| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Case Report
Volume 11, Number 6, December 2022, pages 216-222
Synchronous Presentation of Autoimmune Hepatitis and Multiple Myeloma
Binoy Yohannana, Allen C. Omo-Ogboib, Varaha S. Tammisettic, Adan Riosd, e
aDivision of Hematology and Oncology, McGovern Medical School, The University of Texas Health Science Center at Houston, Huston, TX, USA
bDepartment of Pathology and Laboratory Medicine, McGovern Medical School, The University of Texas Health Science Center at Houston, Huston, TX, USA
cDivision of Diagnostic and Interventional Imaging, McGovern Medical School, The University of Texas Health Science Center at Houston, Huston, TX, USA
dDivision of Hematology and Oncology, The University of Texas Health Science Center at Houston, Huston, TX, USA
eCorresponding Author: Adan Rios, Division of Hematology and Oncology, The University of Texas Health Science Center at Houston, Huston, TX, USA
Manuscript submitted September 2, 2022, accepted October 10, 2022, published online December 1, 2022
Short title: Synchronous Presentation of AIH and MM
doi: https://doi.org/10.14740/jh1049
| Abstract | ▴Top |
Autoimmune hepatitis (AIH) is a rare immune-mediated disease predominantly seen in women and triggered by various environmental factors. Rarely, AIH can be triggered by an underlying malignancy. We report a woman in her 60s who presented with markedly abnormal liver biochemical tests. Serology was positive for anti-smooth muscle antibodies and a liver biopsy confirmed AIH. During the hospital course, she developed sepsis and acute renal failure requiring dialysis support. Serum protein electrophoresis (SPEP) showed a monoclonal IgG kappa protein of 1.92 g/dL and a bone marrow biopsy revealed 7% clonal plasma cells. She had lytic lesions on skeletal survey confirming the diagnosis of a coexisting multiple myeloma (MM). Given her markedly abnormal liver chemistries, we decided to treat the AIH first and use the steroids (an important anti-myeloma therapy) as a bridge to the specific treatment of the MM once her clinical condition improved. She was treated with oral prednisone and azathioprine for AIH. One month later, a marked improvement in liver biochemical test results was noted and she was started on oral ixazomib, lenalidomide and dexamethasone. She received palliative radiotherapy to the lumbar spine (L2), left femur, and ischium lesions. This case highlights a rare co-occurrence of AIH and MM, the underlying mechanism of which is unknown.
Keywords: Multiple myeloma; Autoimmune hepatitis; Abnormal liver chemistries; Paraneoplastic syndrome
| Introduction | ▴Top |
Autoimmune hepatitis (AIH) is a chronic immune-mediated liver disease characterized by hypergammaglobulinemia, transaminitis, interface hepatitis on liver biopsy, and an excellent response to glucocorticoid therapy. AIH is predominantly seen in females, and it has a unique bimodal age distribution, with the first peak seen in children and second peak in the fifth or sixth decade of life [1].The exact trigger of AIH is unknown but is believed to be secondary to various environmental factors, certain medications or viral infections that stimulate the immune system to attack the liver [1]. AIH can also rarely present as a paraneoplastic syndrome secondary to an underlying malignancy. We report an interesting case of a woman in her 60s who presented with markedly abnormal liver chemistries secondary to AIH and was subsequently diagnosed with multiple myeloma (MM).
| Case Report | ▴Top |
Investigations
A woman in her 60s with arterial hypertension presented to our hospital for evaluation of markedly abnormal liver biochemical tests. She noticed yellowish discoloration of the eyes and dark-colored urine 2 weeks prior to admission. She also reported fatigue, nausea, bloating, and indigestion but denied any abdominal pain, fever, or chills. She took cephalexin for 4 days for presumed urinary tract infection without any significant improvement. She denied any arthralgia, rash, pruritus, recent travel, or sick contacts. She denied taking any over-the-counter medications including Chinese or herbal preparations. Her only prescription medication was metoprolol. She had no history of smoking, alcohol abuse, or use of illicit drugs. There was no family history of liver disease or autoimmune disease. On examination, she was alert, awake, and oriented ×4. She had conjunctival pallor and icterus. Respiratory and cardiovascular system examinations were normal. The patient’s abdomen was soft and non-distended. Mild epigastric, and right upper quadrant tenderness were noted. The Murphy sign was negative. She had trace pedal edema. There were no neurological deficits or asterixis noted.
Diagnosis
Admission laboratory test results are shown in Table 1. Serum alcohol, acetaminophen, and salicylate levels were undetectable. Hepatitis and human immunodeficiency virus (HIV) serologies were negative. Serum ceruloplasmin level was 43 mg/dL. Serologies for antinuclear antibodies (ANAs) and antimitochondrial antibodies (AMAs) were negative. Anti-smooth muscle antibody titers were markedly elevated at 1:320 (normal range: < 1:40). Anti-liver kidney microsomal antibodies were negative. Ultrasound of the liver and gall bladder showed gallbladder sludge and cholelithiasis without sonographic evidence of acute cholecystitis. A hepatobiliary iminodiacetic acid (HIDA) scan was negative. She was evaluated by the hepatology consultant and started on N-acetyl cysteine. She underwent a trans-jugular liver biopsy, and pathology showed interface hepatitis with a polyclonal plasma cell infiltrate (Fig. 1). Congo red staining was not performed on the liver biopsy because the tissue morphology did not suggest amyloid deposition in the liver. AIH was diagnosed given markedly elevated serum transaminases, hypergammaglobulinemia, high anti-smooth muscle antibody titers, and classic histological features.
 Click to view | Table 1. Admission Laboratory Test Results |
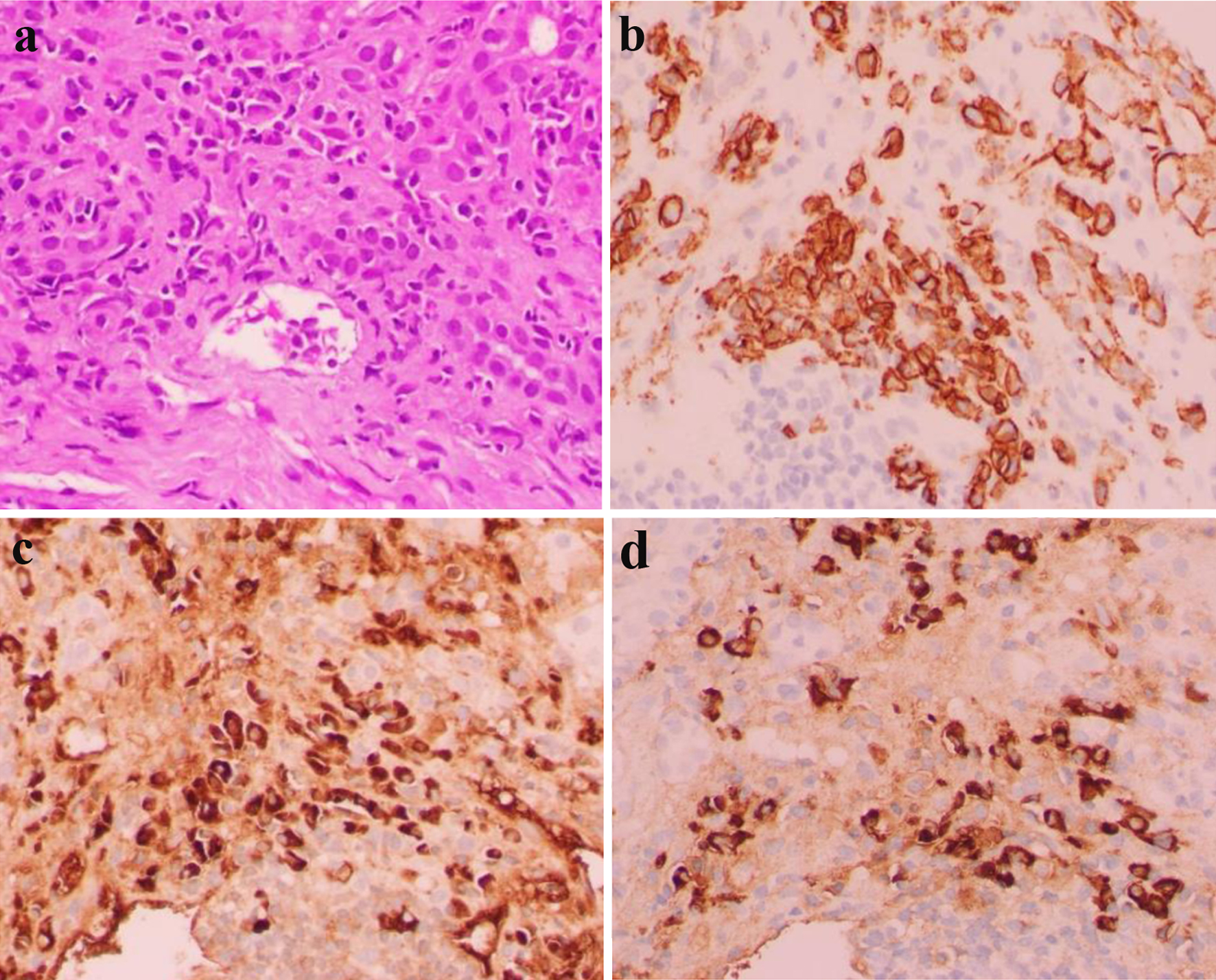 Click for large image | Figure 1. Hematoxylin and eosin stain showing panlobular hepatitis with sheets of lymphoplasmacytic portal and periportal inflammation (× 400 magnification) (a). CD138 immunohistochemical stain highlighting portal and periportal plasma cells (× 400 magnification) (b). Kappa (c) and lambda (d) immunohistochemical stains highlighting polyclonal portal and periportal plasma cells (× 400 magnification). |
SPEP showed a distinct monoclonal band (1.92 g/dL) in the gamma region with distortion, consistent with a monoclonal gammopathy. Serum immunofixation electrophoresis (IFE) showed IgG kappa. Serum kappa and lambda free light chain levels were 565 and 41 mg/L, respectively. The serum kappa/lambda free light chain ratio was 13 (reference range, 0.26 - 1.65). Urine protein electrophoresis showed a monoclonal band (6.6 mg/dL) in the gamma region. Serum IgG was 4,711 mg/dL (reference range, 700 - 1,600 mg/dL). Serum albumin was 2.8 g/dL, beta 2 microglobulin was 19 mg/L and lactate dehydrogenase (LDH) was 387 U/L. Initial bone marrow biopsy showed 5-7% clonal plasma cells by flow cytometry. Immunohistochemistry showed 5-20% clonal plasma cells with expression of CD20 (dim/partial), CD38, CD56, CD138, CD117 (partial), and cytoplasmic kappa. Fluorescence in situ hybridization (FISH) studies did not show any cytogenetic abnormalities. Bone marrow biopsy is shown in Figure 2. Patient was diagnosed with revised International Staging System (R-ISS) stage III myeloma. Computed tomography (CT) of the abdomen showed circumscribed lesions in the sacrum measuring 1.2 cm and right iliac crest lucency measuring 0.4 cm. Magnetic resonance imaging (MRI) of the bone marrow blood supply showed lytic lesions in the lumbar spine (Fig. 3).
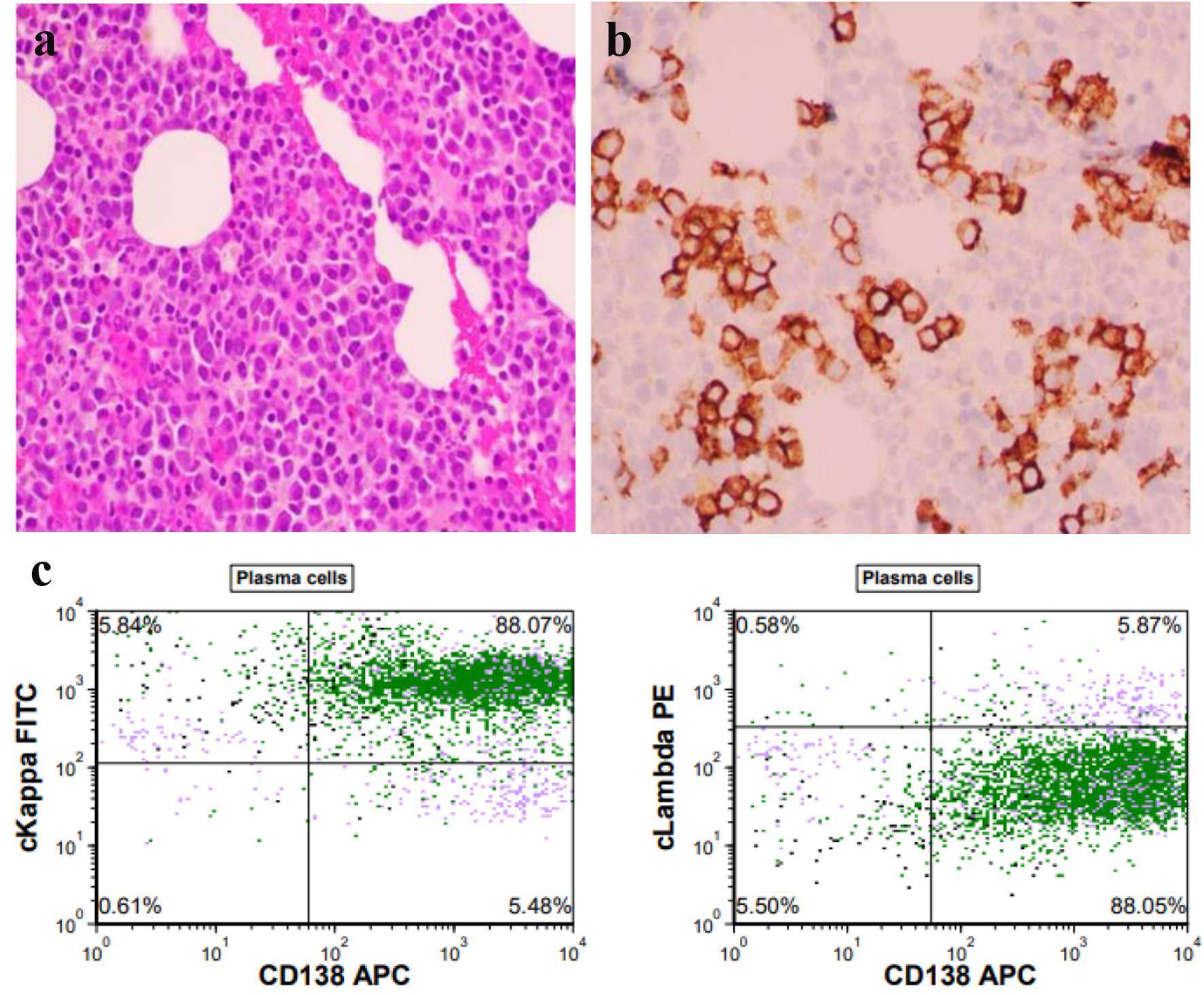 Click for large image | Figure 2. (a) Hematoxylin and eosin stain showing hypercellular marrow and trilineage hematopoiesis with numerous (about 5-20%) plasma cells (× 400 magnification). (b) CD138 immunohistochemical stain highlighting aggregates of plasma cells in the hypercellular bone marrow (× 400 magnification). (c) The flow cytometry image above demonstrating plasma cells which are CD138 positive with a kappa/lambda ratio of 15, indicating a monotypic kappa light chain restriction. |
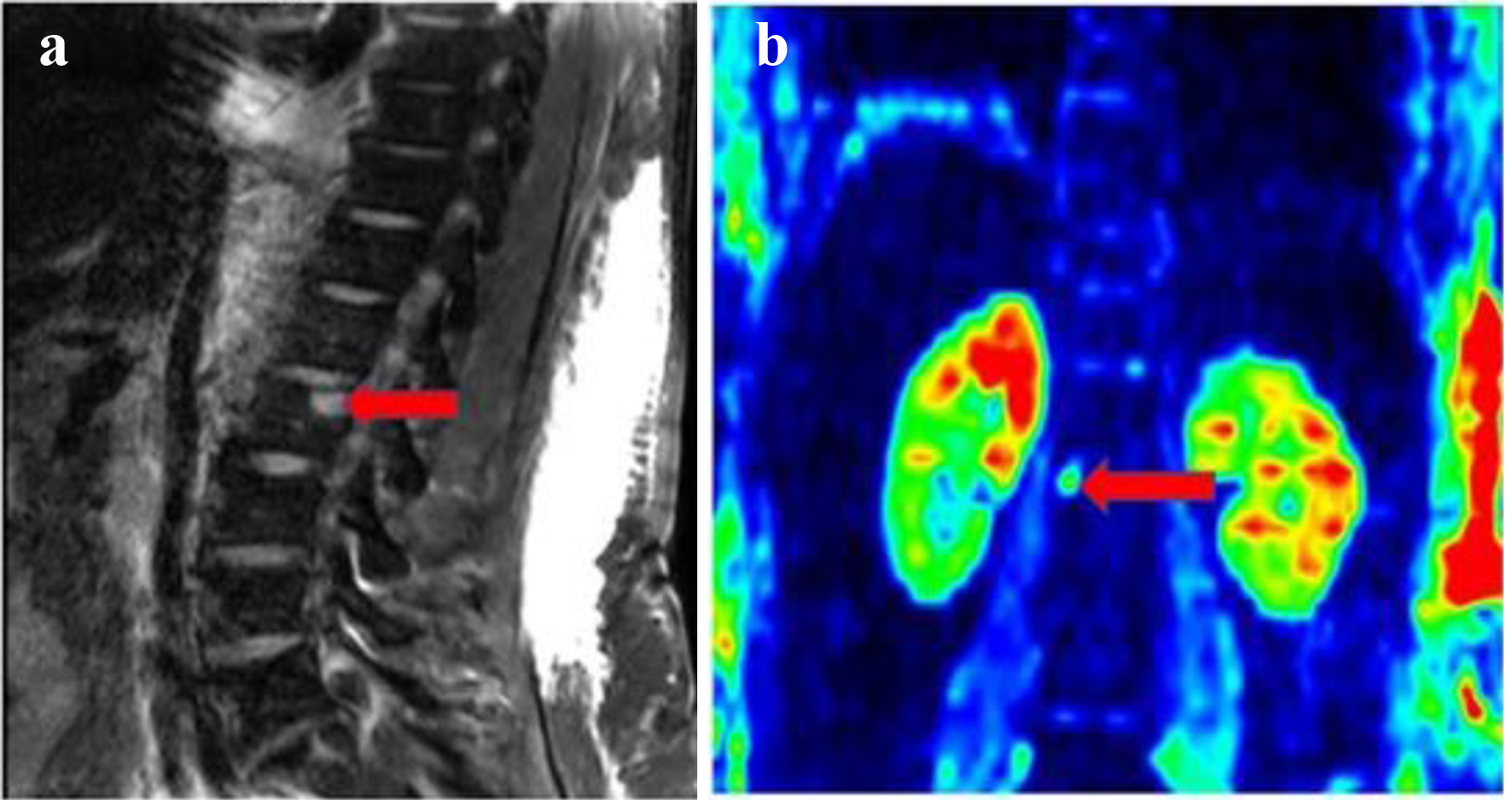 Click for large image | Figure 3. (a) Sagittal T2-weighted imaging (T2W1) of the spine with fat suppression showing L2 vertebral body T2 hyperintense focal lesion (red arrow). (b) Coronal diffusion-weighted imaging (DWI) with b value of 1,000 s/mm2 (in color scale) through the lumbar spine showing marked restricted diffusion of L2 vertebral body focal lesion (red arrow). |
Treatment
The patient was started on oral prednisone 40 mg and azathioprine 50 mg daily with gradual improvement in her liver chemistries over a period of 3 weeks (Table 1). During her hospitalization, she only received steroids for treatment of MM. Proteasome inhibitors and immune modulators were avoided due to hepatic and renal failure. The hospital course was complicated by oliguric renal failure, presumably from myeloma cast nephropathy and hepatorenal syndrome. She required intermittent hemodialysis for 1 month achieving a full renal recovery after 8 weeks.
Follow-up and outcomes
One month following her hospital discharge, she was started on ixazomib and lenalidomide. She received palliative radiotherapy to the lumbar spine (L2), left femur, and ischium lesions at a dose of 25 Gy in 10 fractions. Liver biochemical tests improved significantly during the treatment for AIH. During subsequent follow-up with hepatology, prednisone was weaned off completely in 6 weeks and azathioprine 50 mg daily was continued. Four months after her initial hospitalization, the SPEP showed a monoclonal spike of 1.81 g/dL, a repeat bone marrow showed < 5% clonal plasma cells, and the FISH analysis showed a 17p deletion. Based on the International Myeloma Working Group (IMWG) response criteria, our patient had minimal response. One year later, her liver biochemical tests continue to be within the normal range.
| Discussion | ▴Top |
The association between autoimmune disease and cancer is often bidirectional. Patients with autoimmune disease are at a higher risk for malignancy than the general population [2]. Soderberg et al reported a case-control study from Sweden and observed that the risk of hematological malignancies is significantly increased in individuals with psoriasis, Sjogren syndrome, immune thrombocytopenia, and autoimmune hemolytic anemia [3]. Similarly, the incidence of autoimmune diseases is higher in patients with hematological malignancies [4]. It is often difficult to ascertain whether autoimmunity contributes to the malignancy, or the underlying neoplastic process drives the autoimmune disease.
MM is a clonal plasma cell dyscrasia that arises from a premalignant monoclonal gammopathy of undetermined significance (MGUS). MM has been reported in association with numerous autoimmune hematological and rheumatological conditions [5]. Table 2 provides a summary of autoimmune diseases associated with MM [6-28]. Large population-based studies have suggested that the risk of both MM and MGUS is significantly high in patients with underlying autoimmune disease [29]. By contrast, other studies have proposed that autoimmune diseases do not increase the risk of MM [3]. However, patients with MGUS and autoimmune disease are at a higher risk of progression to MM when compared to those without it [30]. Although the precise mechanism is unknown, it can be hypothesized that chronic antigen stimulation leads to hyperactive B cells that can escape from immune surveillance and transform into neoplastic B-cell clones [3].
 Click to view | Table 2. Autoimmune Conditions Reported in Association With MM |
In addition to myeloma-defining events, patients can also present with hepatomegaly, abnormal liver biochemical tests, jaundice, coagulopathy, and altered mental status [31]. Rarely, patients can present with severe cholestasis and fulminant liver failure [32]. However, AIH as the initial presentation of MM is rare. Thomas et al reported on 64 autopsies of patients with MM and noted hepatic plasma cell infiltrate in 40% of cases; however, overt manifestations with jaundice and ascites were observed in only nine cases (14%) [31]. Perez-Soler et al reported an autopsy series of 128 patients with MM and noted hepatic plasma cell infiltration in 10 of 21 patients [33].
The differential diagnosis of abnormal liver biochemical tests in a patient with MM includes plasma cell infiltration of the liver, amyloidosis, light chain deposition disease (LCDD), and treatment-related effects [31-33]. Although infrequent, autoimmune liver disease should also be considered in the differential diagnosis. Differentiating plasma cell infiltration secondary to MM from AIH is critical because it has important therapeutic implications. Patients with monoclonal plasma cell infiltration of the liver secondary to MM should be treated with standard MM therapy that includes proteasome inhibitors, immunomodulatory drugs, and glucocorticoids. By contrast, patients with AIH have a polyclonal plasma cell infiltrate and should be treated aggressively with glucocorticoids and azathioprine.
LCDD is an infiltrative disease of the liver and can be an incidental finding in a minority of patients with MM [34]. There are a few important differentiating features between LCDD and AL amyloidosis. Patients with LCDD often have a normal bilirubin and transaminases but increased serum alkaline phosphatase. LCDD is usually Congo red negative and without fibrillar structure. Also, LCDD is usually immunofluorescence positive for the kappa light chain, whereas AL amyloidosis mostly stains positive for lambda light chain. Furthermore, the deposits are subendothelial and granular in LCDD compared to randomly arranged fibrils in amyloidosis [35]. Treatment-related effects of MM should also be considered in the differential diagnosis of abnormal liver biochemical tests. In patients with coexisting AIH and MM, it is critical to treat both diseases concomitantly. Lenalidomide, an immunomodulatory drug used in MM, can increase the risk of autoimmunity, and hence should be initially avoided in patients in AIH. Glucocorticoids are the mainstay of treatment for both MM and AIH. Proteasome inhibitors, which are the main pillars of myeloma therapy, are also very effective in refractory antibody-mediated autoimmune diseases [36]. In patients with plasma cell dyscrasia, the presence of a concomitant autoimmune disease is an important prognostic factor. Patients with MM or MGUS and coexisting autoimmune disease are at a higher risk of death than those without autoimmune disease [37]. Parenthetically, 3 months after starting antimyeloma therapy, our patient developed an acquired 17p deletion which is an adverse prognostic marker [38]. In patients with high-risk MM, a quadruplet induction therapy using an anti-CD 38 antibody in combination with proteasome inhibitors, lenalidomide and dexamethasone followed by early consolidation with an autologous stem transplant is preferred [39]. However, our patient was living 200 miles away from the cancer center and had significant logistical challenges with transportation. Hence, we opted to treat her with an oral triplet regimen (ixazomib, lenalidomide, dexamethasone).
In conclusion, AIH can rarely manifest as a paraneoplastic autoimmune disease secondary to underlying MM. Making an accurate diagnosis is essential to providing appropriate treatment and reducing mortality.
Learning points
Autoimmunity and plasma cell dyscrasias are intricately intertwined, and the association is bidirectional.
AIH as the initial manifestation of myeloma is rare, and clinicians should be aware of this rare presentation.
It is critical to differentiate monoclonal hepatic plasma cell infiltrate secondary to MM from polyclonal plasma cell infiltrate in AIH, as this has important therapeutic implications.
Myeloma patients presenting with autoimmune disease tend to have a more aggressive disease biology and are at a higher risk of mortality.
Prompt recognition of AIH and timely initiation of therapy are crucial to improve outcomes.
Acknowledgments
The authors would like to thank Virginia Mohlere for editorial assistance.
Financial Disclosure
None to declare.
Conflict of Interest
The authors have no conflict of interest.
Informed Consent
Informed consent was obtained from the patient.
Author Contributions
BY: concept, design, data collection and initial draft of the manuscript. AR: treating physician and revised the manuscript for important intellectual content. AO: reviewed the pathology slides and revised the draft for important intellectual content. VST: reviewed the radiology images.
Data Availability
The authors declare that data were obtained from electronic medical records.
| References | ▴Top |
- Mack CL, Adams D, Assis DN, Kerkar N, Manns MP, Mayo MJ, Vierling JM, et al. Diagnosis and management of autoimmune hepatitis in adults and children: 2019 practice guidance and guidelines from the American Association for the study of liver diseases. Hepatology. 2020;72(2):671-722.
doi pubmed - Stern M, Buser AS, Lohri A, Tichelli A, Nissen-Druey C. Autoimmunity and malignancy in hematology—more than an association. Crit Rev Oncol Hematol. 2007;63(2):100-110.
doi pubmed - Soderberg KC, Jonsson F, Winqvist O, Hagmar L, Feychting M. Autoimmune diseases, asthma and risk of haematological malignancies: a nationwide case-control study in Sweden. Eur J Cancer. 2006;42(17):3028-3033.
doi pubmed - Martin DN, Mikhail IS, Landgren O. Autoimmunity and hematologic malignancies: associations and mechanisms. Leuk Lymphoma. 2009;50(4):541-550.
doi pubmed - Shimanovsky A, Alvarez Argote J, Murali S, Dasanu CA. Autoimmune manifestations in patients with multiple myeloma and monoclonal gammopathy of undetermined significance. BBA Clin. 2016;6:12-18.
doi pubmed - Kashyap R, Singh A, Kumar P. Prevalence of autoimmune hemolytic anemia in multiple myeloma: A prospective study. Asia Pac J Clin Oncol. 2016;12(2):e319-322.
doi pubmed - Perillie PE. Myeloma and pernicious anemia. Am J Med Sci. 1978;275(1):93-98.
doi pubmed - Guay AT, Tuthill RJ, Woolf PD. Germinal cell aplasia: response of luteinizing hormone (LH), follicle-stimulating hormone (FSH), and testosterone to LH/FSH-releasing hormone with histopathologic correlation. Fertil Steril. 1977;28(6):642-649.
doi - Gupta V, Hegde UM, Parameswaran R, Newland AC. Multiple myeloma and immune thrombocytopenia. Clin Lab Haematol. 2000;22(4):239-242.
doi pubmed - Karapetians A, Bajaj T, Valdes A, Heidari A. A rare case of multiple myeloma presenting as Evan's syndrome. J Investig Med High Impact Case Rep. 2019;7:2324709619852760.
doi pubmed - Aryal MR, Bhatt VR, Tandra P, Krishnamurthy J, Yuan J, Greiner TC, Akhtari M. Autoimmune neutropenia in multiple myeloma and the role of clonal T-cell expansion: evidence of cross-talk between B-cell and T-cell lineages? Clin Lymphoma Myeloma Leuk. 2014;14(1):e19-23.
doi pubmed - Sakano S, Matsuyama H, Ishikawa H, Shindo A, Ii Y, Matsuura K, Mizutani M, et al. Myasthenia gravis with anti-muscle-specific tyrosine kinase antibodies during therapy for multiple myeloma: a case report. BMC Neurol. 2020;20(1):240.
doi pubmed - Nakae Y, Hyuga M, Terada Y, Kishimoto W, Fukunaga A, Tabata S, Maesako Y, et al. Multiple myeloma presenting with autoimmune autonomic ganglionopathy. Intern Med. 2017;56(24):3347-3351.
doi pubmed - Minami A, Iwai A, Watanabe Y, Nagamatsu H, Aono S, Kato S, Kawaguchi A, et al. Two cases of inflammatory bowel disease with multiple myeloma. J Gastroenterol. 1999;34(5):629-633.
doi pubmed - Carvalho LM, Bachour P, Menezes Y, Silva AE, Bombonatti JF, Bordin JO. Lambda Light Chain Multiple Myeloma in a Patient with Primary Biliary Cholangitis: Association or Mere Coincidence? Clin Lymphoma Myeloma Leuk. 2020;20(11):e846-e849.
doi pubmed - Ahmadpour N, Downey M, Frauenhoffer E, Riley T, Schreibman IR. Primary sclerosing cholangitis in association with multiple myeloma. Gastroenterol Hepatol (N Y). 2008;4(8):580-583.
- Trad D, et al. Association between auto-immune hepatitis and myeloma. Annals of Clinical Hepatology. 2019;3(2).
- Long Y, Aljamal AA, Bahmad HF, Yedla N, Herrera GA, Schwartz MA, Layka A. Multiple myeloma presenting as acute tubulointerstitial nephritis. Autops Case Rep. 2021;11:e2021328.
doi pubmed - Thachil J, Sadik W, Shawki H, Abraham KA. Membranous glomerulonephritis—an under-reported histological finding in multiple myeloma. Nephrol Dial Transplant. 2009;24(5):1695-1696.
doi pubmed - Chakraverty R, Rabin N, Peggs K, Robinson S, Duncan JR, Yong K. Dermatomyositis and sarcoid-like reaction associated with multiple myeloma treated effectively by high-dose chemotherapy and autologous peripheral blood stem cell transplantation. Bone Marrow Transplant. 2001;27(11):1215-1217.
doi pubmed - Vallabhajosyula S, Bekur R, Bhat R, Gnanadev N, Belurkar S. Polymyositis associated with non-secretory myeloma - a case report. Australas Med J. 2011;4(4):205-209.
doi pubmed - Lam SM, Ho HH, Dunn P, Luo SF. Association of ankylosing spondylitis with IgA-multiple myeloma: report of a case and pathogenetic considerations. Taiwan Yi Xue Hui Za Zhi. 1989;88(7):726-728.
- Dalamaga M, Karmaniolas K, Papadavid E, Pelecanos N, Migdalis I. Association of thyroid disease and thyroid autoimmunity with multiple myeloma risk: a case-control study. Leuk Lymphoma. 2008;49(8):1545-1552.
doi pubmed - Caimari F, Keddie S, Lunn MP, D'Sa S, Baldeweg SE. Prevalence and Course of Endocrinopathy in POEMS Syndrome. J Clin Endocrinol Metab. 2019;104(6):2140-2146.
doi pubmed - Sendrasoa FA, Ranaivo IM, Rakotoarisaona MF, Raharolahy O, Razanakoto NH, Andrianarison M, Ramarozatovo LS, et al. Pemphigus vulgaris as the first manifestation of multiple myeloma: a case report. J Med Case Rep. 2018;12(1):255.
doi pubmed - Ogg GS, Rosbotham JL, MacDonald DM. Urticaria pigmentosa coexisting with multiple myeloma. Clin Exp Dermatol. 1996;21(5):365-366.
doi pubmed - Bila J, Suvajdzic N, Elezovic I, Colovic M, Boskovic D. Systemic lupus Erythematosus and IgA multiple myeloma: a rare association? Med Oncol. 2007;24(4):445-448.
doi pubmed - Terpos E, Angelopoulou MK, Variami E, Meletis JC, Vaiopoulos G. Sjogren's syndrome associated with multiple myeloma. Ann Hematol. 2000;79(8):449-451.
doi pubmed - Lindqvist EK, Goldin LR, Landgren O, Blimark C, Mellqvist UH, Turesson I, Wahlin A, et al. Personal and family history of immune-related conditions increase the risk of plasma cell disorders: a population-based study. Blood. 2011;118(24):6284-6291.
doi pubmed - Steiner N, Gobel G, Michaeler D, Platz AL, Prokop W, Wolf AM, Wolf D, et al. Rheumatologic diseases impact the risk of progression of MGUS to overt multiple myeloma. Blood Adv. 2021;5(6):1746-1754.
doi pubmed - Thomas FB, Clausen KP, Greenberger NJ. Liver disease in multiple myeloma. Arch Intern Med. 1973;132(2):195-202.
doi pubmed - Barth C, Bosse A, Andus T. Severe acute cholestatic hepatitis by infiltration of monoclonal plasma cells in multiple myeloma. Z Gastroenterol. 2005;43(10):1129-1132.
doi pubmed - Perez-Soler R, Esteban R, Allende E, Tornos Salomo C, Julia A, Guardia J. Liver involvement in multiple myeloma. Am J Hematol. 1985;20(1):25-29.
doi pubmed - Michopoulos S, Petraki K, Petraki C, Dimopoulos MA. Light chain deposition disease of the liver without renal involvement in a patient with multiple myeloma related to liver failure and rapid fatal outcome. Dig Dis Sci. 2002;47(4):730-734.
doi pubmed - Kyle RA, Greipp PR. Amyloidosis (AL). Clinical and laboratory features in 229 cases. Mayo Clin Proc. 1983;58(10):665-683.
- Ratnasingam S, Walker PA, Tran H, Kaplan ZS, McFadyen JD, Tran H, Teh TC, et al. Bortezomib-based antibody depletion for refractory autoimmune hematological diseases. Blood Adv. 2016;1(1):31-35.
doi pubmed - Lindqvist EK, Landgren O, Lund SH, Turesson I, Hultcrantz M, Goldin L, Bjorkholm M, et al. History of autoimmune disease is associated with impaired survival in multiple myeloma and monoclonal gammopathy of undetermined significance: a population-based study. Ann Hematol. 2017;96(2):261-269.
doi pubmed - Lakshman A, Painuly U, Rajkumar SV, Ketterling RP, Kapoor P, Greipp PT, Dispenzieri A, et al. Impact of acquired del(17p) in multiple myeloma. Blood Adv. 2019;3(13):1930-1938.
doi pubmed - Zamagni E, Barbato S, Cavo M. How I treat high-risk multiple myeloma. Blood. 2022;139(19):2889-2903.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


