| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website http://www.thejh.org |
Case Report
Volume 5, Number 4, December 2016, pages 142-150
Severe Gestational Thrombocytopenia: A Case Report and Brief Review of the Literature
Mohamad Khaled Ramadana, b, d, Manal Hubeicha, Saad Eddine Itania, Anas Mogharbilc
aDepartment of Obstetrics and Gynecology, Makassed General Hospital, Beirut, Lebanon
bDivision of Maternal-Fetal-Medicine, Makassed General Hospital, Beirut, Lebanon
cDepartment of Internal Medicine (Division of Hematology-Oncology), Makassed General Hospital, Beirut, Lebanon
dCorresponding Author: Mohamad Khaled Ramadan, Department of Obstetrics and Gynecology, Makassed General Hospital, Beirut, Lebanon
Manuscript accepted for publication December 16, 2016
Short title: Severe Gestational Thrombocytopenia
doi: https://doi.org/10.14740/jh308w
| Abstract | ▴Top |
Thrombocytopenia (TCP) is a common medical finding in obstetric population at term. The majority of new-onset TCP cases are mild, asymptomatic and diagnosed accidentally on routine antenatal screening. The most common causes at term are gestational thrombocytopenia (GT), preeclampsia/HELLP syndrome and immune-mediated thrombocytopenia (ITP). Preeclampsia/HELLP syndrome is accompanied with well-defined clinical characteristics and specific laboratory findings, while the other two are usually asymptomatic and are impossible to distinguish from one another. We encountered a case of new-onset TCP at 40 weeks gestation with negative history and a platelet count of 33 × 109/L, yet, who had a fast spontaneous postpartum recovery. Her second pregnancy was also complicated by TCP of 77 × 109/L at 37 weeks gestation. The newborn platelet count was normal in both instances. She was considered to have GT after a lapse of 4 years, being consistently healthy with normal platelet counts. After excluding other serious causes of severe new-onset TCP at term, management should be oriented towards securing hemostasis in preparation for delivery without wasting precious time and resources trying to discern between GT and ITP.
Keywords: Severe new-onset thrombocytopenia in pregnancy; Workup; Gestational and immune thrombocytopenia
| Introduction | ▴Top |
Thrombocytopenia (TCP) is a common finding encountered among pregnant women at term [1] with rates varying according to the cutoff level used to define this hematologic condition. Diagnosis is usually reached after a thorough and laborious workup intended primarily to exclude serious diseases known to inflict adverse maternal and neonatal outcomes. Almost all causes of TCP are associated with a constellation of signs and symptoms and abnormal laboratory or pathologic findings except for gestational thrombocytopenia (GT) and immune thrombocytopenia (ITP). These two disorders make the majority of cases, yet both are diagnosed by exclusion and are predominantly asymptomatic except for bleeding associated with severe ITP cases. Differentiating between them is generally inaccurate and very difficult during pregnancy [2].
Pregnancy represents a challenge in the management of TCP where fetal considerations, safeguarding hemostatic requirements of invasive procedures like regional anesthesia and delivery, and the familiarity with managing pregnancy-related diseases are added concerns exclusively encountered during pregnancy. This is usually reflected by the inconsistent and controversial obstetric management of this condition by many obstetricians [3]. Furthermore, occasionally there is limited time to dwell on detailed investigations or even to have adequate treatment response as with women presenting in labor and imminent delivery rendering urgent yet proper management difficult in some cases. In the following text, we will describe an unusual case of severe GT and present a brief review of the literature. A search of the Medline database for “incidental”, “gestational”, “pregnancy-associated thrombocytopenia” and “ITP in pregnancy” was done for articles published between 2000 and 2016 in English language. The search yielded 407 articles, of which 84 were considered relevant. Information pertaining to the management of new-onset TCP during pregnancy was extracted from these and other older land-mark articles that were considered to be essential to the understanding of the management process.
| Case Report | ▴Top |
A 17-year-old primigravida presented to labor and delivery unit at 40 + 3/7 weeks with frank rupture of membranes and early labor pains. A complete blood count and differential (CBCD) taken on admission showed normal hemogram except for severe TCP of 33 × 109/L. This was confirmed with peripheral blood smear (PBS) exam which was consistent with isolated immune TCP. Her antenatal course was smooth with a platelet count of 188 × 109/L at 13 weeks gestation. Her past medical and family histories were negative for any bleeding tendency or TCP or medications. Physical examination revealed normal blood pressure, absence of petechiae, bruising or bleeding anywhere. Laboratory investigations were extended to exclude disseminated intravascular coagulopathy (DIC) profile, infections immune status, liver function tests and autoimmune disorders. She was delivered by cesarean section due to dystocia under general anesthesia directly following platelets transfusion of one unit single-donor in the operating theater. The operation was smooth without bleeding accidents. Platelet count reached 70 × 109/L 12 h postpartum and 139 × 109/L on day 6 at discharge. The newborn platelet count was 157 × 109/L. She was followed postpartum by a hematologist and all her laboratory investigations were normal.
Three months later, the patient underwent laparoscopic cholecystectomy during which her platelet count was 188 × 109/L and 195 × 109/L directly before and 24 h after the operation, respectively.
Two years later, she conceived again and on regular monthly antenatal visits her platelet count was found to be 120 × 109/L at 35 weeks to drop a week later to 99 × 109/L. At 36 + 6/7 weeks, the patient presented with uterine contractions and a platelet count of 77 × 109/L. She was delivered uneventfully by secondary cesarean section under general anesthesia without any treatment and without unusual blood loss. Platelet count 12 h postpartum increased to 89 × 109/L to reach 120 × 109/L on day 3 at discharge (Fig. 1). Once again, the newborn had normal platelet count of 216 × 109/L. Two years later, she was found to be healthy with normal platelet count.
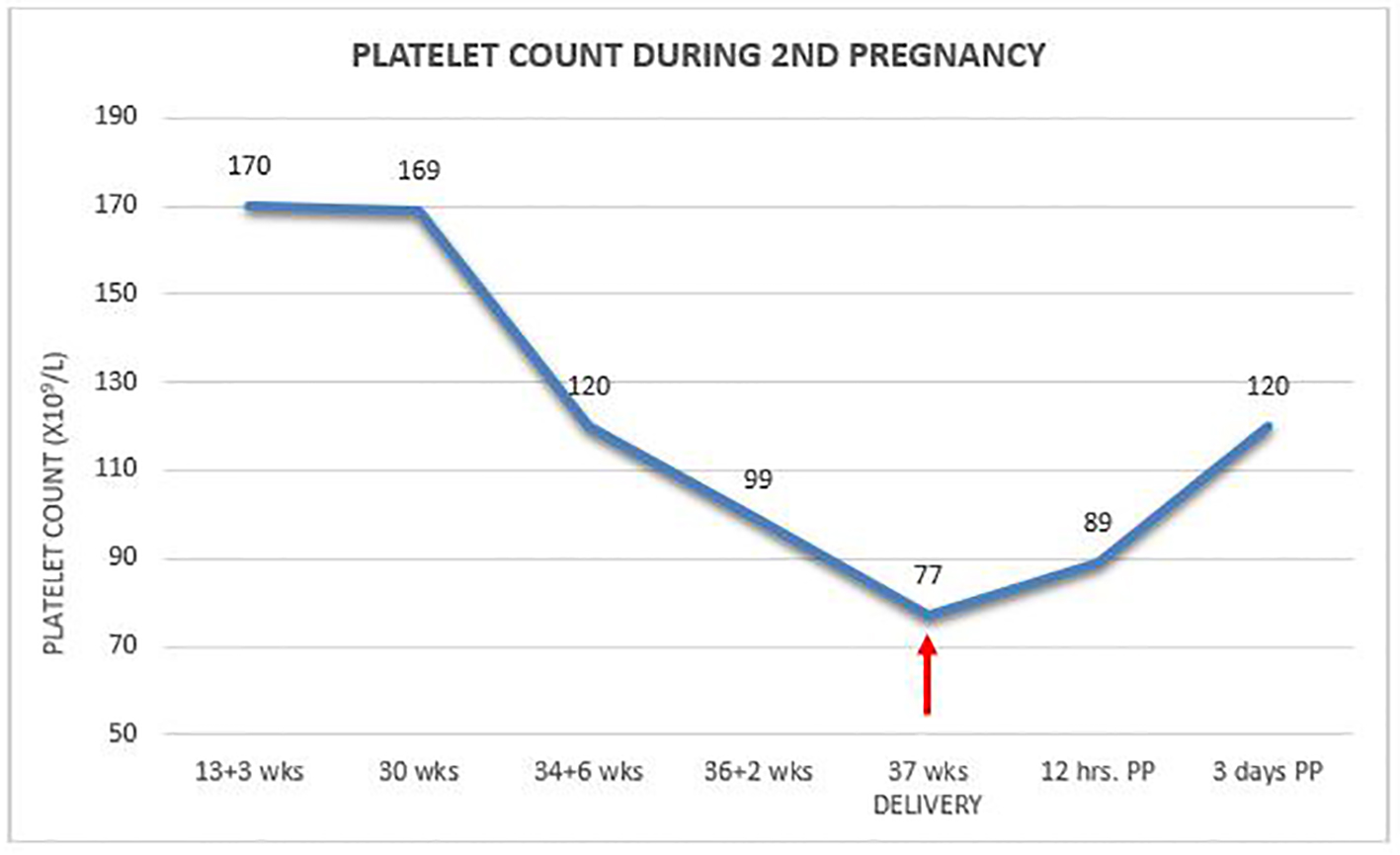 Click for large image | Figure 1. Platelet count during second pregnancy. |
| Discussion | ▴Top |
A TCP of platelet counts < 100 × 109/L, as adopted by the International Working Group, seems to better reflect clinical significance rather than mathematical calculation of percentiles in healthy individuals [4]. Using this latter cutoff level, TCP is observed to affect only 1% of obstetric population at term [5], and here levels of < 70 × 109/L are usually used to define severity. Pregnancy is associated with a physiologic decline in the mean platelet count of about 10% [6], leading to the appearance of mild-moderate asymptomatic TCP in some healthy pregnant women at term. Some patients are known to have medical illnesses that cause TCP, while in others, pregnancy is the first encounter of TCP. This represents a diagnostic and management challenge as in our case. At term, TCP is mostly due to GT (75%), hypertensive disorders of pregnancy (21%) disorders with autoimmune background as ITP and systemic lupus erythematosus (SLE) (4%), while other causes are rare and account only for < 1% of cases [7].
Our patient with severe new-onset TCP at term and imminent delivery was provisionally diagnosed to have new-onset ITP. All the efforts were oriented towards securing a platelet count compatible with the hemostatic requirements of cesarean delivery. Here, as her platelet count level was lower than the minimal threshold recommended for safe cesarean delivery [8, 9], platelet transfusion was the sound choice of emergent and rapid therapy. Interestingly, her postpartum course was not as expected for ITP (showing rapid and complete spontaneous recovery). On the other hand, it was also unfamiliar for GT to present with such severe TCP. Long-term follow-up provided more information about the nature of her TCP having had a normal platelet count in the intervals before and following her two pregnancies. The patient did not develop complications during her pregnancies and neonatal platelet count was also normal in both instances. These developments compelled us to change the diagnosis to severe GT. Severe GT has become more increasingly encountered during our practice with rates reaching in some reports up to 21.0-30.8% of all GT cases [10, 11], which demands fellow obstetricians to be well-informed and prepared to manage such challenging cases.
A workup plan for the management of new-onset TCP with emphasis on distinguishing GT from ITP
Familiarity and acquaintance with the clinical presentation and the most prominent laboratory hallmarks of diseases, especially those exclusive to pregnancy, is crucial in the workup needed for early diagnosis and institution of proper treatment in cases of new-onset TCP. Some causes are specific to pregnancy while others can affect non-pregnants as well, yet with increased frequency during pregnancy [12]. TCP can be the only hematologic abnormality of some diseases as with GT and ITP, while in other disorders, it is only one aspect of a systemic illness as with thrombotic thrombocytopenic purpura (TTP)/hemolytic uremic syndrome (HUS), preeclampsia/HELLP syndrome or leukemias. The primary intention of the workup is to reach a diagnosis and to exclude serious illnesses known to cause catastrophic maternal or neonatal outcomes. While all diseases that can cause TCP during pregnancy including preeclampsia/HELLP are associated with specific signs and symptoms and abnormal laboratory studies, GT and ITP can be asymptomatic, insidious, give the same picture on PBS examination and do not have specific confirmatory laboratory tests except for isolated TCP. Furthermore, no reliable laboratory test exists to differentiate between GT and ITP, hence, the need to explore any available clinical differences between these two disorders.
Confirming the diagnosis
The initial step in the management of new-onset TCP is to inspect carefully the CBCD for the presence of abnormalities in other cell-lines such as pancytopenia, vitamin B12, folate or iron deficiency anemia. It is probably wise to repeat the test on a different sample or machine or to use citrated blood samples. Final diagnosis of isolated ITP, however, should be made after confirmation by PBS examination [13].
PBS examination
Careful PBS examined by experienced hematologist is recommended as the initial step in the differential diagnosis workup [13] and is favored by many even in mild TCP [14]. It can exclude platelet clumping and aggregation causing pseudo-thrombocytopenia. Furthermore, it can ascertain the diagnosis of isolated TCP and assist in the early exclusion of many abnormal and serious disorders. This might prove crucial in ameliorating the course of otherwise fatal illnesses as with TTP/HUS or myelodysplastic disorders. The diagnosis of isolated TCP with immune background entails the presence of abundant normally appearing megakaryocytes and predominantly normal platelets of all generations, while other cell-lines especially red blood cells must be normal. It might be necessary to repeat the PBS examination if the clinical course of the disease changes or resistance to conventional treatment develops [13].
Past medical, obstetrical and family history
A detailed past medical and family history is of extreme importance in eliminating some serious causes like hereditary TCP or transfusion-related infections. History of receiving a vaccine or a new medication can be the only clue to the presence of drug-induced thrombocytopenia (DITP) or heparin-induced thrombocytopenia (HIT). History of menorrhagia, easy bruising or bleeding such as epistaxis or gingival bleeds, either spontaneously or following minor traumas can point to a chronic disease antedating current pregnancy such as ITP. Obstetric and neonatal outcome of previous pregnancies is extremely valuable in providing information about the etiology of the underlying cause of TCP. A relevant example would be maternal or neonatal history of bleeding diathesis which could point to ITP or recurrent mid-pregnancy fetal deaths which is common with antiphospholipid antibody (APLA) syndrome. Family history of bleeding tendencies can also be of assistance in exploring and investigating such disorders. Inquiry about life-style can unveil increased risks for human immunodeficiency virus (HIV) and hepatitis B virus (HBV).
Physical examination
TCP can be an isolated finding in cases such as ITP or GT, but might also be one feature of a precarious underlying disease and here physical examination is concerned with the collection of all the present signs that might be of aid in the diagnosis. A meticulous physical examination and blood pressure assessment will guide to the exclusion of more serious disorders as preeclampsia/HELLP syndrome. Patients with counts of > 50 × 109/L are often asymptomatic while those with lower counts are mostly sick and usually exhibit the manifestations of a systemic disease [15]. Finding signs of bleeding can sometimes give approximate information about the duration and severity of TCP. Webert et al described three forms of hemorrhagic complications [16]. Minor bleeds as purpuric rash are commonly seen in dependent parts of the lower limbs. Moderate bleeds as epistaxis, bleeding following minor traumas, while, gastrointestinal bleeding, hematuria and rarely intracranial hemorrhage are considered severe forms of bleeding. Submucous mouth blisters are specific to TCPs and are commonly seen in low counts [3]. Furthermore, the identification of splenomegaly can point to sequestration etiology of TCP and may thus speak against ITP. TCP can also be the initial manifestation of a serious illness, while the remaining features appear gradually afterwards, hence the importance of repeating the physical examination, at intervals determined by the clinical picture, for detection of any changes in the clinical course of the disease.
Contents of the workup
As in non-pregnant patients, platelet count level is the principle determinant of the contents of workup, the frequency of follow-up visits, and the need to initiate or modify treatment. Detailed history and physical examination with a follow-up of the clinical course of the disease together with cord blood and postpartum platelet testing are thought by many to be sufficient when platelet count is > 100 × 109/L [17] or > 115× 109/L [5]. More detailed investigations are needed when platelet counts range between 75 and 115 × 109/L to include CBCD, PBS examination, liver function tests, HIV, HCV and other clinically oriented tests in the presence of normal history and physical examination. Severe TCP (< 70 × 109/L), however, warrants different and more detailed investigations which should include PBS exam and other clinically oriented laboratory studies, yet short of bone marrow or antiplatelet antibodies testing, unless clinically indicated [8, 9]. The only indication for bone marrow testing, as in non-pregnant status, is the lack of response to conventional treatment or the presence of abnormal findings on PBS or physical examination [8, 9]. In this group, and in spite of substantial overlap in the clinical manifestations, it is expected to diagnose a sizable proportion of the serious disorders as reported by Boehlen et al who could reach diagnosis in 53% of cases with severe TCP < 75 × 109/L compared to only 3% in cases with mild TCP [5]. Some consider full and detailed investigations only when the patient is discovered in early pregnancy, with a platelet count level < 75 × 109/L, development of complications or in the postpartum period for cases who did not show normalization of their platelet counts [2].
Treatment guidelines
Treatment is reserved for cases known or suspected to have ITP either with a strong history or clinical grounds suggestive of ITP. Though TCP by itself can rarely cause bleeding, yet, it can aggravate bleedings secondary to surgery or trauma when counts are < 50 × 109/L [3]. Serious spontaneous bleeding is usually limited to cases with TCP < 10 × 109/L, yet is rare even in known cases of severe ITP owing to a compensatory action exerted by the physiologic increase of most coagulation factors, thus rendering pregnancy as hypercoagulative status intended to counterbalance the usual blood loss anticipated at delivery [14].
Furthermore, GT should not require any treatment. Nevertheless, in cases suspected to have GT and needed treatment, not enough data exist on treatment modality of any proven efficacy and the few cases who received either steroids or intravenous immunoglobulins (IVIG) showed no response [18, 19]. These cases should be treated as being ITP [13]. The aim of treatment during pregnancy is to maintain a safe maternal platelet count necessary for hemostasis rather than inducing prolonged remission [20]. Women with counts of > 70 × 109/L do not need treatment. Cases with lower counts are managed as in non-pregnant individuals. With levels above 50 × 109/L remote from term, no treatment is started but close observation is due. In cases with counts 20 - 50× 109/L, no treatment is indicated unless complicated with bleeding or approaching delivery. Treatment is also indicated if the count is < 10 - 20 × 109/L in first trimester or < 20 - 30 × 109/L in other trimesters, even in asymptomatic patients [8, 9]. Here treatment is intended to prevent spontaneous maternal bleeding, though no effect is anticipated on fetal/neonatal platelet counts. Women known to have ITP before pregnancy might need less treatment than cases with new-onset ITP [16]. In general, 30-50% of ITP cases might need treatment during pregnancy [16].
Distinction between GT and ITP
No specific diagnostic test exists for either GT or ITP and diagnosis in both remains largely that of exclusion [21]. The diagnosis of primary ITP, however, entails the exclusion of other causes that might induce TCP of immune background (secondary ITP) such as SLE, APLA, HIT, DITP and viral infections [4]. In women with mild incidentally discovered asymptomatic TCP who do not need treatment, the distinction of GT from ITP may not be important as both carry favorable maternal and neonatal outcomes [17]. With lower counts, it might be reasonable to attempt distinguishing between these two close entities for several reasons. Mainly because ITP is linked to increased risks of severe neonatal TCP in up to 9-15% of all cases, and can be associated with maternal and neonatal bleeding. Another reason is to minimize the load of unnecessary investigations or interventions and finally due to its extreme value for antenatal management of subsequent pregnancies. Distinction is based on clinical and laboratory features that are inaccurate most of the times due to close similarity and considerable overlap in the presentation of both disorders (Table 1 [3, 6, 8-11, 13, 16, 18, 19, 22-27]). It is noteworthy that some parameters related to GT such as the time of onset in pregnancy and platelet count levels are imprecise with many exceptions, while other parameters such as neonatal TCP, of any severity, in association with maternal GT are not clear. Furthermore, history of TCP antedating pregnancy is of little help in 30% of newly diagnosed ITP. Likewise, a history of TCP during previous pregnancy can be present in both GT and ITP and cannot be used to discriminate between them. The tendency of progressive decline in counts towards term is seen among GT patients if diagnosed early during mid-pregnancy and this is also seen in at least 50% of ITP patients rendering this feature of limited value [22]. However, a past obstetric history of maternal or neonatal TCP associated with bleeding is a strong indicator in favor of ITP. The postpartum course and long-term follow-up seem to be the most constant forms of assessment, yielding unequivocal distinction [11]. Another interesting observation is that GT tends to appear more frequently in twin pregnancies [11]. Nevertheless, for practical reasons, in severe new-onset TCP, there is no point in making the differential as all cases who need intervention should be treated the same as if being ITP [13, 28].
 Click to view | Table 1. Differential Diagnosis of GT and ITP in Pregnancies With New-Onset Thrombocytopenia |
Antenatal surveillance
Asymptomatic cases with platelet counts of ≥ 70 × 109/L can be followed closely at intervals of 4 weeks for any changes in the clinical course (detection of bleeding or hypertension), while lower platelet counts might be repeated every 2 weeks with advancement of pregnancy [20]. Furthermore, the close follow-up allows the detection of new features related to different serious illnesses as HELLP syndrome [29]. Lower counts mandate visits with closer intervals. Beyond 34 weeks, visits and platelet count estimation are scheduled on weekly bases [20, 28].
Proximity to delivery
Remote from delivery, minimal risks are expected even in cases known to have ITP and counts of 30 - 50× 109/L are considered safe and do not require to be higher. In the last few weeks of pregnancy, initiation or modification of treatment should be done as of 36 weeks gestation to ensure a platelet count in the 50 - 70 × 109/L range as labor and delivery might ensue suddenly. If cesarean delivery or regional anesthesia is entertained, higher counts of 80 - 100 × 109/L are targeted [8].
Delivery considerations
Route of delivery should be determined according to standard obstetric considerations where most recent reports did not find increased neonatal bleeding risks associated with normal vaginal delivery [8, 9]. Invasive procedures to acquire fetal platelet counts by cordocentesis or fetal scalp blood sampling are of no benefit, not recommended and are currently abandoned by most obstetricians [8]. Attention should be paid for preparing the parturient for delivery by ensuring platelet count levels of ≥ 50 × 109/L to meet normal vaginal delivery requirement [8, 9] and a level of ≥ 80 × 109/L in cases anticipated to deliver by the cesarean route [8]. If the current count does not meet these standards, treatment should be initiated as soon as possible if time allows. For those already receiving treatment, this should be augmented to meet the platelet count requirements and is usually started at 36 - 37 weeks. When patients present in emergent conditions such as preterm premature rupture of membranes (PPROM) or preterm birth where time does not allow medical treatment to elevate the count, platelet transfusion alone or combined with IVIG or high-dose parenteral corticosteroids should be initiated urgently [13]. The transfusion of 6 - 10 units of random donor platelets, in spite of a short-lived effect [30], can usually yield an increase of 10 × 109/L per unit [31]. Other modalities of treatment will not result in similar immediate response. The response to corticosteroids is usually elicited within 4 - 14 days and reaches a peak within 1 - 4 weeks, while IVIG starts to exert its effect within 1 - 3 days and peaks within 2 - 7 days [4]. It is recommended also to avoid the use of fetal scalp electrodes or delivery with vacuum application in ITP patients. Likewise, the use of salicylates and non-steroidal analgesics is discouraged.
Obstetric outcome
A common finding of most studies and case series of new-onset or GT has been the lack of long-term follow-up surveillance to ascertain that TCP was related and limited to pregnancy and that the patient did not develop other disorders that can cause TCP such as SLE or ITP. This led many to be skeptical about the very existence of any “GT” if platelet counts < 75 × 109/L, when associated with maternal bleeding or was accompanied with neonatal TCP or hemorrhage [13, 22].
Very few studies explored the course of pregnancy and obstetric complications among patients with TCP. Parnas et al studied 199 pregnancies with moderate to severe TCP and found that when the underlying etiology was limited to GT or ITP, the outcome was favorable while adverse pregnancy outcome was linked to other etiologies such as preeclampsia/HELLP syndrome [32]. This finding was also confirmed by Ozdemir et al [33]. Postpartum hemorrhage, any adverse maternal outcome, intrapartum fetal distress, intrauterine fetal death (IUFD), cesarean delivery, low Apgar scores, NICU admissions, neonatal intracranial hemorrhage or neonatal death were not increased in cases of severe TCP when compared to moderate disease [10]. Kasai et al compared two groups of TCP patients, those with GT and others with ITP, and found that maternal outcome was favorable in both groups in spite of the need for treatment among ITP patients [11]. ITP patients on steroids might be at increased risk of developing gestational diabetes and hypertensive disorders. The common finding of most studies was that patients with mild TCP due to any etiology have favorable pregnancy outcome. Even with severe forms of TCP, when the etiology is limited to GT or ITP, these patients are at no increased risk of developing complications when compared to regular obstetric population and hence no need for specific interventions other than close observation.
Fetal/neonatal considerations
The management of TCP, when ITP is suspected, is essentially the same as in non-pregnant patients except for few considerations. The choice of using corticosteroids during first trimester as first-line treatment of ITP is hindered by a possible yet minimal teratogenicity linked to increased risks of clefting [34]. Its use during the rest of pregnancy warrants caution due to definite, dose-dependent, increased risk of gestational diabetes, weight gain, hypertension, intrauterine growth restriction (IUGR), bone loss, preterm labor, preterm birth [23] and possibly placental abruption. Furthermore, the use of several medications like vinca alkaloids, danazol and cyclophosphamide used in resistant cases is contraindicated during first trimester for fear of potential teratogenicity [35]. The use of these agents during the rest of pregnancy is linked to IUGR, impaired hematopoiesis and developmental delay [36]. Mild TCP whether due to GT or ITP is always associated with favorable neonatal outcome, yet there are two views concerning new-born infant surveillance. Shehata et al are proponents of non-intervention as the need for detailed newborn investigations other than cord platelet count is not necessary especially in GT cases [24]. Pourrat et al, on the other hand, recommend close monitoring of platelet counts for every infant born to a mother with TCP including cases with GT [25]. Nevertheless, it is advised for new-born infants with TCP of any degree to be investigated for other etiologies such as infections and alloimmune TCP [37]. ITP is associated with neonatal TCP due to passage of maternal antigens into the fetal circulation. This might not manifest at birth and several days may pass before the appearance of neonatal TCP, with the nadir occurring within the first 2 weeks [16]. This can reach < 50 × 109/L in 10-20% of cases and < 20 × 109/L in 5% of newborns in mothers with severe ITP [37]. Bleeding can affect 25-50% of newborns with severe TCP due to ITP, but severe bleeding especially intracranial hemorrhage affects only 1-2% [2, 3]. Some studies pointed to less neonatal TCP in association with new-onset ITP when compared to pregestational ITP [26]. Newborns who developed severe neonatal TCP were invariably sick and had other associated problems such as IUGR or prematurity [16]. Furthermore, Fujimura in his nationwide study from Japan reported that neonatal TCP was more frequent among splenectomized mothers [23]. No reliable risk factor can identify neonates at risk for this complication including maternal platelet counts, route of delivery or level of maternal antiplatelet antibodies [37]. The only reliable risk factor, however, remains the delivery of severely thrombocytopenic newborn infant in previous pregnancy [20].
The principal debatable and major concern remains whether GT can cause neonatal TCP. Most studies refuted this association and presented evidence that rates were similar to those found in non-thrombocytopenic pregnancies of 2% [2, 10]. Others, however, reported that neonatal TCP of various degrees including counts < 50 × 109/L can also be associated with GT [25, 27]. Similarly, a study by Gasparovic et al pointed to possible association between the severity of GT and the occurrence of neonatal TCP [10]. Jensen et al also confirmed this association [38]. This could be in accordance with the assumption that GT might be a mild and transient form of ITP [13]. Neonatal TCP among infants born to patients with GT, when present, was not reported to be associated with neonatal bleeding and was consistently transient resolving in couple of weeks without the need for any treatment [25, 27].
Postpartum surveillance
Physiologic changes in platelet counts induced by pregnancy are expected to revert to normal 3 - 5 days postpartum after showing an initial drop in the first 24 - 48 h [39, 40]. Similarly counts are reported to normalize spontaneously and rapidly in GT while in ITP it might recover but typically stays in the TCP level for non-pregnants with possible relapses after a while [11]. Postpartum monitoring of platelet count can be repeated every 1 - 3 months until normalization [20, 28]. This period is of utmost importance in making the indisputable diagnosis of GT. There is however, no agreement on the duration of postpartum observation period needed to monitor these patients. According to ACOG most cases will show normal counts within days and almost all will do so within 2 - 12 weeks postpartum [3]. It might be better to continue monitoring cases with new-onset TCP for longer periods after delivery even after normalization of their platelet count as some patients, after having rapid spontaneous postpartum recovery, might experience fluctuations and relapses several months later [11, 19]. Monitoring should include platelet counts, CBCD, clinical or laboratory evidence of autoimmune disorders and probably platelet life-span testing [41]. Furthermore, some patients with asymptomatic mild TCP needed more than 12 weeks to recover spontaneously before being finally diagnosed as GT [27]. Cases with known ITP should not be counseled against future pregnancy and breast feeding should be allowed [20], though, with caution due to few anecdotal reports of neonatal TCP associated with breastfeeding [42].
Recommendations
With the current available evidence, we recommend the following upon managing new-onset TCP during pregnancy. 1) New-onset TCP in asymptomatic and healthy pregnant women will eventually belong to either GT or ITP. 2) Management of new-onset TCP should preferably be carried out within multispecialty team approach. 3) Mild new-onset TCP caused by GT or ITP is associated with favorable outcome; thus there is no need to discern between them. Here limited workup is needed after confirming the diagnosis by CBCD, PBS, detailed history and physical exam. Full workup is better reserved for cases with counts of < 75 × 109/L. 4) All maternal or obstetric complications are related to causes other than GT or ITP, thus every effort should be oriented towards excluding these serious illnesses. 5) During pregnancy, in severe new-onset TCP, and after exclusion of different serious etiologies, the distinction between GT and ITP might not be accurate and when treatment is indicated, these cases should be considered as ITP. If no response to the conventional treatment was elicited, platelet transfusion should be used especially in emergent situations as with very low platelet counts or emergent delivery. 6) TCP by itself is not associated with coagulation disturbances and does not cause postpartum hemorrhage, placental abruption or DIC. These conditions when present, however, can exhaust the coagulation factors and if compounded by TCP can worsen the hemostatic system integrity. We thus advise that when these situations develop in patients with TCP, initiation of treatment measures with platelets and other blood constituents should take place early enough before the development of major hemorrhagic catastrophes. 7) In severe new-onset TCP at term, making the distinction between GT and ITP is of no instant value and management should be directed towards providing the appropriate hemostasis needed for safe imminent delivery. 8) In any case with TCP, postpartum surveillance of platelet and other blood components (CBCD) should better be done at spaced intervals extended up to 1 year. 9) Finally, we recommend not relying on cord-blood platelet count alone before excluding neonatal TCP, but rather to continue monitoring for at least 2 weeks even in mild asymptomatic maternal TCP. Alloimmune and other etiologies must be excluded in every case of severe neonatal TCP.
Conclusion
We encountered a case of new-onset TCP at term with platelet count of < 70 × 109/L, the bench-mark usually used to do the distinction between GT and ITP. Irrespective of the exact underlying etiology, management was directed towards excluding serious systemic causes of TCP and to secure the hemostatic functions necessary to do an expedited cesarean delivery. We herein also report the long-term outcome of this case, where 4 years later, she was found to be healthy with a normal platelet count which allowed us to diagnose this case as GT in her two consecutive pregnancies. With the absence of a reliable laboratory test, observation of remote postpartum course, where diversion becomes more obvious between GT and ITP, can be utilized as a reliable clinical method to make the diagnosis with certainty.
Acknowledgments
We are grateful to the assistance and the valuable efforts provided by Ms. Loubna Sinno MPH (the research coordinator at Makassed General Hospital) in performing the Medline search and preparing the final manuscript of this review.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Abbreviations
APLA: antiphospholipid antibody; CBCD: complete blood count and differential; DITP: drug-induced thrombocytopenia; GT: gestational thrombocytopenia; HBV: hepatitis B virus; HCV: hepatitis C virus; HIT: heparin-induced thrombocytopenia; HIV: human immunodeficiency virus; ITP: immune thrombocytopenia; IUFD: intrauterine fetal death; IUGR: intrauterine growth restriction; IVIG: intravenous immunoglobulins; PBS: peripheral blood smear; PPROM: preterm premature rupture of membranes; SLE: systemic lupus erythematosus
| References | ▴Top |
- Valera MC, Parant O, Vayssiere C, Arnal JF, Payrastre B. Physiologic and pathologic changes of platelets in pregnancy. Platelets. 2010;21(8):587-595.
doi pubmed - Boehlen F. Thrombocytopenia during pregnancy. Importance, diagnosis and management. Hamostaseologie. 2006;26(1):72-74; quiz 75-78.
pubmed - Practice Bulletin No. 166: Thrombocytopenia in Pregnancy. Obstet Gynecol. 2016;128(3):e43-53.
doi pubmed - Rodeghiero F, Stasi R, Gernsheimer T, Michel M, Provan D, Arnold DM, Bussel JB, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;113(11):2386-2393.
doi pubmed - Boehlen F, Hohlfeld P, Extermann P, Perneger TV, de Moerloose P. Platelet count at term pregnancy: a reappraisal of the threshold. Obstet Gynecol. 2000;95(1):29-33.
doi - Verdy E, Bessous V, Dreyfus M, Kaplan C, Tchernia G, Uzan S. Longitudinal analysis of platelet count and volume in normal pregnancy. Thromb Haemost. 1997;77(4):806-807.
pubmed - Sainio S, Kekomaki R, Riikonen S, Teramo K. Maternal thrombocytopenia at term: a population-based study. Acta Obstet Gynecol Scand. 2000;79(9):744-749.
doi pubmed - Guidelines for the investigation and management of idiopathic thrombocytopenic purpura in adults, children and in pregnancy. Br J Haematol. 2003;120(4):574-596.
doi - George JN, Woolf SH, Raskob GE, Wasser JS, Aledort LM, Ballem PJ, Blanchette VS, et al. Idiopathic thrombocytopenic purpura: a practice guideline developed by explicit methods for the American Society of Hematology. Blood. 1996;88(1):3-40.
pubmed - Elvedi Gasparovic VE, Beljan P, Skrablin S, Gveric Ahmetasevic SN. Effect of severe gestational thrombocytopenia to perinatal outcome. Signa Vitae. 2014;9(Suppl. 1):49-53.
- Kasai J, Aoki S, Kamiya N, Hasegawa Y, Kurasawa K, Takahashi T, Hirahara F. Clinical features of gestational thrombocytopenia difficult to differentiate from immune thrombocytopenia diagnosed during pregnancy. J Obstet Gynaecol Res. 2015;41(1):44-49.
doi pubmed - McCrae KR. Thrombocytopenia in pregnancy: differential diagnosis, pathogenesis, and management. Blood Rev. 2003;17(1):7-14.
doi - George JN. Platelets. Lancet. 2000;355(9214):1531-1539.
doi - Kam PC, Thompson SA, Liew AC. Thrombocytopenia in the parturient. Anaesthesia. 2004;59(3):255-264.
doi pubmed - Gauer RL, Braun MM. Thrombocytopenia. Am Fam Physician. 2012;85(6):612-622.
pubmed - Webert KE, Mittal R, Sigouin C, Heddle NM, Kelton JG. A retrospective 11-year analysis of obstetric patients with idiopathic thrombocytopenic purpura. Blood. 2003;102(13):4306-4311.
doi pubmed - Burrows RF, Kelton JG. Incidentally detected thrombocytopenia in healthy mothers and their infants. N Engl J Med. 1988;319(3):142-145.
doi pubmed - Win N, Rowley M, Pollard C, Beard J, Hambley H, Booker M. Severe gestational (incidental) thrombocytopenia: to treat or not to treat. Hematology. 2005;10(1):69-72.
doi pubmed - Anteby E, Shalev O. Clinical relevance of gestational thrombocytopenia of < 100,000/microliters. Am J Hematol. 1994;47(2):118-122.
doi pubmed - Gernsheimer TB. Thrombocytopenia in pregnancy: is this immune thrombocytopenia or...? Hematology Am Soc Hematol Educ Program. 2012;2012:198-202.
- George JN, el-Harake MA, Raskob GE. Chronic idiopathic thrombocytopenic purpura. N Engl J Med. 1994;331(18):1207-1211.
doi pubmed - Kelton JG. Idiopathic thrombocytopenic purpura complicating pregnancy. Blood Rev. 2002;16(1):43-46.
doi pubmed - Fujimura K, Harada Y, Fujimoto T, Kuramoto A, Ikeda Y, Akatsuka J, Dan K, et al. Nationwide study of idiopathic thrombocytopenic purpura in pregnant women and the clinical influence on neonates. Int J Hematol. 2002;75(4):426-433.
doi pubmed - Shehata N, Burrows R, Kelton JG. Gestational thrombocytopenia. Clin Obstet Gynecol. 1999;42(2):327-334.
doi pubmed - Pourrat O, Valere G, Pierre F. Is incidental gestational thrombocytopaenia really always safe for the neonate? J Obstet Gynaecol. 2014;34(6):499-500.
doi pubmed - Samuels P, Bussel JB, Braitman LE, Tomaski A, Druzin ML, Mennuti MT, Cines DB. Estimation of the risk of thrombocytopenia in the offspring of pregnant women with presumed immune thrombocytopenic purpura. N Engl J Med. 1990;323(4):229-235.
doi pubmed - Ruggeri M, Schiavotto C, Castaman G, Tosetto A, Rodeghiero F. Gestational thrombocytopenia: a prospective study. Haematologica. 1997;82(3):341-342.
pubmed - Stasi R. How to approach thrombocytopenia. Hematology Am Soc Hematol Educ Program. 2012;2012:191-197.
- Minakami H, Kohmura Y, Izumi A, Watanabe T, Matsubara S, Sato I. Relation between gestational thrombocytopenia and the syndrome of hemolysis, elevated liver enzymes, and low platelet count (HELLP syndrome). Gynecol Obstet Invest. 1998;46(1):41-45.
doi pubmed - Neunert C, Lim W, Crowther M, Cohen A, Solberg L, Jr., Crowther MA. The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood. 2011;117(16):4190-4207.
doi pubmed - Levy JA, Murphy LD. Thrombocytopenia in pregnancy. J Am Board Fam Pract. 2002;15(4):290-297.
pubmed - Parnas M, Sheiner E, Shoham-Vardi I, Burstein E, Yermiahu T, Levi I, Holcberg G, et al. Moderate to severe thrombocytopenia during pregnancy. Eur J Obstet Gynecol Reprod Biol. 2006;128(1-2):163-168.
doi pubmed - Ozdemir S, Gorkemli H, Acar A, Celik C, Kayikcioglu E. A Comparison of Maternal and Fetal Outcomes of Pregnancies Complicated by Moderate to Severe Thrombocytopenia Caused by Gestational Thrombocytopenia Preeclampsia/HELLP Syndrome and Immune Thrombocytopenic Purpura. Gynecology Obstetrics & Reproductive Medicine. 2016;14(3):154-158.
- Pradat P, Robert-Gnansia E, Di Tanna GL, Rosano A, Lisi A, Mastroiacovo P. First trimester exposure to corticosteroids and oral clefts. Birth Defects Res A Clin Mol Teratol. 2003;67(12):968-970.
doi pubmed - Gernsheimer T, McCrae KR. Immune thrombocytopenic purpura in pregnancy. Curr Opin Hematol. 2007;14(5):574-580.
doi pubmed - Temprano KK, Bandlamudi R, Moore TL. Antirheumatic drugs in pregnancy and lactation. Semin Arthritis Rheum. 2005;35(2):112-121.
doi pubmed - Burrows RF, Kelton JG. Fetal thrombocytopenia and its relation to maternal thrombocytopenia. N Engl J Med. 1993;329(20):1463-1466.
doi pubmed - Jensen JD, Wiedmeier SE, Henry E, Silver RM, Christensen RD. Linking maternal platelet counts with neonatal platelet counts and outcomes using the data repositories of a multihospital health care system. Am J Perinatol. 2011;28(8):597-604.
doi pubmed - Ygge J. Changes in blood coagulation and fibrinolysis during the puerperium. Am J Obstet Gynecol. 1969;104(1):2-12.
pubmed - Bonnar J, McNicol GP, Douglas AS. Coagulation and fibrinolytic mechanisms during and after normal childbirth. Br Med J. 1970;2(5703):200-203.
doi pubmed - Ajzenberg N, Dreyfus M, Kaplan C, Yvart J, Weill B, Tchernia G. Pregnancy-associated thrombocytopenia revisited: assessment and follow-up of 50 cases. Blood. 1998;92(12):4573-4580.
pubmed - Hauschner H, Rosenberg N, Seligsohn U, Mendelsohn R, Simmonds A, Shiff Y, Schachter Y, et al. Persistent neonatal thrombocytopenia can be caused by IgA antiplatelet antibodies in breast milk of immune thrombocytopenic mothers. Blood. 2015;126(5):661-664.
doi pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


