| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website http://www.thejh.org |
Case Report
Volume 6, Number 2-3, September 2017, pages 68-71
Hypertrophic Herpes Simplex Virus Type 1 Infection in a Patient With Acute Myeloid Leukemia
Yusuke Ogawaa, Takakazu Higuchib, d, Ryosuke Koyamadab, Satoru Araic, Sadamu Okadab
aInternal Medicine, St. Luke’s International Hospital, Tokyo, Japan
bDivision of Hematology, St. Luke’s International Hospital, Tokyo, Japan
cDepartment of Dermatology, St. Luke’s International Hospital, Tokyo, Japan
dCorresponding Author: Takakazu Higuchi, Dokkyo Medical University Koshigaya Hospital, 2-1-50, Minami-Koshigaya, Koshigaya, Saitama 343-8555, Japan
Manuscript submitted June 14, 2017, accepted June 29, 2017
Short title: Hypertrophic HSV-1 in Acute Leukemia
doi: https://doi.org/10.14740/jh333w
| Abstract | ▴Top |
A 64-year-old woman had a transformation from polycythemia vera to acute myeloid leukemia. While she was treated with azacitidine and prednisolone, a nodule at the left angle of the mouth developed, which was biopsied and diagnosed with hypertrophic herpes simplex virus type 1 (HSV-1) infection. The nodule resolved completely with aciclovir. While HSV type 2 virus occasionally forms mass or tumoral lesions in immunocompromised, especially acquired immunodeficiency syndrome, patients, it is extremely rare that HSV-1 infection leads to similar lesions. The hematological conditions and the therapies given may have contributed to the rare manifestation of HSV-1 infection.
Keywords: Hyperplastic herpes; Herpes simplex virus type 1; Acute myeloid leukemia; Azacitidine; Immunocompromise
| Introduction | ▴Top |
Herpes simplex virus (HSV) is one of the most prevalent viral infections of oral and anogenital regions. HSV type 1 (HSV-1) primarily infects oral cavity and causes gingivostomatitis and then resides in the sensory ganglion, resulting in a latent infection [1]. Reactivation of HSV-1 occurs with various trigger factors and usually forms red macules on the mucocutaneous junction of the lips which rapidly become vesicular, then form pustular scabs and ulcers. HSV-1 may form atypical lesions in immunocompromised patients; however, it is extremely rare that it forms mass, tumoral, or hypertrophic lesions as occasionally seen in the anogenital regions of patients infected with human immunodeficiency virus (HIV) due to HSV type 2 (HSV-2) infection [2-7].
Here, we report a case of acute myeloid leukemia (AML) transformed from polycythemia vera (PV) who developed a nodule at the left angle of the mouth due to HSV-1 infection while receiving extended corticosteroids and azacitidine. The nodule resolved completely with aciclovir (ACV). The occurrence of such hypertrophic chronic HSV-1 infection in AML patients has not been reported in the literature and this case demonstrates that it is important to think of a possibility of manageable tumor even in patients with myeloid malignancies, in whom leukemic cells often form myeloid sarcoma, and to attempt a biopsy.
| Case Report | ▴Top |
A 64-year-old woman had been diagnosed with PV in 1994 and treated with hydroxycarbamide until September 2013, when she had a transformation to AML. She had no history of oral or genital herpes. The laboratory data showed the white blood cell (WBC) count to be 1.0 × 109/L with 30.0% blasts, the red blood cell count 4.04 × 1012/L with 1.67% reticulocytes, the hemoglobulin 11.9 g/dL, the hematocrit 40.2%, and the platelet count 144 × 109/L. The bone marrow (BM) was hypoplastic with dysplastic changes of the hematopoietic cells and the myeloblasts accounted for 20% of the nucleated cells. The patient was therefore diagnosed with AML transformed from PV.
Two courses of intensive chemotherapy with idarubicin and cytarabine followed by a course of high-dose cytarabine resulted in a partial remission. In April 2014, when the BM blasts accounted for 3.0% of the nucleated cells, subcutaneous azacitidine was started at 75 mg/m2 for 5 days and repeated every 4 or 5 weeks. Although the peripheral blast cells were noted in August 2014 and gradually increased, azacitidine was continued. She was complicated with organizing pneumonia in December 2014 and treated with corticosteroids. After the resolution of the pneumonia, oral prednisolone was continued at 20 - 30 mg/day. In February 2015, she noticed multiple tender ulcers in the oral cavity and another ulcer with dark crust at the left angle of the mouth. The latter gradually formed a painful nodule of 1.5 cm in size with black necrotic tissue (Fig. 1a, b). The WBC count was 25.8 × 109/L with 32.0% blasts and the lymphocytes accounted for 2.5% of the WBC with the absolute T-lymphocyte count as low as 12.9 × 106/L.
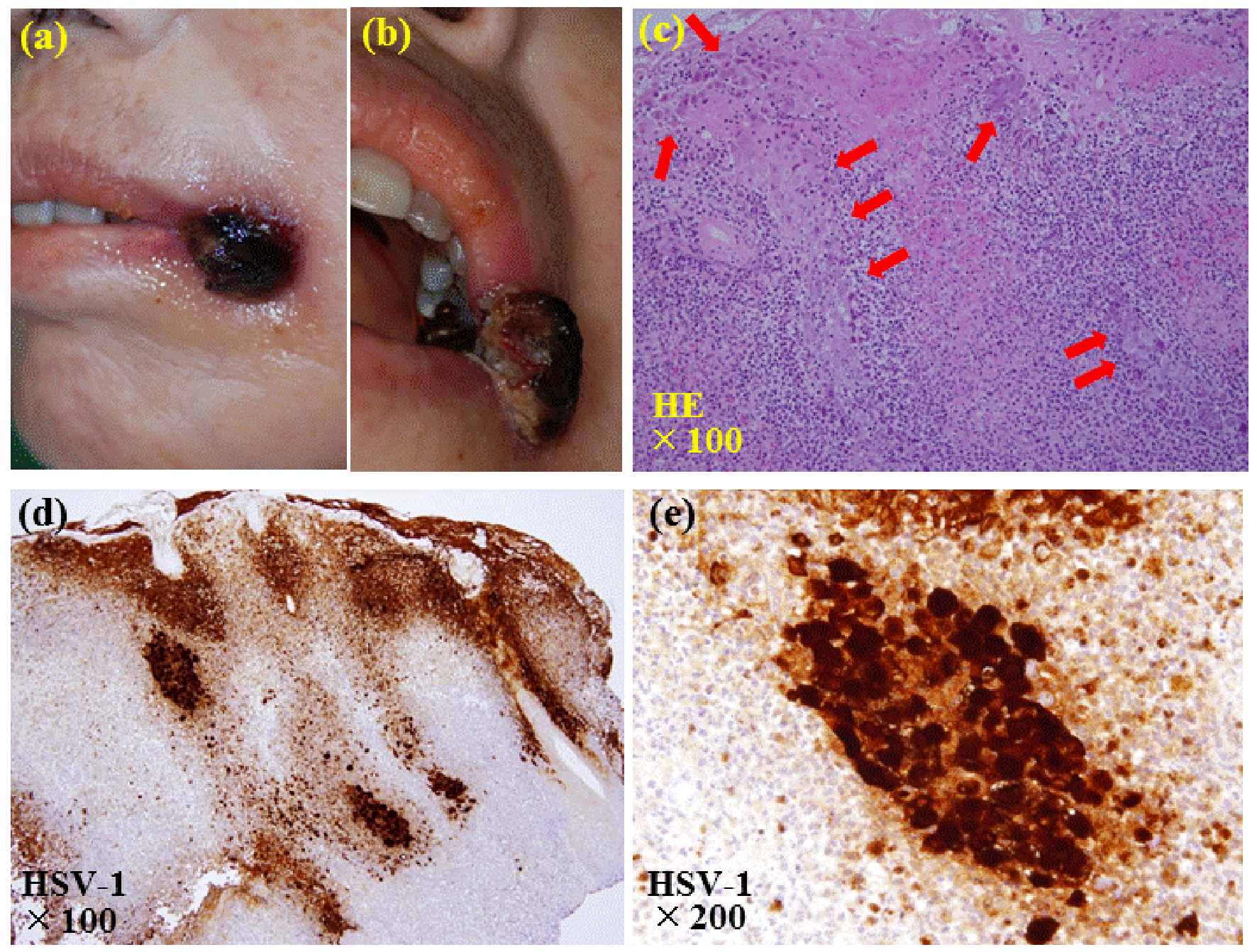 Click for large image | Figure 1. Pictures and pathological findings of the nodule at the left angle of the mouth. (a, b) A painful nodule of 1.5 cm in size with black necrotic tissue is seen at the left angle of the mouth. (c) The biopsy of the nodule reveals ulcerative lesions with necrosis of the epithelium and vesicular formation. Enlarged epithelial cells whose nuclei are enlarged with moldering and displacement of chromatin to the periphery, and intranuclear inclusion bodies are seen (arrows) (hematoxylin and eosin stain, × 100). (d, e) Immunostaining shows that these epithelial cells are positive with antibody against anti-herpes simplex virus type 1 (d: × 100; e: × 200). |
Biopsy of the nodule was performed. The hematoxylin and eosin stain of the biopsy sample revealed ulcerative lesions with necrosis of the epithelium and vesicular formation (Fig. 1c). Enlarged epithelial cells whose nuclei were enlarged with moldering and displacement of chromatin to the periphery were seen, and intranuclear inclusion bodies were also observed. Immunostaining with antibody against HSV-1 or HSV-2 (DAKO Corporation, Carpinteria, CA, USA) showed that these cells were stained positive with both antibodies (Fig. 1d, e). Reactive granulation tissues with extensive neutrophilic infiltration and abscess formation were observed in the dermis. No evidence of infiltration of the leukemic cells was seen. These findings were considered to be a hypertrophic HSV infection complicated with ulceration and abscess formation. The diagnosis was supported by the serological findings showing positive anti-HSV immunoglobulin (Ig) G titer of 48.7 (EIA) (reference range < 2.0) and negative anti-HSV IgM. As the antibodies against HSV-1 and HSV-2 might react not only with HSV-1 and HSV-2 specific antigens, respectively, but also with antigens common for HSV-1 and HSV-2, differentiation between HSV-1 and HSV-2 infections could not be made based on the results of the immunostaining studies. However, serological tests revealed that the anti-HSV-1 IgG antibody (NT) was positive at 4 (reference range < 4) and anti-HSV-2 antibody (NT) was negative, thus the lesion was diagnosed to be a chronic HSV-1 lesion. Positive staining with anti-HSV-2 antibody was considered to be due to the cross-reactivity of the antibody. Anti-HIV antibody was negative.
ACV was given intravenously at 5 mg/kg three times a day for 10 days and resulted in a complete resolution of the nodule and oral ulcers. ACV was continued at 200 mg orally and the nodule did not reappear thereafter until her death 6 months later due to the progression of AML.
| Discussion | ▴Top |
Recurrent infection of HSV-1 is caused by reactivation of the virus maintained latently in the sensory ganglion and the lesions typically occur on the mucocutaneous junction of the face as red macules that rapidly become vesicular [1]. Although it is known that recurrent HSV-1 infection may cause atypical, more extensive and/or aggressive lesions in immunocompromised patients than in immunocompetent individuals, it is very rare that HSV-1 infection leads to formation of mass or tumoral hypertrophic lesions as seen in HSV-2 infection in immunocompromised patients. Occasional cases of HSV-2 infection in HIV-infected patients have been reported in whom HSV-2 causes formation of mass or tumoral lesions in the anogenital regions [5, 7, 8]. While similar HSV-2 infection in the genital region has been reported in patients with other immunocompromised conditions such as common variable immunodeficiency and organ transplant recipients, such reports are extremely rare [6, 9]. HSV-1 infection that leads to a formation of hypertrophic lesions is very rare and, to our knowledge, only four such cases have been reported in the literature. One patient with Hodgkin lymphoma was treated with multidrug chemotherapy and palliative radiation therapy and on prednisone and another patient who had orthotopic heart transplantation was on prednisone, tacrolimus, and mycophenolate mofetil [2, 3]. Both patients developed tongue masses due to HSV-1 infection. The third patient with restrictive lung disease without apparent immunocompromise developed endotracheal mass caused by HSV-1 [4]. The last patient with acquired immunodeficiency syndrome developed nodules in the anal area and the immunochemistry revealed HSV-1 infection [5]. The present case was the first case of hypertrophic HSV-1 infection forming a nodular lesion in a patient with AML in whom differentiation between myeloid sarcoma and other etiologies was important but difficult.
It is conceivable that the patient was immunocompromised due to AML, long-term hydroxycarbamide, intensive chemotherapy courses, prolonged corticosteroids, and, possibly, azacitidine therapy. Azacitidine is a hypomethylating agent and the first drug which was shown to extend the overall survival of high-risk myelodysplastic syndrome (MDS) patients compared to conventional care [10]. Azacitidine has shown efficacy in treating AML with low BM blast counts and therapy-related myeloid neoplasms [10-12].
The lymphocyte count was profoundly reduced when the hypertrophic HSV-1 lesion developed. It is likely that the long-term steroid therapy is largely responsible for the immunocompromised state of the present case, providing a background for the formation of the atypical hypertrophic HSV-1 lesion. In addition, it appears possible that azacitidine might contribute to some extent as the effects of azacitidine on T-lymphocytes have been reported [13]. Azacitidine treatment reduces the numbers of regulatory T (Treg), T-helper 1, and T-helper 2 cells in MDS patients. On the other hand, it is assumed that the imbalance in cytokine release secondary to the immune alterations could be responsible for the abnormal response to HSV-2 infection leading to hypertrophic HSV-2 lesions in HIV-infected patients [14]. In vitro studies have shown that azacitidine reduces the function of Treg cells and that the inhibitory effects of Treg cells on proliferation of T-effector cells and on cytokine secretion are subsequently decreased, resulting in an increased secretion of proinflammatory cytokines [13]. Thus, it is possible that increased secretion of the proinflammatory cytokines associated with azacitidine therapy may have contributed to the development of the hypertrophic HSV-1 lesions in the present case. However, these assumptions are highly speculative at present and the accumulation and further investigation of the similar cases are obviously needed.
Recent clinical application of diverse and profound immunosuppression may cause atypical reactions to pathogens in patients who are already immunocompromised as exemplified by the present case. This case also exemplifies that it is important to consider the possibility of manageable infectious tumoral lesions even in patients with preexisting malignancies.
Conflicts of Interest
All the authors have no conflicts of interest to disclose.
| References | ▴Top |
- Arduino PG, Porter SR. Herpes Simplex Virus Type 1 infection: overview on relevant clinico-pathological features. J Oral Pathol Med. 2008;37(2):107-121.
doi pubmed - Leming PD, Martin SE, Zwelling LA. Atypical herpes simplex (HSV) infection in a patient with Hodgkin's disease. Cancer. 1984;54(12):3043-3047.
doi - Tabaee A, Saltman B, Shutter J, Hibshoosh H, Markowitz A. Recurrent oral herpes simplex virus infection presenting as a tongue mass. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97(3):376-380.
doi pubmed - Upadya A, Tilluckdharry L, Nagy CD, Ravichandran P, Manthous C. Endobronchial pseudo-tumour caused by herpes simplex. Eur Respir J. 2005;25(6):1117-1120.
doi pubmed - Nadal SR, Calore EE, Manzione CR, Horta SC, Ferreira AF, Almeida LV. Hypertrophic herpes simplex simulating anal neoplasia in AIDS patients: report of five cases. Dis Colon Rectum. 2005;48(12):2289-2293.
doi pubmed - Hanjani NM, Foster DC, Scott GA, Mercurio MG. A genital mass due to herpes simplex virus in a renal transplant recipient. J Low Genit Tract Dis. 2007;11(3):173-176.
doi pubmed - Sbidian E, Battistella M, LeGoff J, Lafaurie M, Bezier M, Agbalika F, Simon F, et al. Recalcitrant pseudotumoral anogenital herpes simplex virus type 2 in HIV-infected patients: evidence for predominant B-lymphoplasmocytic infiltration and immunomodulators as effective therapeutic strategy. Clin Infect Dis. 2013;57(11):1648-1655.
doi pubmed - Leeyaphan C, Surawan TM, Chirachanakul P, Prasertworonun N, Punyaratabandhu P, Omcharoen V, Jiamton S. Clinical characteristics of hypertrophic herpes simplex genitalis and treatment outcomes of imiquimod: a retrospective observational study. Int J Infect Dis. 2015;33:165-170.
doi pubmed - Beasley KL, Cooley GE, Kao GF, Lowitt MH, Burnett JW, Aurelian L. Herpes simplex vegetans: atypical genital herpes infection in a patient with common variable immunodeficiency. J Am Acad Dermatol. 1997;37(5 Pt 2):860-863.
doi - Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Finelli C, Giagounidis A, Schoch R, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol. 2009;10(3):223-232.
doi - Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Gattermann N, Germing U, Sanz G, et al. Azacitidine prolongs overall survival compared with conventional care regimens in elderly patients with low bone marrow blast count acute myeloid leukemia. J Clin Oncol. 2010;28(4):562-569.
doi pubmed - Fianchi L, Criscuolo M, Lunghi M, Gaidano G, Breccia M, Levis A, Finelli C, et al. Outcome of therapy-related myeloid neoplasms treated with azacitidine. J Hematol Oncol. 2012;5:44.
doi pubmed - Costantini B, Kordasti SY, Kulasekararaj AG, Jiang J, Seidl T, Abellan PP, Mohamedali A, et al. The effects of 5-azacytidine on the function and number of regulatory T cells and T-effectors in myelodysplastic syndrome. Haematologica. 2013;98(8):1196-1205.
doi pubmed - Deza G, Martin-Ezquerra G, Curto-Barredo L, Villar Garcia J, Pujol RM. Successful treatment of hypertrophic herpes simplex genitalis in HIV-infected patient with topical imiquimod. J Dermatol. 2015;42(12):1176-1178.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


