia were observed as detailed in Table 3. Additionally, CLOF was tolerated well in post-HSCT cases where only one recipient reported sinusoidal obstruction syndrome while two patients developed fatal acute respiratory distress syndrome. There were three deaths that were attributed to treatment with CLOF, which is comparable to reported treatment-related deaths by Inaba et al and Hijiya et al [14, 15].
Our report is limited by the small cohort which is often the case for refractory/relapsed leukemia studies. However, to our knowledge it is the first report from the region demonstrating comparable efficacy of CLOF-based regimens with international reports. Even more remarkable in our experience is the successful HSCT in 90% of patients with CR. While efficacious, CLOF-based regimens are associated with the significant burden of infectious complications and sepsis-related deaths. Thus, rigorous protocols of antimicrobial prophylaxis (antibiotic, antifungal), surveillance for organisms, support with granulocyte colony-stimulating factor (G-CSF), and aggressive management of infections or febrile episodes are warranted. CLOF continues to be investigated in salvage regimens in refractory leukemias (NCT04002115, NCT03136146, NCT02441803), however its role in the evolving immunotherapy era is yet to be determined. It remains to be considered a powerful agent for refractory disease, particularly when immunotherapy is not readily available.
We continue to hope for further advances in the outcome for this fragile group of patients, and the facilitation of novel agents with enhanced benefit and reduced toxicities.
| Supplementary Material | ▴Top |
Suppl 1. Cytogenetics and molecular expressions of the cohort.
Acknowledgments
None to declare.
Financial Disclosure
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of Interest
The authors declare no conflict of interest.
Informed Consent
As the study was designed as a retrospective review, no consent/assent was taken from patients/parents. A waiver of informed consent/assent was sought from the IRB and was duly granted.
Author Contributions
IG, OE and KS has designed and performed the study. SR, OE, IG and KS have drafted the manuscript and did critical editing. OE and OS have assisted and supported in sample collection while subsequent analysis with statistics was done by KS. SK, HA, AA, AAA, AAJ and MA have carefully supervised this manuscript preparation and writing.
Data Availability
The data used in this work is available from the corresponding author upon reasonable request. However, some restrictions may apply.
Abbreviations
ALL: acute lymphoblastic leukemia; AML: acute myeloid leukemia; CAR: chimeric antigen receptor; HSCT: hematopoietic stem cell transplantation; BM: bone marrow; CLOF: clofarabine; WBC: white blood cell; Ara-CTP: triphosphate cytarabine; MRD: minimum residual disease; CR: complete response; G-CSF: granulocyte colony-stimulating factor; CTCAE: Common Terminology Criteria for Adverse Events; OS: overall survival; IRB: Institutional Review Board; ICU: intensive care unit
| References | ▴Top |
- Hunger SP, Mullighan CG. Acute lymphoblastic leukemia in children. N Engl J Med. 2015;373(16):1541-1552.
doi pubmed - Zwaan CM, Kolb EA, Reinhardt D, Abrahamsson J, Adachi S, Aplenc R, De Bont ES, et al. Collaborative efforts driving progress in pediatric acute myeloid leukemia. J Clin Oncol. 2015;33(27):2949-2962.
doi pubmed - Hayashi RJ, Winter SS, Dunsmore KP, Devidas M, Chen Z, Wood BL, Hermiston ML, et al. Successful outcomes of newly diagnosed T lymphoblastic lymphoma: results from children's oncology group AALL0434. J Clin Oncol. 2020;38(26):3062-3070.
doi pubmed - McCall D, Jabbour E, Roth M, Nunez C, Cuglievan B. Mini-hyper CVD + CRIB (condensed rituximab, inotuzumab ozogamicin, and blinatumomab) for refractory pediatric B-acute lymphoblastic leukemia. Pediatr Blood Cancer. 2023;70(1):e29939.
doi pubmed - Pui CH, Jeha S. Clofarabine. Nat Rev Drug Discov. 2005;Suppl:S12-S13.
doi - Jeha S, Gaynon PS, Razzouk BI, Franklin J, Kadota R, Shen V, Luchtman-Jones L, et al. Phase II study of clofarabine in pediatric patients with refractory or relapsed acute lymphoblastic leukemia. J Clin Oncol. 2006;24(12):1917-1923.
doi pubmed - Jeha S, Gandhi V, Chan KW, McDonald L, Ramirez I, Madden R, Rytting M, et al. Clofarabine, a novel nucleoside analog, is active in pediatric patients with advanced leukemia. Blood. 2004;103(3):784-789.
doi pubmed - Hijiya N, Gaynon P, Barry E, Silverman L, Thomson B, Chu R, Cooper T, et al. A multi-center phase I study of clofarabine, etoposide and cyclophosphamide in combination in pediatric patients with refractory or relapsed acute leukemia. Leukemia. 2009;23(12):2259-2264.
doi pubmed - Locatelli F, Testi AM, Bernardo ME, Rizzari C, Bertaina A, Merli P, Pession A, et al. Clofarabine, cyclophosphamide and etoposide as single-course re-induction therapy for children with refractory/multiple relapsed acute lymphoblastic leukaemia. Br J Haematol. 2009;147(3):371-378.
doi pubmed - Cooper T, Ayres M, Nowak B, Gandhi V. Biochemical modulation of cytarabine triphosphate by clofarabine. Cancer Chemother Pharmacol. 2005;55(4):361-368.
doi pubmed - Faderl S, Gandhi V, O'Brien S, Bonate P, Cortes J, Estey E, Beran M, et al. Results of a phase 1-2 study of clofarabine in combination with cytarabine (ara-C) in relapsed and refractory acute leukemias. Blood. 2005;105(3):940-947.
doi pubmed - Cooper TM, Alonzo TA, Gerbing RB, Perentesis JP, Whitlock JA, Taub JW, Horton TM, et al. AAML0523: a report from the Children's Oncology Group on the efficacy of clofarabine in combination with cytarabine in pediatric patients with recurrent acute myeloid leukemia. Cancer. 2014;120(16):2482-2489.
doi pubmed - Cooper TM, Razzouk BI, Gerbing R, Alonzo TA, Adlard K, Raetz E, Gamis AS, et al. Phase I/II trial of clofarabine and cytarabine in children with relapsed/refractory acute lymphoblastic leukemia (AAML0523): a report from the Children's Oncology Group. Pediatr Blood Cancer. 2013;60(7):1141-1147.
doi pubmed - Inaba H, Bhojwani D, Pauley JL, Pei D, Cheng C, Metzger ML, Howard SC, et al. Combination chemotherapy with clofarabine, cyclophosphamide, and etoposide in children with refractory or relapsed haematological malignancies. Br J Haematol. 2012;156(2):275-279.
doi pubmed - Hijiya N, Thomson B, Isakoff MS, Silverman LB, Steinherz PG, Borowitz MJ, Kadota R, et al. Phase 2 trial of clofarabine in combination with etoposide and cyclophosphamide in pediatric patients with refractory or relapsed acute lymphoblastic leukemia. Blood. 2011;118(23):6043-6049.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


