| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website http://www.thejh.org |
Original Article
Volume 4, Number 2, June 2015, pages 157-163
Effect of Preoperative Autologous Blood Donation Coupled With Fluid Resuscitation on Transfusion Requirements Following Orthopedic Surgery
Diane Hazela, Murray Berna, b, c, e, James Bonod, Donald Reillyd, Claire Robbinsd
aDivision of Research, New England Baptist Hospital, 125 Parker Hill Avenue, Boston, MA 02120, USA
bDepartment of Medicine, New England Baptist Hospital, 125 Parker Hill Avenue, Boston, MA 02120, USA
cUniversity of New Mexico Cancer Center, 1201 Camino de Salud, NE, Albuquerque, NM 87505, USA
dDepartment of Orthopedic Surgery, New England Baptist Hospital, 125 Parker Hill Avenue, Boston, MA 02120, USA
eCorresponding Author: Murray Bern, University of New Mexico Cancer Center, 1201 Camino de Salud NE, Albuquerque, NM 87131, USA
Manuscript accepted for publication June 19, 2015
Short title: Preoperative Autologous Blood Donation
doi: http://dx.doi.org/10.14740/jh208w
| Abstract | ▴Top |
Background: Preoperative auto-blood donation has been shown to increase the likelihood of developing postoperative anemia following orthopedic surgery. This study was to assess the additive effect of intraoperative plus postoperative fluid resuscitation upon the relationship between preoperative donation and the frequency of postoperative transfusion.
Methods: In this retrospective, single institution case-controlled study, hemoglobin levels, fluid administration, and incidence of transfusion were reviewed among 182 patients (91 donated blood preoperatively and 91 did not) undergoing total hip arthroplasty (THA) or total knee arthroplasty (TKA).
Results: Thirty-two (35.2%) donors and 18 (19.8%) non-donors received transfusion for adjusted risk of 2.817 (1.301 - 6.100) among donors versus non-donors. Donors are more affected by the hemodilution effects associated with fluid infusion and are transfused earlier and more frequently than non-donors.
Conclusion: Preoperative autologous donation and fluid administration increase the risk for receiving postoperative transfusion.
Keywords: Hemodilution; Transfusion; Hip replacement; Knee replacement; Autologous donation
| Introduction | ▴Top |
Many hospitals utilize preoperative autologous blood donations, along with other techniques, to reduce the frequency of allogeneic blood transfusions with the associated risks of blood-transmitted diseases and mismatch errors for patients undergoing total hip arthroplasty (THA) or total knee arthroplasty (TKA) [1-4]. Autologous donation programs also reduce costs and protect the general blood supply.
One multi-center study reported auto-donated units were the most commonly used blood product for total joint arthroplasty in a review of 9,482 patients reported from 330 surgeons in 235 sites. In this survey, 46% of the hip arthroplasty patients and 39% of the knee arthroplasty patients received breakthrough allogeneic blood transfusion, thus nullifying the goals of the auto-donation program [5]. Bern et al found that patients who did not auto-donate received fewer transfusions following hip and knee arthroplasty than those who did donate [6]. Increased number of auto-donated units correlated with increased likelihood that the patient would require transfusion postoperatively. When stratified by baseline hemoglobin ≥ 13.0 g/dL, only 30.8% of the patients were transfused, whereas 48.8% of those with baseline hemoglobin less than 13.0 g/dL were transfused. Cohen and Brecher using a mathematical model predicted this same sequence [7]. Other investigators described similar findings [8-12].
The objective of this current retrospective, case-controlled, single institution study was to assess the association between auto-donation and intraoperative and postoperative intravenous (IV) fluid management on the frequency of such transfusions following THA and TKA.
| Materials and Methods | ▴Top |
The donor and non-donor patients were selected on a one-to-one ratio from two surgeons, one of whom never includes autologous donation in the preoperative plan and the other who usually advises patients to have autologous donation. Eligible patients included those having primary, unilateral, elective TKA or THA. Auto-donations were within 1 - 4 weeks prior to the scheduled surgery. Oral iron replacement therapy was recommended to each patient who donated blood. Patients with a baseline hemoglobin value below 12.0 g/dL within 6 weeks of surgery and those having other surgery within 8 weeks of the index surgery were excluded from this study. Age, gender, race and weight were not considered in patient selection.
The surgeons involved in this study were very experienced in performing these procedures. They used very similar surgical techniques. Intraoperative red blood cell savers were used, and those cells were reinfused. The surgeons participate in the same postoperative management and rehabilitation protocols, managed on a daily basis by the permanent hospitalist staff. General anesthesia was used for all patients. Hydroxyethyl starch 6% (HES) was used at the discretion of the anesthesiologist when needed for intravascular volume expansion. A restrictive transfusion policy was in place, with transfusions given based upon clinical need when hemoglobin was less than 9.0 g/dL. Autologous blood units were returned to the respective donors preferentially over allogeneic blood.
Patients’ medical records were reviewed for age, gender, height, weight, body mass index (BMI), estimated blood loss (EBL) including losses in the operating room and post-anesthesia care unit (PACU), type and volume of intravenous fluids infused, daily hemoglobin values, the number of autologous and allogeneic transfusions given, and trigger hemoglobin prior to transfusion. All data were collected and managed using the Research Electronic Data Capture [13]. This protocol was approved by the hospital Institutional Review Board.
Statistical analysis
Sample size calculations determined that a minimum requirement of 77 patients was needed in each group in order to detect an anticipated 25% difference in transfusion rates between donors and non-donors at a power level of 0.9. There were 685 eligible patients from the surgeon who utilizes preoperative autologous donation and 289 eligible patients from the surgeon who does not. Using the SAS statistical package, a random sample of 96 patients was generated from each surgeon in order to meet the required minimum of 77 patients in each arm after an estimated 20% reduction due to missing data. However, only five patients were removed from each group due to incomplete records, resulting in a final sample of 182 eligible patients with complete study records with 91 patients for each surgeon.
Student’s t-tests were calculated to determine the differences in EBL, volume of fluids administered, and hemoglobin change from preoperative to postoperative levels between the two patient groups. The same comparisons were made between patients who received at least one unit of transfused blood and patients who did not receive transfusion. A multiple, stepwise, logistic regression was used to calculate adjusted relative risks for several anticipated risk factors for receiving transfusion. Analysis of variance (ANOVA) was utilized to measure differences in fluid resuscitation between multiple levels of BMI. Data were analyzed using SAS statistical package version 9.3 (SAS Institute, Cary, NC).
| Results | ▴Top |
The non-donor and donor cohorts were similar with respect to age, BMI, gender, and operative joint (Table 1).
 Click to view | Table 1. Baseline Characteristics |
Transfusion rates
Fifty of the 182 total patients received one or more transfusions during the 7-day postoperative period. Among the 91 patients who donated, 32 (35.1%) received one or more transfusions compared to 18 of the 91 (19.8%) patients who did not donate. Thus, donating patients were almost twice as likely to require postoperative transfusion (relative risk (RR) = 1.778, 95% CI: 1.0793 - 2.9282). After adjusting for age, BMI, use of intraoperative HES, and operative joint (knee vs. hip), the risk of requiring transfusion became larger for donor vs. non-donor patients (RR = 2.817, 95% CI: 1.301 - 6.100) (Table 2). Among the 32 donor patients who required transfusions, seven subsequently required additional allogeneic transfusions following depletion of their stored autologous units. A total 26 units of allogeneic blood were distributed among 16 non-donor patients who required transfusion. The other two non-donor patients used a combined 14 units and were removed from this analysis due to complex underlying conditions with unexpected postsurgical complications.
 Click to view | Table 2. Relative Risk (RR) for Receiving at Least 1 Unit of Transfused Blood Postoperatively |
HES
Patients receiving HES had average postoperative hemoglobin of 11.2 g/dL whereas patients who did not receive starch had average hemoglobin of 12.2 g/dL (P < 0.001), suggesting greater dilution among the former. Patients who received HES lost on average 2.58 (P = 0.001) times more blood during surgery.
Blood hemoglobin concentrations
Hemoglobin concentrations comparing donors to non-donors for preoperative through postoperative day 2 are demonstrated in Table 3. Donor patients had a higher baseline hemoglobin but equal trough hemoglobin compared to non-donors. There were statistically significant, but clinically insignificant, lower hemoglobin for donors vs. non-donors in the recovery room and on postoperative day 1. By postoperative day 2 all such differences disappeared. While the trough hemoglobin level was the same for both groups, the donor group reached nadir hemoglobin concentration earlier compared to non-donors (day 1.99 vs. day 2.54, P < 0.001).
 Click to view | Table 3. Hemoglobin Values in Perioperative Period |
The trigger hemoglobin level leading to transfusion was significantly higher for the donor group.
There was no significant difference in blood loss comparing donors and non-donors (Table 4). For patients who required transfusion, the EBL was more than twice the amount compared to those who did not require transfusion, regardless of donation status (477.4 ± SD 510.4 mL compared to 232.4 ± SD 219.9 mL, P = 0.002).
 Click to view | Table 4. Blood Loss and Fluid Administration |
Fluid administration
When comparing transfused vs. non-transfused patients, there were no differences in fluid administration, nor was there difference when comparing the donor vs. non-donor cohorts (Table 4). However, among patients who received transfusion, the non-donor patients received nearly 1 L of fluids more than donors before receiving blood products (4,980.6 mL ± SD 1,643.7 mL compared to 4,011.5 mL ± SD 1,348.4 mL, P = 0.029).
BMI
Logistic regression analysis revealed a protective effect against being transfused by having an increased BMI (Table 2 and Fig. 1). Figure 1 displays changes in hemodilution across four BMI quartiles. In absolute terms the morbidly obese patients received an average additional 1,270 mL of intravenous fluid preceding the first transfusion. However, when calculated as mL/kg body weight, there was a decrease in milliliters of infused intravenous fluid as BMI increased prior to the first transfusion. Lean patients received an additional 26.14 mL/kg of body weight compared to morbidly obese patients. Despite these differences in fluid administration, the triggering hemoglobin for first transfusion was nearly equal, with no significant differences, among the four BMI quartiles.
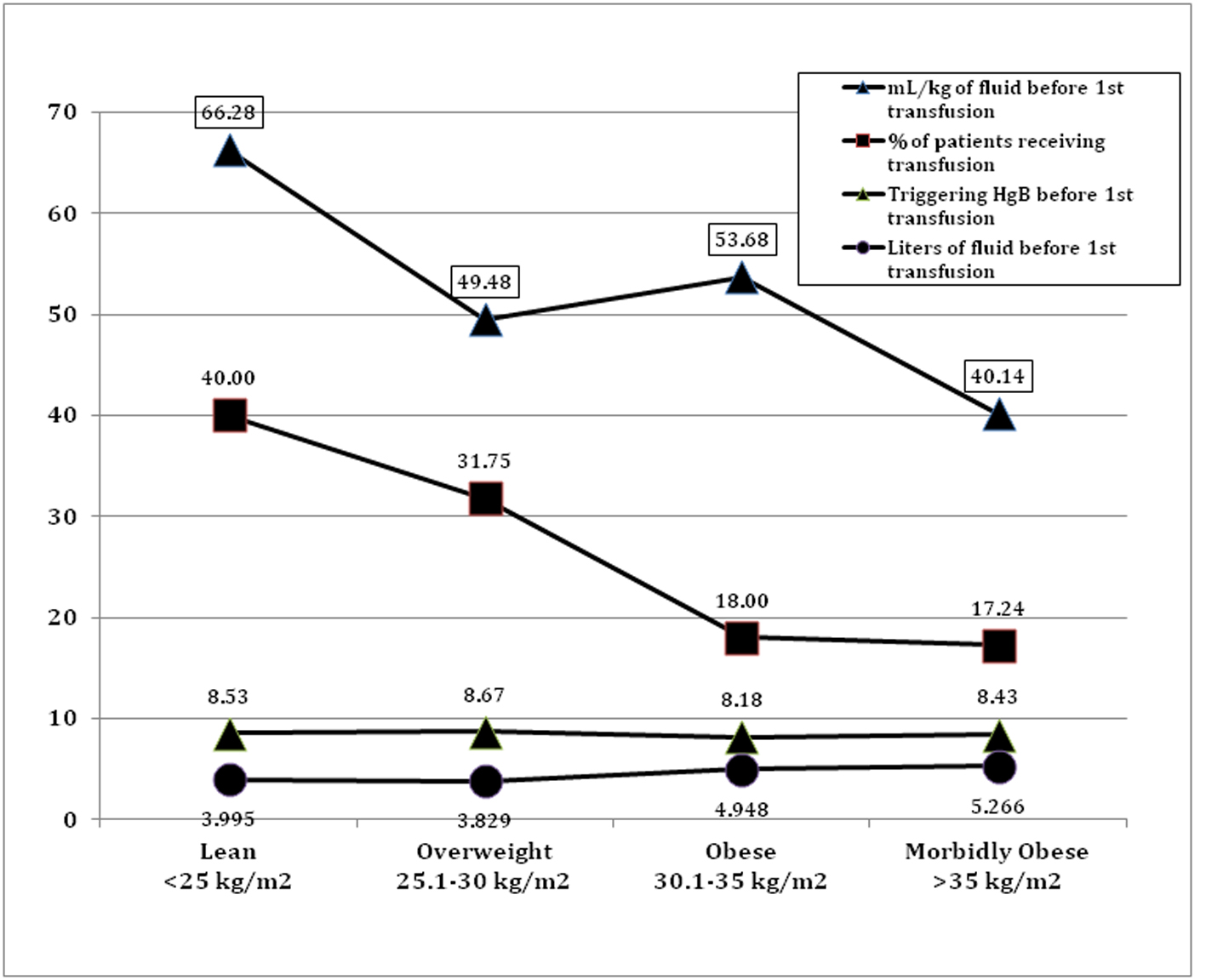 Click for large image | Figure 1. Relationship between BMI, fluids, and risk of transfusion. |
| Discussion | ▴Top |
The hemoglobin concentration of whole blood falls following surgery due to a combination of hemorrhagic blood loss and hemodilution following IV crystalloid fluid resuscitation. Preoperative auto-donation becomes part of the combined hemorrhagic blood loss when there is inadequate time for the patient to reconstitute the shed red cell mass. In some situations auto-donation creates additional physiological stress by causing reduced red cell mass that cannot be replaced with autologous infusion alone [14]. These factors, taken together, can lead to more blood transfusions than would have occurred had no preoperative auto-donations occurred [6, 12].
In the current study all patients regardless of donation status reached nearly equal trough hemoglobin values during the immediate postoperative period; however, donors reached this trough faster than non-donors leading to earlier transfusions. Furthermore, while the average trough hemoglobin was comparable, the average hemoglobin for triggering transfusion was higher among donors. One could speculate that surgeons elect to transfuse donor patients with their stored autologous blood due to its availability, while non-donor patients received crystalloid fluids for longer periods of time. Further study would be needed to determine whether non-donor patients better tolerated the added iatrogenic hemodilution without actually requiring early transfusion.
Those patients undergoing THA were at greater risk for requiring transfusion compared to those undergoing TKA. This is due in large part to the discrepancy in blood loss, most likely due to the application of tourniquet during the knee arthroplasty [15, 16]. THA patients lost an average of 489.3 ± 386.1 mL of blood compared to 105.9 ± 107.4 mL in the TKA patient cohort.
Preoperative anemia and allogeneic transfusions are independently associated with postoperative adverse outcomes. Thus, even though 25 (78%) of the donor patients who received transfusion were able to avoid the risks associated with allogeneic transfusion they were still placed at greater risk of adverse outcomes from iatrogenic anemia [17].
Use of intraoperative HES was associated with increased incidence of postoperative transfusion. There are at least two explanations for this association. The decision to administer the HES may be precipitated by a disproportionately higher volume of surgical blood loss, possibly worsened by the mild coagulopathy associated with HES [18, 19]. HES also causes a shift of extravascular fluid into the intravascular space creating an additional approximate mL for mL expansion of the plasma volume, thus adding approximately another liter of hemodilution for the 500 mL HES given [20].
The data in this study revealed a steadily decreasing risk for receiving transfusion as BMI increased. Obese patients received more volume replacement in absolute terms, but less replacement fluid when measured as mL/kg body weight prior to the initial transfusion as compared to lean patients [21]. With this, however, the trough hemoglobin was not significantly different between any of the groups, suggesting less hemodilution among overweight patients. There are other physiologic factors to be considered. Patients who are overweight have a larger volume of intravascular space, thereby allowing large volumes of fluid to distribute with minimal impact on blood concentrations [22, 23]. Also, patients with more adipose tissue shift excess fluids from the intravascular space into the interstitial tissues, thereby maintaining intravascular blood concentration equal to lean patients [23].
Many factors are to be considered when determining the management of controlled blood volume depletion, as occurs with the THA and TKA discussed here. Authors have attempted to create formulas for predicting hemoglobin change with surgery [24, 25]. Because only one-eighth of total body fluid is located within intravascular circulation, it has been proposed that the type of fluid and corresponding degree to which these fluids are retained in circulation has only a small impact on hemoglobin [26]. Others argue that estimates must be adjusted for type of fluid [23]. Gender and weight are also significant contributing variables [27]. Results from the current study suggest that BMI should be included in algorithms for predicting the impact of blood loss and subsequent fluid infusions.
An extreme example of iatrogenic hemodilution is found with isovolemic hemodilution. In this technique, patients develop reduced hemoglobin concentrations in their whole blood as a result of preoperative autologous donation and subsequent infusion of crystalloid and/or colloid solutions to maintain circulating volume and adequate tissue perfusion [24, 26, 28-32]. Shander and Rijhwani proposed that this acute iatrogenic hemodilution causes a temporary overcompensation and increased oxygen delivery to the tissues [31]. They posit that systemic oxygen uptake can remain adequate with hemodilution down to hematocrit of 10-12% or hemoglobin of 4.5 - 5 g/dL. Madjdpour et al proposed similar benefits but with a more conservative hemoglobin of 7.0 g/dL as a threshold for maintaining adequate perfusion and oxygenation [32]. Spahn et al further discuss the physiological adaptations that allow for greater blood flow due to reduction in blood viscosity occurring between 10 and 15 g/dL of hemoglobin. There is also increased sympathetic stimulation to the heart, increased venous return, decreased vascular resistance, and decreased after load, all resulting in greater cardiac output. Other vital organs will increase oxygen consumption to support vital function [28]. These studies suggest that surgical blood loss may be effectively treated with crystalloid or colloid infusions only for volume maintenance without compromising tissue integrity even at low hemoglobin concentrations.
The results of this study support prior work that preoperative autologous blood donation prior to total joint arthroplasty increases the risk for receiving one or more blood transfusions [6, 12, 33, 34]. Other risk factors include the use of HES and obesity as they relate to alterations in intra- and extravascular fluid dynamics.
There are large variations in the transfusion practices among orthopedic surgeons. A systematic review of the medical literature to better understand these variations was minimally successful due to the generally weak quality of the studies of this field, the heterogeneity of the surgical procedures under study, and the lack of prospective studies. Among the risk factors most identified were low hemoglobin and increasing age, but also mentioned were surgical complexity, comorbidities, female gender, and low body weight [35]. No attention was given to the effect of iatrogenic hemodilution, unless low body weight is a surrogate for this by way of putting too much IV fluid into smaller patients leads to lower hemoglobin levels. Having no autologous preoperative donated units available may be a predictor for patient having allogeneic blood transfusion for THA but not TKA [36].
Further study could provide insight into the necessity of critically evaluating the proper use of such blood management programs in individual healthcare settings. Researchers should strive to further clarify whether auto-donation should be recommended for patients whose baseline hemoglobin is high enough that postoperative anemia requiring transfusion is unlikely. The data may help support the trend of performing total joint arthroplasty in a surgical day setting for qualified patients [37]. While there is certainly a need for intra- and postoperative IV fluid infusions, we would argue that more judicious use of those IV crystalloids would obviate against excessive hemodilution with its attendant fall of hemoglobin, and thus reduce the tendency to transfuse either auto- or allogeneic blood.
Author Contributions
Diane Hazel and Murray Bern created the initial protocol, analyzed the data and wrote the manuscript. James Bono and Donald Reilly contributed patients for the study. Claire Robbins contributed to manuscript preparation.
Funding
This work was supported by New England Baptist Hospital, Boston, Massachusetts, the Foundation for Hematology Research, Boston, Massachusetts and the Cancer Center Research Foundation, Boston, Massachusetts.
| References | ▴Top |
- Shander A. Emerging risks and outcomes of blood transfusion in surgery. Semin Hematol. 2004;41(1 Suppl 1):117-124.
doi pubmed - Feagan BG, Wong CJ, Lau CY, Wheeler SL, Sue AQG, Kirkley A. Transfusion practice in elective orthopaedic surgery. Transfus Med. 2001;11(2):87-95.
doi pubmed - Regis D, Corallo F, Franchini M, Rosa R, Ricci M, Bartolozzi P. Preoperative autologous blood donation in primary total knee arthroplasty: critical review of current indications. Chir Organi Mov. 2008;91(1):41-44.
doi pubmed - Gee AO, Garino JP, Lee GC. Autologous blood reinfusion in patients undergoing bilateral total hip arthroplasty. J Orthop Surg (Hong Kong). 2011;19(2):181-184.
- Bierbaum BE, Callaghan JJ, Galante JO, Rubash HE, Tooms RE, Welch RB. An analysis of blood management in patients having a total hip or knee arthroplasty. J Bone Joint Surg Am. 1999;81(1):2-10.
pubmed - Bern MM, Bierbaum BE, Katz JN, Losina E. Autologous blood donation and subsequent blood use in patients undergoing total knee arthroplasty. Transfus Med. 2006;16(5):313-319.
doi pubmed - Cohen JA, Brecher ME. Preoperative autologous blood donation: benefit or detriment? A mathematical analysis. Transfusion. 1995;35(8):640-644.
doi - Keating EM, Meding JB, Faris PM, Ritter MA. Predictors of transfusion risk in elective knee surgery. Clin Orthop Relat Res. 1998;(357):50-59.
doi pubmed - Faris PM, Spence RK, Larholt KM, Sampson AR, Frei D. The predictive power of baseline hemoglobin for transfusion risk in surgery patients. Orthopedics. 1999;22(1 Suppl):s135-140.
pubmed - Sculco TP, Gallina J. Blood management experience: relationship between autologous blood donation and transfusion in orthopedic surgery. Orthopedics. 1999;22(1 Suppl):s129-134.
pubmed - Salido JA, Marin LA, Gomez LA, Zorrilla P, Martinez C. Preoperative hemoglobin levels and the need for transfusion after prosthetic hip and knee surgery: analysis of predictive factors. J Bone Joint Surg Am. 2002;84-A(2):216-220.
pubmed - Jakovina Blazekovic S, Bicanic G, Hrabac P, Tripkovic B, Delimar D. Pre-operative autologous blood donation versus no blood donation in total knee arthroplasty: a prospective randomised trial. Int Orthop. 2014;38(2):341-346.
doi pubmed - Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381.
doi pubmed - Cushner FD, Hawes T, Kessler D, Hill K, Scuderi GR. Orthopaedic-induced anemia: the fallacy of autologous donation programs. Clin Orthop Relat Res. 2005;(431):145-149.
doi pubmed - Callaghan JJ, O'Rourke MR, Liu SS. Blood management: issues and options. J Arthroplasty. 2005;20(4 Suppl 2):51-54.
doi pubmed - Courtney JB, Cushner F, Long WJ, Nett MP. An effective bloodless surgery protocol. Techniques in Knee Surgery. 2011;10:188-197.
doi - Spahn DR. Anemia and patient blood management in hip and knee surgery: a systematic review of the literature. Anesthesiology. 2010;113(2):482-495.
doi pubmed - Schramko A, Suojaranta-Ylinen R, Kuitunen A, Raivio P, Kukkonen S, Niemi T. Hydroxyethylstarch and gelatin solutions impair blood coagulation after cardiac surgery: a prospective randomized trial. Br J Anaesth. 2010;104(6):691-697.
pubmed - Haisch G, Boldt J, Krebs C, Suttner S, Lehmann A, Isgro F. Influence of hydroxyethyl starch preparation (HES 130/0.4) on coagulation in cardiac surgical patients. J Cardiothorac Vasc Anesth. 2001;15(3):316-321.
doi - Moggio RA, Rha CC, Somberg ED, Praeger PI, Pooley RW, Reed GE. Hemodynamic comparison of albumin and hydroxyethyl starch in postoperative cardiac surgery patients. Crit Care Med. 1983;11(12):943-945.
doi pubmed - Nelson J, Billeter AT, Seifert B, Neuhaus V, Trentz O, Hofer CK, Turina M. Obese trauma patients are at increased risk of early hypovolemic shock: a retrospective cohort analysis of 1,084 severely injured patients. Crit Care. 2012;16(3):R77.
doi pubmed - Messerli FH, Christie B, DeCarvalho JG, Aristimuno GG, Suarez DH, Dreslinski GR, Frohlich ED. Obesity and essential hypertension. Hemodynamics, intravascular volume, sodium excretion, and plasma renin activity. Arch Intern Med. 1981;141(1):81-85.
doi pubmed - Raison J, Achimastos A, Bouthier J, London G, Safar M. Intravascular volume, extracellular fluid volume, and total body water in obese and nonobese hypertensive patients. Am J Cardiol. 1983;51(1):165-170.
doi - Bourke DL, Smith TC. Estimating allowable hemodilution. Anesthesiology. 1974;41(6):609-612.
doi - Meier J, Kleen M, Habler O, Kemming G, Messmer K. New mathematical model for the correct prediction of the exchangeable blood volume during acute normovolemic hemodilution. Acta Anaesthesiol Scand. 2003;47(1):37-45.
doi pubmed - Sehat KR, Evans RL, Newman JH. Hidden blood loss following hip and knee arthroplasty. Correct management of blood loss should take hidden loss into account. J Bone Joint Surg Br. 2004;86(4):561-565.
pubmed - George TJ, Beaty CA, Kilic A, Haggerty KA, Frank SM, Savage WJ, Whitman GJ. Hemoglobin drift after cardiac surgery. Ann Thorac Surg. 2012;94(3):703-709.
doi pubmed - Spahn DR, Leone BJ, Reves JG, Pasch T. Cardiovascular and coronary physiology of acute isovolemic hemodilution: a review of nonoxygen-carrying and oxygen-carrying solutions. Anesth Analg. 1994;78(5):1000-1021.
doi pubmed - Kleen M, Habler O, Hutter J, Podtschaske A, Tiede M, Kemming G, Welte M, et al. Effects of hemodilution on gastric regional perfusion and intramucosal pH. Am J Physiol. 1996;271(5 Pt 2):H1849-1855.
pubmed - Weiskopf RB, Viele MK, Feiner J, Kelley S, Lieberman J, Noorani M, Leung JM, et al. Human cardiovascular and metabolic response to acute, severe isovolemic anemia. JAMA. 1998;279(3):217-221.
doi pubmed - Shander A, Rijhwani TS. Acute normovolemic hemodilution. Transfusion. 2004;44(12 Suppl):26S-34S.
doi pubmed - Madjdpour C, Spahn DR, Weiskopf RB. Anemia and perioperative red blood cell transfusion: a matter of tolerance. Crit Care Med. 2006;34(5 Suppl):S102-108.
doi pubmed - Gonzalez-Porras JR, Colado E, Conde MP, Lopez T, Nieto MJ, Corral M. An individualized pre-operative blood saving protocol can increase pre-operative haemoglobin levels and reduce the need for transfusion in elective total hip or knee arthroplasty. Transfus Med. 2009;19(1):35-42.
doi pubmed - Ulrich SD, Kyle B, Johnson AJ, Zywiel MG, Mont MA. Strategies to reduce blood loss in lower extremity total joint arthroplasty. Surg Technol Int. 2010;20:341-347.
pubmed - Barr PJ, Donnelly M, Cardwell C, Alam SS, Morris K, Parker M, Bailie KE. Drivers of transfusion decision making and quality of the evidence in orthopedic surgery: a systematic review of the literature. Transfus Med Rev. 2011;25(4):304-316 e301-306.
- Yoshihara H, Yoneoka D. Predictors of allogeneic blood transfusion in total hip and knee arthroplasty in the United States, 2000-2009. J Arthroplasty. 2014;29(9):1736-1740.
doi pubmed - Berend ME, Berger RA, Hartzband MA. Outpatient Arthroplasty: Same Day, Home Safe. American Academy of Orthopedic Surgeons Annual Meeting, 2014. Instructional course: March 12, 2014.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


