| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website http://www.thejh.org |
Case Report
Volume 4, Number 4, December 2015, pages 231-234
Rare Respiratory and Neurologic Adverse Reactions to Azacitidine in the Treatment of Myelodysplastic Syndrome of Patients Treated at the Ottawa Hospital
Azin Ahraria, Mitchell Sabloffb, e, Chris Bredesonb, Smita Pakhalec, Carolina Souzad, Jocelyn Zwickerc, Dawn Sheppardb
aUniversity of Ottawa, Ottawa, Ontario, Canada
bDivision of Hematology, Department of Medicine, University of Ottawa, and Ottawa Hospital Research Institute, Ottawa, Canada
cDepartment of Medicine, The Ottawa Hospital Research Institute, and University of Ottawa, Ottawa, Canada
dDepartment of Medical Imaging, The Ottawa Hospital, and University of Ottawa, Ottawa, Canada
eCorresponding Author: Mitchell Sabloff, Division of Hematology, Department of Medicine, University of Ottawa, and Ottawa Hospital Research Institute, Ottawa, Canada
Manuscript accepted for publication October 13, 2015
Short title: Adverse Reactions to Azacitidine
doi: http://dx.doi.org/10.14740/jh227w
| Abstract | ▴Top |
Myelodysplastic syndromes (MDSs) are a heterogeneous group of disorders negatively affecting the bone marrow and resulting in multiple cytopenias. MDS ultimately progresses to acute myeloid leukemia (AML). Azacitidine was the first hypomethylating agent approved in Canada for patients with high risk MDS and patients with AML with 20-30% blasts. Azacitidine has a relatively low toxicity profile and is well tolerated by most age groups. We conducted a retrospective chart review of patients who received azacitidine at our institution, over a 3.5-year period. To our knowledge, this report presents the first case of peripheral neuropathy and an additional case of pneumonitis as rare adverse effects of azacitidine.
Keywords: Myelodysplastic syndromes; Hypomethylating agents; Pneumonitis; Peripheral neuropathy; Azacitidine
| Introduction | ▴Top |
Myelodysplastic syndromes (MDSs) are a heterogeneous group of disorders characterized by cytopenias of one or more peripheral blood cell types, abnormal hematopoiesis and often acquired genetic abnormalities in the bone marrow. This is followed by a progressive impairment in growth and differentiation and eventually evolution to acute myeloid leukemia (AML), which occurs at a variable rate, depending on risk stratification [1-3].
Hypermethylation has been recognized as one of the epigenetic abnormalities in patients with myelodysplasia, as well as other cancers [4-6]. A new class of drugs known as hypomethylating agents has been recently developed demonstrating positive effects on cytopenias, decreased transfusion requirements and a survival advantage compared to supportive care [7, 8]. One of these agents, azacitidine, was approved by Health Canada in April 2009, for treatment of adult patients who, based on the International Prognostic Scoring System (IPSS), have intermediate-2 and high risk MDS or AML with 20-30% blasts and multi-lineage dysplasia, and who are not eligible for stem cell transplantation [9].
Azacitidine has a relatively low toxicity profile and is well tolerated among elderly patients. The most commonly reported adverse effects of this medication are myelosuppression, gastrointestinal symptoms (nausea, vomiting, diarrhea, constipation and anorexia) and injection site reactions such as erythema [10]. Rare serious or fatal complications from the drug have not been well described [11].
We retrospectively reviewed our use of azacitidine from September 2009 to December 2012, approved by the Ottawa Health Science Network Research Ethics Boar, and identified two cases of rare pulmonary and neurologic adverse events suspected to be secondary to azacitidine.
| Case Reports | ▴Top |
Case 1
A 73-year-old male was seen in leukemia clinic for an abnormal complete blood count (CBC). He was anemic (hemoglobin of 89 g/L) and thrombocytopenic (platelet count of 63 × 109/L). His white blood count was 13.6 × 109/L with a neutrophil count of 5.92 × 109/L, and a blast count of 1.78 × 109/L, in the peripheral blood. Following a bone marrow aspirate and biopsy, the patient was diagnosed with a MDS, specifically, refractory anemia with excess blasts-2. Cytogenetic testing showed a normal karyotype. Therefore, the resultant IPSS score of 2.0 placed him in the intermediate-2 risk group, which indicated that his median survival was 1.2 years. He began azacitidine, 75 mg/m2/day subcutaneous (SC) for 7 days every 4 weeks. At the start of the third cycle, he started to experience intermittent fevers, chills and night sweats. He was started on levofloxacin. Blood cultures demonstrated Mycobacterium fortuitum, and antibiotics were switched to clarithromycin and ciprofloxacin. Four weeks later, after the fourth cycle of azacitidine, a chest radiograph showed bilateral hilar enlargement and bilateral perihilar ground-glass opacities. Four weeks later, after the fifth cycle of azacitidine, progression of the pulmonary infiltrates was evident on the chest radiograph. Also, the susceptibility testing of the Mycobacterium fortuitum organism demonstrated resistance to clarithromycin. Therefore, trimethoprim/sulfamethoxazole was added. A repeat bone marrow, at that time, demonstrated a reduction in the leukemic blasts from 15% to less than 5%. Four weeks later, after the sixth cycle, a repeat chest radiograph was stable, and he was continued on his antibiotics. However, because of persistent and worsening symptoms, a computed tomography (CT) of the chest was performed after the seventh cycle. Results showed bilateral ground-glass opacities with reticulation in the mid- and upper lung zones and patchy peripheral airspace consolidation in a pattern suggestive of drug toxicity, possibly alveolar proteinosis (Fig. 1). An infectious etiology was deemed unlikely based on these findings. He was subsequently seen by the respirology service, and a bronchoscopy was performed after the eighth cycle. The bronchoalveolar lavage culture did not grow any fungal, viral or bacterial organisms strongly suggesting that an infectious cause was not responsible for progression of lung disease. Follow-up blood cultures were also all negative for Mycobacterium fortuitum. He was admitted to hospital because of worsening hypoxia, requiring 4 L/min of oxygen to maintain an oxygen saturation of 92%. A bronchial wash showed a significant amount of eosinophils and no pathogens, which suggested an eosinophilic pneumonia or inflammatory response. Antibiotics were discontinued, and he was started on high dose prednisone. Azacitidine was also discontinued, as it was suspected to have contributed to patient’s pulmonary compromise. While tapering the dose of prednisone over a 4-week period he became more short of breath, and his oxygen saturation dropped to 85% on 3 L/min of oxygen. A repeat chest radiograph was reported as stable. A CT of the chest, 2 months after stopping azacitidine, demonstrated a similar pattern with worsening airspace consolidation and reticulation. No changes were made to the differential diagnosis which again included drug reaction and alveolar proteinosis (Fig. 2). The temporal evolution was felt to be atypical for infection and pulmonary hemorrhage. While trying to further characterize his illness, the patient deteriorated further and was admitted to hospital, 3 months after stopping azacitidine. During his stay at the hospital, his condition deteriorated, and the goals of care became palliative. The patient passed away in hospital, 1 year after starting azacitidine and 5 months after discontinuing it.
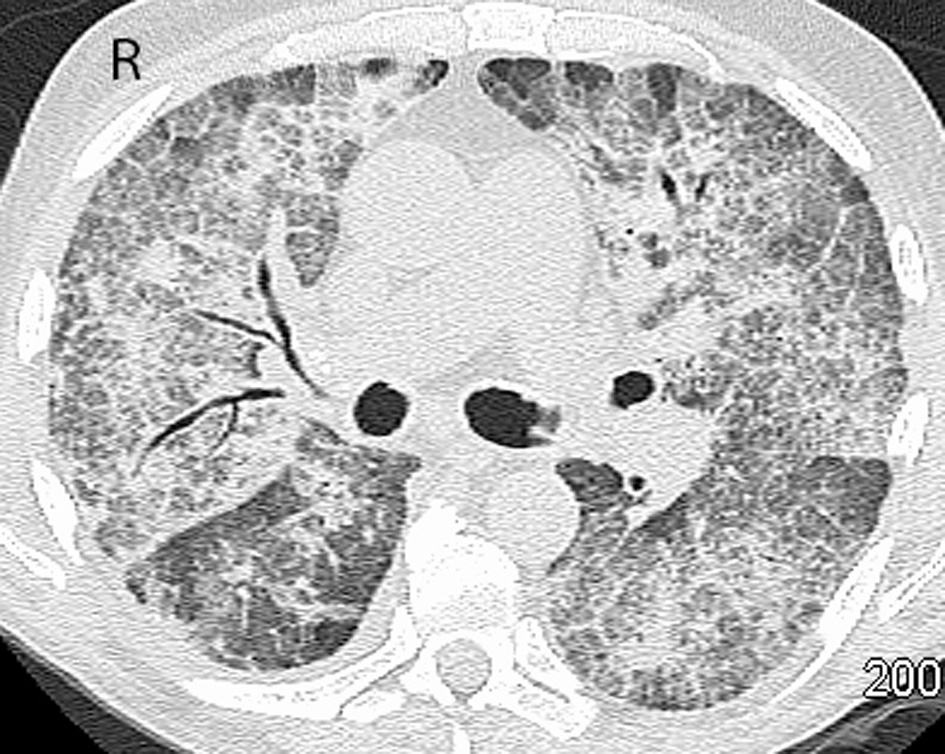 Click for large image | Figure 1. CT of the chest after receiving seven cycles of azacitidine. Initial CT scan of the chest demonstrates bilateral ground-glass opacities with reticulation and patchy peripheral airspace consolidation. Findings had a predominant mid- and upper lobe distribution. |
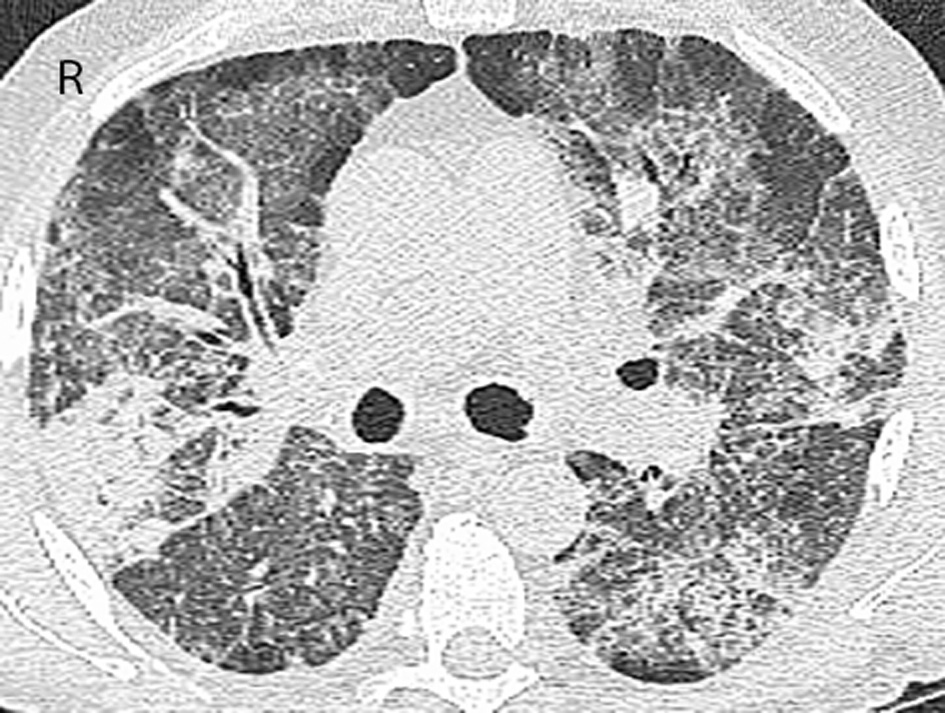 Click for large image | Figure 2. CT of the chest 2 months after discontinuing azacitidine. CT scan of the chest 3 months after previous chest CT demonstrates significant progression of ground-glass opacities and reticulation with new and more widespread areas of consolidation. Findings were again more severe in the mid- and upper lobes. |
Case 2
A 60-year-old male was seen in the lymphoma clinic for an asymptomatic pancytopenia and a previous bone marrow demonstrating a non-Hodgkin’s lymphoma with loss of chromosome 7. He was anemic (hemoglobin of 77 g/L) with a white blood cell count of 3.2 × 109/L and a platelet count of 86 × 109/L. A repeat bone marrow biopsy showed a refractory cytopenia with multi-lineage dysplasia with < 5% blast and a small monoclonal B cell infiltration. The patient was initially treated with 4-week cycles of single agent rituximab in an attempt to reduce the lymphoma tumor burden and improve peripheral cell lines. However, he continued to require blood transfusions, and 2 months after receiving the rituximab treatment, he remained pancytopenic. A repeat bone marrow aspirate and biopsy was performed demonstrating the refractory anemia with multi-lineage dysplasia, as reported previously. He continued to require regular blood transfusions. His IPSS was intermediate-2. A stem cell transplant was considered; however, no appropriate donor was identified. Since no bone marrow donor was available, treatment with azacitidine was initiated 5 months after the initial diagnosis. After three cycles of azacitidine, he required fewer transfusions (from 6 units/month prior to azacitidine to 2 units for over 2 months), and his platelet count normalized. However, he started to have numbness and pain involving bilateral lower legs between cycles 2 and 3 of azacitidine treatment. Atorvastatin was stopped in an attempt to improve this pain, but the neuropathy progressed. Deferasirox, which had been initiated due to iron overload, secondary to blood transfusions, was also discontinued. The frequency of azacitidine was reduced from every 4 to every 5 weeks. His transfusion dependence increased again to 6 units/month. Electromyogram (EMG) studies showed low amplitude bilateral motor responses with slowed conduction velocity in lower and upper extremities consistent with a motor sensory polyneuropathy. The patient subsequently became febrile and was not able to control the neuropathic pain with his analgesic regimen (acetylsalicylic acid, codeine, baclofen, pregabalin and hydromorphone). Therefore, he was subsequently admitted to the hospital where a regimen including gabapentin and nortriptyline improved the patient’s symptoms. Azacitidine was held at this point after completion of four cycles. A repeat EMG, 3 months after the first one, indicated generalized peripheral neuropathy. Compared to the previous study, there had been significant worsening. In consultation with neurology, they noted that there were elements of both axon loss and demyelination similar to a chronic inflammatory demyelinating polyneuropathy (CIDP) syndrome. Physical examination revealed normal cranial nerves. There was atrophy of upper extremity muscles and diminished reflexes in both upper and lower limbs. Pinprick and light touch sensation was reduced in the feet. The patient also experienced worsening weakness and neuropathic pain in the legs as well as bilateral foot drop. Family history for neuropathy was negative, and other causes of neuropathy such as hepatitis C, cryoglobulins, vitamin B12 deficiency and thyroid abnormalities were eliminated. After stopping azacitidine, symptoms plateaued. Therefore, drug-induced neuropathy due to azacitidine cytotoxicity was the only likely explanation. Intravenous immunoglobulin (IVIg) was started to treat a possible immune-mediated neuropathy. Shortly after starting the IVIg, 11 months after the diagnosis and 6 months after the start of azacitidine, the patient was admitted to the hospital for neutropenia and a bacterial pneumonia. He passed away in hospital as a result of this infection.
| Discussion | ▴Top |
Herein, we present two cases illustrating potential rare toxicities of azacitidine. The first case illustrates a case of drug-induced lung toxicity. Diagnosis of drug-induced lung disease is based on temporal relation of drug exposure and onset of respiratory symptoms. There are several case reports of pulmonary toxicity due to azacitidine. Symptoms typically developed within 8 weeks of initiation of azacitidine.
All cases of azacitidine-induced pulmonary toxicity have been described as pneumonitis and eosinophilic pneumonia which are characterized by cough, lung infiltrates, and the presence of eosinophils in the alveolar spaces and pulmonary interstitium [12-16]. Chest CT in all cases showed bilateral airspace disease, nodular opacities, bilateral patchy infiltrates, and enlargement of mediastinal and hilar lymph nodes. Bronchoalveolar lavage showed numerous eosinophils and no pathogens. However, this is not necessary for making the diagnosis.
In the case described here, the patient experienced fever, chills and night sweats, 8 weeks after initiation of azacitidine. Chest radiograph findings at the time were consistent with interstitial lung disease. Positive blood cultures for Mycobacterium fortuitum and treatment with antibiotics, delayed the identification of the association between azacitidine and potential lung toxicity. The dry cough and dyspnea worsened over time, and bronchoscopy identified no pathogens. Furthermore, the chest CT showed progressive ground-glass opacities, reticulation, and consolidation predominantly in the peripheral and upper lobes. These findings were similar to the reported cases of lung toxicity caused by azacitidine. No other etiology for pneumonitis was obvious. According to the Naranjo adverse drug reaction probability scale, the probability that this adverse effect was due to azacitidine was “probable” [17]. However, because of the delay in discontinuing azacitidine the damage was extensive in the lung tissue, and discontinuing azacitidine and treatment with prednisone did not reverse the process. In three reported cases of azacitidine-induced lung toxicity, changes were reversible with IV steroid administration and immediate discontinuation of azacitidine. In two cases, azacitidine-induced lung toxicity led to the patient’s demise.
The second case presented another patient with an advanced myelodysplasia who was responding to azacitidine with an improvement in blood counts and reduced need of transfusions. He developed progressive polyneuropathy which was improved with gabapentin and nortriptyline. Since other medications possibly causing symptoms were discontinued and all other possible causes had been eliminated, we were left with azacitidine as the remaining culprit. Reducing and stopping azacitidine only reduced pain; however, the numbness persisted. This patient eventually succumbed to progression of his myelodysplasia, being admitted with febrile neutropenia.
A case of CIDP has been reported in abstract form at the American Society of Hematology annual meeting in 2009, with a similar presentation [18]. A neurologic syndrome has also been previously described when azacitidine was used in high dose for induction chemotherapy in the mid 1970s [19]. CIDP has been reported to be associated with myelodysplasia as a paraneoplastic phenomenon [20, 21]. However, in this case the patient did not present with symptoms of CIDP at diagnosis, rather the neurologic syndrome only became evident after exposure to azacitidine. Therefore, the likelihood that it was associated with the underlying myelodysplasia is low. This is the first reported case of azacitidine-associated CIDP in Canada. In our patient, the temporal relationship between exposures to azacitidine, development of symptoms and lack of other known cause for the symptoms formed the basis of this diagnosis. According to the Naranjo probability scale, the diagnosis was “probable”.
Although the hypomethylating agents have advanced the treatment for MDS in those who previously had few options for treatment, it is important to be aware of some of its potential, but rare, serious complication which, if identified early, may be reversible.
Acknowledgement
Many thanks to Dr. Gary Garber for his intellectual input and revising the manuscript critically.
Competing Interests
The authors declare that they have no competing interests.
Abbreviations
MDS: myelodysplastic syndrome; AML: acute myeloid leukemia; IPSS: International Prognostic Scoring System; CBC: complete blood count; CT: computed tomography; EMG: electromyogram; CIDP: chronic inflammatory demyelinating polyneuropathy
| References | ▴Top |
- Cheson BD, Greenberg PL, Bennett JM, Lowenberg B, Wijermans PW, Nimer SD, Pinto A, et al. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood. 2006;108(2):419-425.
doi pubmed - Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G, Sanz M, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89(6):2079-2088.
pubmed - Greenberg PL, Tuechler H, Schanz J, Sanz G, Garcia-Manero G, Sole F, Bennett JM, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012;120(12):2454-2465.
doi pubmed - Hattori N, Ushijima T. Compendium of aberrant DNA methylation and histone modifications in cancer. Biochem Biophys Res Commun. 2014;455(1-2):3-9.
doi pubmed - Jiang Y, Dunbar A, Gondek LP, Mohan S, Rataul M, O'Keefe C, Sekeres M, et al. Aberrant DNA methylation is a dominant mechanism in MDS progression to AML. Blood. 2009;113(6):1315-1325.
doi pubmed - Ruter B, Wijermans PW, Lubbert M. DNA methylation as a therapeutic target in hematologic disorders: recent results in older patients with myelodysplasia and acute myeloid leukemia. Int J Hematol. 2004;80(2):128-135.
doi pubmed - Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Finelli C, Giagounidis A, Schoch R, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol. 2009;10(3):223-232.
doi - Estey EH. Epigenetics in clinical practice: the examples of azacitidine and decitabine in myelodysplasia and acute myeloid leukemia. Leukemia. 2013;27(9):1803-1812.
doi pubmed - Summary Basis of Decision (SBD) PrVIDAZA. Health Canada . 6-22-2010.
- Vigil CE, Martin-Santos T, Garcia-Manero G. Safety and efficacy of azacitidine in myelodysplastic syndromes. Drug Des Devel Ther. 2010;4:221-229.
doi pubmed - Sullivan M, Hahn K, Kolesar JM. Azacitidine: a novel agent for myelodysplastic syndromes. Am J Health Syst Pharm. 2005;62(15):1567-1573.
doi pubmed - Nair GB, Charles M, Ogden L, Spiegler P. Eosinophilic pneumonia associated with azacitidine in a patient with myelodysplastic syndrome. Respir Care. 2012;57(4):631-633.
doi pubmed - Sekhri A, Palaniswamy C, Kurmayagari K, Kalra A, Selvaraj DR. Interstitial lung disease associated with azacitidine use: a case report. Am J Ther. 2012;19(2):e98-e100.
doi pubmed - Adams CD, Szumita PM, Baroletti SA, Lilly CM. Azacitidine-induced interstitial and alveolar fibrosis in a patient with myelodysplastic syndrome. Pharmacotherapy. 2005;25(5):765-768.
doi pubmed - Hayashi M, Takayasu H, Tada M, Yamazaki Y, Tateno H, Tazawa S, Wakabayashi A, et al. Azacitidine-induced pneumonitis in a patient with myelodysplastic syndrome: first case report in Japan. Intern Med. 2012;51(17):2411-2415.
doi pubmed - Kuroda J, Shimura Y, Mizutani S, Nagoshi H, Kiyota M, Chinen Y, Maegawa S, et al. Azacitidine-associated acute interstitial pneumonitis. Intern Med. 2014;53(11):1165-1169.
doi pubmed - Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, Janecek E, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239-245.
doi pubmed - Harvey PE, Nierodzik ML, Wu J. Guillain-Barre Syndrome Following Azacitidine for Treatment of Myelodysplastic Syndrome. ASH Annual Meeting Abstracts 2009;114:4861.
- Levi JA, Wiernik PH. Combination therapy with 5-azacytidine (NSC-102816) and methyl-GAG (NSC-32946) in previously treated adults with acute nonlymphocytic leukemia. Cancer Chemother Rep. 1975;59(5):1043-1045.
pubmed - Isoda A, Sakurai A, Ogawa Y, Miyazawa Y, Saito A, Matsumoto M, Sawamura M. Chronic inflammatory demyelinating polyneuropathy accompanied by chronic myelomonocytic leukemia: possible pathogenesis of autoimmunity in myelodysplastic syndrome. Int J Hematol. 2009;90(2):239-242.
doi pubmed - Saif MW, Hopkins JL, Gore SD. Autoimmune phenomena in patients with myelodysplastic syndromes and chronic myelomonocytic leukemia. Leuk Lymphoma. 2002;43(11):2083-2092.
doi pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


