| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website http://www.thejh.org |
Case Report
Volume 8, Number 3, September 2019, pages 137-140
Gamma-Delta T-Cell Acute Lymphoblastic Leukemia/Lymphoma: Immunophenotype of Three Adult Cases
Wei Wanga, Yan Lia, Min Ou-Yanga, Meixiang Zhanga, Linjun Zhaob, Jianxin Liua, Yao Zhanga, Wenqing Hua, Bin Jianga, c
aDepartment of Hematology, Peking University International Hospital, Beijing, China
bDepartment of Lymphoma, Peking University International Hospital, Beijing, China
cCorresponding Author: Bin Jiang, Department of Hematology, Peking University International Hospital, No. 1, Zhong-Guan-Cun Life Science Park Road, Changping District, Beijing 102206, China
Manuscript submitted June 16, 2019, accepted August 16, 2019
Short title: Gamma-Delta T-ALL
doi: https://doi.org/10.14740/jh535
| Abstract | ▴Top |
Gamma-delta (γδ) T-cell acute lymphoblastic leukemia/lymphoma (T-ALL) is not commonly observed in adult patients. We report three adult cases and describe their immunophenotypes. Two of these cases were diagnosed as γδ T-ALL; one was diagnosed as a mixture of T-ALL and T-cell non-Hodgkin lymphoma (T-NHL). We also discussed the differential diagnoses.
Keywords: γδ T-ALL; Immunophenotype; Diagnosis
| Introduction | ▴Top |
There are four types of gamma-delta (γδ) T-cell neoplasms, namely, γδ T-cell acute lymphoblastic leukemia/lymphoma (T-ALL), skin and mucosal γδ T-cell lymphoma, hepatosplenic T-cell lymphoma, and γδ T-cell large granular lymphocytic leukemia [1-4]. The incidence of γδ T-ALL is low [1, 2]. It is characterized by the expression of γδ T-cell antigen receptors (TCRs). Here, we report the clinical and pathological characteristics of three adult patients with γδ T-ALL.
| Case Reports | ▴Top |
As shown in Table 1, case 1 was untreated, while cases 2 and 3 were refractory.
 Click to view | Table 1. Clinical Presentations |
Case 1
A 66-year-old woman with a 3-month history of gradually increasing tiredness came to our hospital. The vital signs were normal. The blood routine test was normal. Positron emission tomography/computed tomography (PET/CT) showed an irregular mediastinal mass with hypermetabolism signal. A mediastinal mass biopsy was performed. The immunohistochemistry result showed CD99+, CD5+, TdT+, CD3+, CD117+, CD30+, Ki-67+ (90%), CK-, CD20-, and CD15-. She was diagnosed as T-ALL.
Case 2
A 31-year-old woman with a 3-month history of tiredness and low fever presented to another hospital. The vital sign showed the heart rate of 110 beats per minute (bpm). The hemoglobin was 99 g/L, and platelet was 79 × 109/L. The bone marrow (BM) film showed plenty of abnormal lymphoblasts (84%). The immunophenotype of the BM specimen showed CD7+, cytoplasmic (c) and surface (s) CD3+, and cTdT+. She was diagnosed as T-ALL. The chemotherapy regimens were cyclophosphamide, vindesine, doxorubicin, and dexamethasone (CHOP), methotrexate and pegaspargase in time sequence. After two cycles of chemotherapy, the patient presented to our hospital. The BM aspirate showed lymphoblasts (17%) this time.
Case 3
A 29-year-old man with a 1-month history of cough and enlargement of cervical lymph nodes was admitted to another hospital. He had generalized cervical lymphadenopathy. Platelet was 69 × 109/L. The CT showed lymphadenopathy in multiple superficial lymph nodes. A cervical lymph node biopsy was performed. The immunohistochemistry result showed CD3+, CD5+, TdT+, and Ki-67+ (80%). The BM film showed plenty of abnormal lymphoblasts (41%). He was diagnosed as T-ALL. The chemotherapy regimen was vincristine, daunorubicin, cyclophosphamide, and prednisone (VDCP) for one cycle. Then the patient presented to our hospital. The BM aspirate showed abnormal lymphoblasts (25%).
BM specimens of all patients were collected. A total of 5 × 105 nucleated cells per tube were incubated with 0.1% bovine serum albumin solution and then stained with monoclonal antibodies (mAbs). Samples were stained as described before [5]. All samples were detected by BD FACSCalibur and analyzed by Cell Quest (all are from BD).
The gating strategy was as follows: live cells were gated on the forward scatter/side scatter (FSC/SSC) dot plot. Then, each cellular population was delineated on the SSC/CD45 dot plot. Abnormal γδ T cells were gated on the CD45/TCR γδ or CD45/CD3 dot plot. The definitions of the expression of a certain marker were described previously [6].
TCR rearrangements were detected by the ABI 7500 Fast Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). The methods have been previously described [7].
The abnormal T cells in all cases were highly positive for CD7 and CD99, and positive for TCR γδ, CD45, and c/sCD3. For other pan-T-cell markers, all cases were positive for CD5; case 2 was dimly positive for CD8; case 3 was positive for CD2 and partially positive for CD8 (75.4%). For markers of immature cells, a fraction of abnormal T cells in cases 1 and 2 was positive for CD34 (22.9% and 46.1%, respectively); the abnormal T cells in case 1 were dimly positive for cTdT; a fraction of abnormal T cells in case 1 was positive for CD1a (15.47%); and a fraction of abnormal T cells in case 2 and case 3 was dimly positive for cTdT (75.2% and 24.6%, respectively). For the other markers, the abnormal T cells were negative for cMPO, CD11c, CD14, CD64, DR, CD13, CD15, CD33, cCD22, cCD79a, CD16, CD56, and CD57. The results of flow cytometry and PCR are presented in Table 2 and Figure 1.
 Click to view | Table 2. Immunophenotype and TCR Rearrangement |
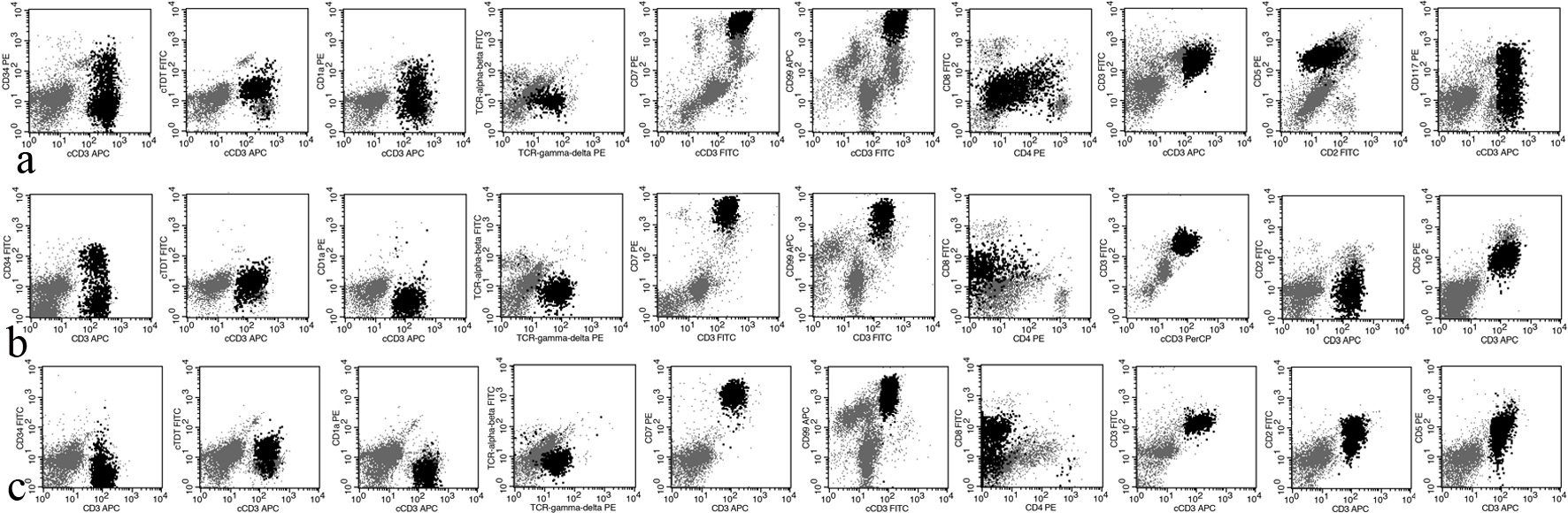 Click for large image | Figure 1. The expression profile of T-ALL cells in cases 1, 2, and 3. T-ALL: T-cell acute lymphoblastic leukemia/lymphoma. |
| Discussion | ▴Top |
Generally, T-ALL lacks TCR expression, and only rare cases are positive for TCR γδ. The diagnosis of T-ALL is based on the immunophenotype, that is simultaneous positivity for c/sCD3 and blastic markers (cTdT, CD34, or CD1a) [8]. First, we defined the lineage of these abnormal cells, and the three cases were all positive for cCD3 and sCD3 and negative for cMPO, CD11c, CD14, CD64, CD19, CD10, cCD22, and cCD79a. Therefore, these cells were definitely T cells. Second, we needed to clarify the maturation stage. Based on the expression of sCD3 and CD1a, the World Health Organization (WHO) separates T-ALL into three categories: early T-ALL (CD1a-, sCD3-), thymic T-ALL (CD1a+, sCD3-), and mature T-ALL (CD1a-, sCD3+). T-ALL cells in case 1 dimly expressed cTdT, and some cells also expressed CD34 (22.9%) and CD1a (32.1%). A fraction of T-ALL cells in case 2 expressed CD34 (46.1%) and dimly expressed cTdT (75.2%). In case 3, some of these cells dimly expressed cTdT (24.6%). All these data show that these abnormal T cells are immature. Considering these data, case 1 was diagnosed as thymic T-ALL, while the other two as mature T-ALL.
The diagnosis of case 3 is worthy of being discussed in more detail. All the abnormal T cells expressed sCD3. Approximately 25% of the abnormal T cells dimly expressed cTdT, while approximately 75% of the abnormal T cells expressed CD8. These data suggest that there were two abnormal T-cell populations in this sample, and the majority of abnormal T cells were more mature than others. Based on the developmental stage, T cells can be divided into four types: pro-T, pre-T, cortical-T, and mature-T [9]. Therefore, in case 3, the majority of abnormal T cells were in the mature T-cell stage, while the minority were somewhere between the cortical T-cell stage and mature T-cell stage.
Early T-cell precursor T-ALL (ETP-ALL) is a subtype of T-ALL [10]. These cells deriving from hematopoietic stem cells migrate from the BM to the thymus [11]. Approximately 54% of T-ALL cells in case 1 expressed CD117, and thus we need to distinguish it from ETP-ALL. We have pieces of evidence to support the diagnosis of γδ T-ALL. First, the abnormal T cells were positive for CD5 (100% of blasts) and sCD3. Second, some abnormal T cells were positive for CD1a. Third, these T cells lacked the expression of other myeloid or stem cell markers. Fourth, CD117 can rarely be detected in T-NHL and T-ALL (2.2% and 11.4%, respectively) [8]. The immunophenotype shows that these T-ALL cells were more mature than ETP-ALL cells. Therefore, we excluded the diagnosis of ETP-ALL [10-12].
We were also interested in other immunophenotypic characteristics of these γδ T-ALL cases. CD7 and CD99 are universally highly expressed in all cases. For the other pan-T-cell markers, at least some T-ALL cells positively or dimly express CD2, CD5, or CD8. Although normal γδ T cells are double negative for CD4 and CD8, some γδ T-ALLs express CD4, CD8, or both [2]. These findings suggest that γδ T-ALL exhibits asynchronous antigen expression. In addition, CD10 is a common marker of lineage infidelity, the same finding in αβ T-ALL.
Here, we report three cases of γδ T-ALL. More studies are needed to clarify the unique clinical and laboratory characteristics.
Acknowledgments
We thank all the patients.
Financial Disclosure
None to declare.
Conflict of Interest
All authors have no conflict of interest to disclose.
Informed Consent
All the data and specimens were collected with informed consent.
Author Contributions
WW wrote the manuscript and analyzed the results of flow cytometry; YL performed the flow cytometry; MOY, MZ, LZ, JL, YZ, and WH treated the patients, and provided clinical information and other lab results; BJ treated the patients and revised the manuscript.
| References | ▴Top |
- Wei EX, Leventaki V, Choi JK, Raimondi SC, Azzato EM, Shurtleff SA, Ong MG, et al. Gammadelta T-Cell acute lymphoblastic leukemia/lymphoma: discussion of two pediatric cases and its distinction from other mature gammadelta T-Cell malignancies. Case Rep Hematol. 2017;2017:5873015.
doi pubmed - Matos DM, Rizzatti EG, Fernandes M, Buccheri V, Falcao RP. Gammadelta and alphabeta T-cell acute lymphoblastic leukemia: comparison of their clinical and immunophenotypic features. Haematologica. 2005;90(2):264-266.
- Rego EM, Garcia AB, Viana SR, Falcao RP. Characterization of acute lymphoblastic leukemia subtypes in Brazilian patients. Leuk Res. 1996;20(4):349-355.
doi - Schott G, Sperling C, Schrappe M, Ratei R, Martin M, Meyer U, Riehm H, et al. Immunophenotypic and clinical features of T-cell receptor gammadelta+ T-lineage acute lymphoblastic leukaemia. Br J Haematol. 1998;101(4):753-755.
doi pubmed - Wang W, Gao L, Gong M, Tang Y, Li Y, Zhang WT, Huang FZ, et al. Non-malignant T-cells lacking multiple pan-T markers can be found in lymph nodes. Leuk Lymphoma. 2018;59(1):155-161.
doi pubmed - Kraus TS, Sillings CN, Saxe DF, Li S, Jaye DL. The role of CD11c expression in the diagnosis of mantle cell lymphoma. Am J Clin Pathol. 2010;134(2):271-277.
doi pubmed - van Dongen JJ, Langerak AW, Bruggemann M, Evans PA, Hummel M, Lavender FL, Delabesse E, et al. Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: report of the BIOMED-2 Concerted Action BMH4-CT98-3936. Leukemia. 2003;17(12):2257-2317.
doi pubmed - Gorczyca W. Flow cytometry in neoplastic hematology morphologic - immunophenotypic correlation. 3rd ed. Boca Raton (FL): Taylor & Francis Group. 2017:94-98.
doi - Bene MC, Castoldi G, Knapp W, Ludwig WD, Matutes E, Orfao A, van't Veer MB. Proposals for the immunological classification of acute leukemias. European Group for the Immunological Characterization of Leukemias (EGIL). Leukemia. 1995;9(10):1783-1786.
- Coustan-Smith E, Mullighan CG, Onciu M, Behm FG, Raimondi SC, Pei D, Cheng C, et al. Early T-cell precursor leukaemia: a subtype of very high-risk acute lymphoblastic leukaemia. Lancet Oncol. 2009;10(2):147-156.
doi - Jain N, Lamb AV, O'Brien S, Ravandi F, Konopleva M, Jabbour E, Zuo Z, et al. Early T-cell precursor acute lymphoblastic leukemia/lymphoma (ETP-ALL/LBL) in adolescents and adults: a high-risk subtype. Blood. 2016;127(15):1863-1869.
doi pubmed - Zhang J, Ding L, Holmfeldt L, Wu G, Heatley SL, Payne-Turner D, Easton J, et al. The genetic basis of early T-cell precursor acute lymphoblastic leukaemia. Nature. 2012;481(7380):157-163.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


