| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Original Article
Volume 11, Number 5, October 2022, pages 167-175
Incidence of Venous Thromboembolism in Hospitalized COVID-19 Patients Receiving Thromboprophylaxis
Jimmy Huanga, Jenny Martineza, Daniel Diaza, William R. Wolowichb
aDepartment of Pharmacy, Mount Sinai Medical Center, Miami Beach, FL, USA
bCollege of Pharmacy, Nova Southeastern University, Fort Lauderdale, FL, USA
cCorresponding Author: Jimmy Huang, Department of Pharmacy, Mount Sinai Medical Center, Miami Beach, FL 33140, USA
Manuscript submitted July 27, 2022, accepted October 3, 2022, published online October 31, 2022
Short title: VTE in Hospitalized COVID-19 Patients
doi: https://doi.org/10.14740/jh1036
| Abstract | ▴Top |
Background: The purpose of this study was to investigate the association between anticoagulant dosing intensity in coronavirus disease 2019 (COVID-19) infected patients and its outcomes on venous thromboembolism (VTE) and all-cause mortality.
Methods: This is a retrospective observational study that examined different anticoagulation regimens among COVID-19 patients for prophylaxis of VTE. Primary outcomes of the study were VTE incidence and all-cause mortality for patients receiving prophylaxis-intensity (PPX) and therapeutic-intensity (TX) anticoagulation. Secondary outcomes were incidence of hemorrhagic events and hospital length of stay. Patients were matched (1:1) based on age and Charlson comorbidity score. Sub-group analyses evaluated outcomes within critically ill patients, between specific anticoagulant agents and comorbid conditions.
Results: The primary outcome of VTE occurred in six patients within the prophylactic dose group and eight patients in the therapeutic-intensity dose group (risk ratio (RR): 2.02 (95% confidence interval (CI): 0.7 - 5.2); P = 0.2). Bleeding occurred in 15 (11%) patients in the prophylactic group and 27 (19%) patients in the therapeutic group (RR: 0.5 (95% CI: 0.3 - 1.0); P < 0.049). Hospital length of stay was shorter by 4 days in those treated with prophylactic-intensity anticoagulation (P = 0.003). Intensive care unit admission and ventilation were negatively correlated with mortality in a multivariate analysis.
Conclusions: Among hospitalized COVID-19 patients, the use of therapeutic-intensity anticoagulation did not show any benefits in reducing the occurrence of VTE. An increase in mortality and in the incidence of hemorrhagic events was statistically significant in the therapeutic-intensity group. Future prospective studies are warranted to evaluate anticoagulation therapy in COVID-19 infected patients.
Keywords: Anticoagulation; Heparin; Low-molecular-weight heparin; Coronavirus; SARS; COVID-19; D-dimer; Coagulopathy
| Introduction | ▴Top |
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has infected more than 180 million individuals worldwide and caused more than 4 million deaths [1]. Hypercoagulability presents as a major complication particularly among hospitalized coronavirus disease 2019 (COVID-19) patients and it significantly impacts mortality. From early observational studies, the incidence of venous thromboembolism (VTE) was reported up to 25% in hospitalized COVID-19 infected patients, who were not receiving thromboprophylaxis [2]. A recent multicentered study has shown an incidence of VTE as high as 31% despite patients being on standard thromboprophylaxis [3]. The prevalence of VTE will vary by severity of the disease and it is more notable among the critically ill.
While still in early stages of understanding the hematologic manifestations of COVID-19, new therapeutic management has emerged from the pandemic due to the observance of VTE events despite anticoagulation therapy. Low-molecular-weight heparin (LMWH) is known to reduce the risk of VTE in hospitalized patients and it may have anti-inflammatory properties. Based on this finding and the understanding of COVID-19, patients at high risk for VTE (those with high inflammatory markers), are often treated with higher doses of anticoagulation. Evidence suggests that higher anticoagulation targets should be evaluated as critically ill COVID-19 patients on anticoagulation developed thrombotic complications [4]. The World Health Organization (WHO) and the International Society of Thrombosis and Hemostasis (ISTH) currently recommend that all hospital admitted COVID-19 patients receive standard prophylaxis doses of LMWH or unfractionated heparin (UFH) unless otherwise contraindicated [5].
COVID-19 infection is associated with several biochemical abnormalities including leukocytosis, neutrophilia, and elevated ferritin, creatinine kinase, and troponin. D-dimer, a fibrin degradation product, has become a significant biomarker in identifying risk of increased morbidity and it is being used to guide therapy on anticoagulation due to the association with an increase of thrombin formation [6]. Among other markers, prothrombin time and platelets are also key monitoring parameters in directing anticoagulant management decisions.
With the lack of robust randomized control trials and guideline recommendations, there is still much left to uncover regarding the management of VTE prophylaxis. The utilization of treatment doses of anticoagulation as empiric prophylaxis remains controversial as the data lack beneficial outcomes with the potentially higher risk of bleeding. This study aims to determine the association between different anticoagulation level prophylaxis (PPX) and treatment (TX) in COVID-19 infected patients and their outcomes on VTE occurrence and in-hospital all-cause mortality.
| Materials and Methods | ▴Top |
Study design
This retrospective single-center cohort study reviewed patients admitted to Mount Sinai Medical Center for COVID-19 between April 2020 and August of 2020. Patients were included in this study if they were 18 years of age or older, had a SARS-CoV-2 infection confirmed by reverse transcriptase-polymerase chain reaction (RT-PCR), were admitted for the inpatient treatment of COVID-19, and received anticoagulation for the prevention of VTE. Pertinent data collected for analysis included patient demographics (age, gender, race), structured clinical data (medications administered, vital signs, International Classification of Diseases, 10th Edition (ICD-10) diagnoses), and unstructured clinical data (physician notes) from the electronic health record. Patients were placed into two groups based on anticoagulation intensity (PPX, TX) and then matched (1:1) by age and Charlson Comorbidity Index. Patients who were admitted for less than 48 h or on anticoagulation for less than 48 h were excluded from this study. Datasets were assembled including key covariates and outcomes measures derived from structured and unstructured clinical data. This study obtained approval from Mount Sinai Medical Center’s Institutional Review Board and was conducted in compliance with the ethical standards of the responsible institution on human subjects.
Study groups
This study compared prophylactic-intensity (PPX) and therapeutic-intensity (TX) doses of anticoagulation. The most common agents used in the study were UFH and enoxaparin. The PPX patient group received subcutaneous UFH at a dose of 5,000 units every 8 h; or enoxaparin at a dose of 40 mg daily or 30 mg daily if creatinine clearance (CrCl) < 30 mL/min. The therapeutic-intensity patient group (TX) received enoxaparin 1 mg/kg twice a day or UFH continuous infusion to a targeted aPTT goal. The choice of anticoagulant was not randomized but it was driven by prescriber preference, patient specific factors, and institutional availability of medications during the study period. A decision-making tool was available for practitioners to guide anticoagulation therapy. Therapeutic anticoagulation was suggested to be used among those that were considered high-risk COVID patients (defined by oxygen saturations less than 90%, respiratory rate greater than 24 breaths per minute (BPM), increase in supplemental oxygen requirements, elevated D-dimer, or elevated C-reactive protein levels), while those that were not high-risk received standard prophylaxis doses.
Outcomes
The primary outcome of this study was the incidence of VTE (deep vein thrombosis (DVT), pulmonary emboli (PE), and in-hospital all-cause mortality. VTE events were confirmed via imaging studies (ultrasound Dopplers, computed tomography (CT) pulmonary angiograms). Secondary outcomes were incidence of bleeding events and hospital length of stay. Bleeding events in this study encompassed both minor and major bleeds that were confirmed either via physician documentation noted among diagnostic examinations, overt bleeding events, or a hemoglobin below 7.0 g/dL requiring multiple transfusions. Diagnostic tests were not routinely performed and were done as per the treating physician’s clinical judgement. Length of stay was defined as time from admission to death or discharge from hospital.
Statistical analysis
Descriptive statistics were used to analyze general characteristics of the sample population for categorical and continuous variables. Chi-squared test was used for dichotomous and categorical covariates. Primary outcome VTE incidence by study group and mortality were assessed with restriction at 30 days and unrestricted at the end of the study. Hospital length of stay (survival time when the censor is death or discharge) was analyzed using Kaplan-Meier Survival analysis and restricted mean survival time (RMST) analysis. The association between the significant factors from the univariate analysis and the primary and secondary outcomes was assessed in Cox regression analyses. All statistical tests used an alpha for significance of 0.05.
| Results | ▴Top |
Patient baseline characteristics
From the cases reviewed, 282 patients met the inclusion criteria for this study. The characteristics of the patients at baseline are described in Table 1. The median age in the study was 65 years old, and more than half of the sample were males (69%). The median body mass index (BMI) of patients within the study was 29 kg/m2 (interquartile range (IQR): 26 - 34). A comparable amount of prior anticoagulation use (12% vs 9%) was noted between both groups for which indications of anticoagulation was not documented. Most patients had one or more underlying chronic conditions, hypertension (n = 172, 60%) and diabetes (n = 122, 42%). Other pertinent chronic conditions noted were chronic obstructive pulmonary disease (COPD) which had a higher baseline “prevalence” in the TX group (n = 19) compared with the PPX group (n = 10), and end-stage renal disease (ESRD) which was higher in the PPX group (n = 5) compared to the TX group (n = 0). Median D-dimer was 50% higher in the TX group.
 Click to view | Table 1. Baseline Characteristics |
Study outcomes
Primary
This study compared the incidence of VTE and all-cause mortality in 282 hospitalized COVID-19 patients who received pharmacological anticoagulation. The data were analyzed using a 30-day cut-off and unrestricted length of stay for all-cause mortality and occurrence of DVT. Regardless of length of stay restriction, no significant difference in the incidence of VTE was observed between the two study groups (Table 2). At 30 days, three of 141 (2.2%) patients in the PPX group experienced a VTE, while five of 141 (3.9%) occurred in the TX group (odds ratio (OR): 0.56 (95% confidence interval (CI): 0.2 - 2.0); P = 0.5). At 30 days, all-cause mortality was 13.5% overall (36/266). All-cause mortality was not impacted by length of stay. A significant difference was found for all-cause mortality; seven (5.0%) patients in the PPX group and 33 (23.4%) patients in the TX group expired. The odds ratio showed the risk of mortality was 0.16 (0.08 - 0.39; P < 0.0001) less in the PPX group.
 Click to view | Table 2. Primary and Secondary Outcomes |
Time to VTE and death were assessed with Kaplan-Meier survival analysis. Figure 1 displays the occurrences of VTE as a function of a length of stay up to 30 days by treatment group. Figure 2 demonstrates the corresponding KM curves for unrestricted length of stay. Overall, no significant differences were noted between PPX and TX anticoagulation in the time to VTE events. This conclusion is supported by the restricted mean survival analysis presented in Table 3. Time to death is explored in Figures 3 and 4. In Figure 3, the 30-day survival plot indicates a 50% survival at 22 days in the TX group, 50% survival was not reached in the PPX group. When the 30-day time restriction is removed, the RMST analysis reveals a significantly shorter (27 vs. 35 days, P = 0.009) time to death in the TX group.
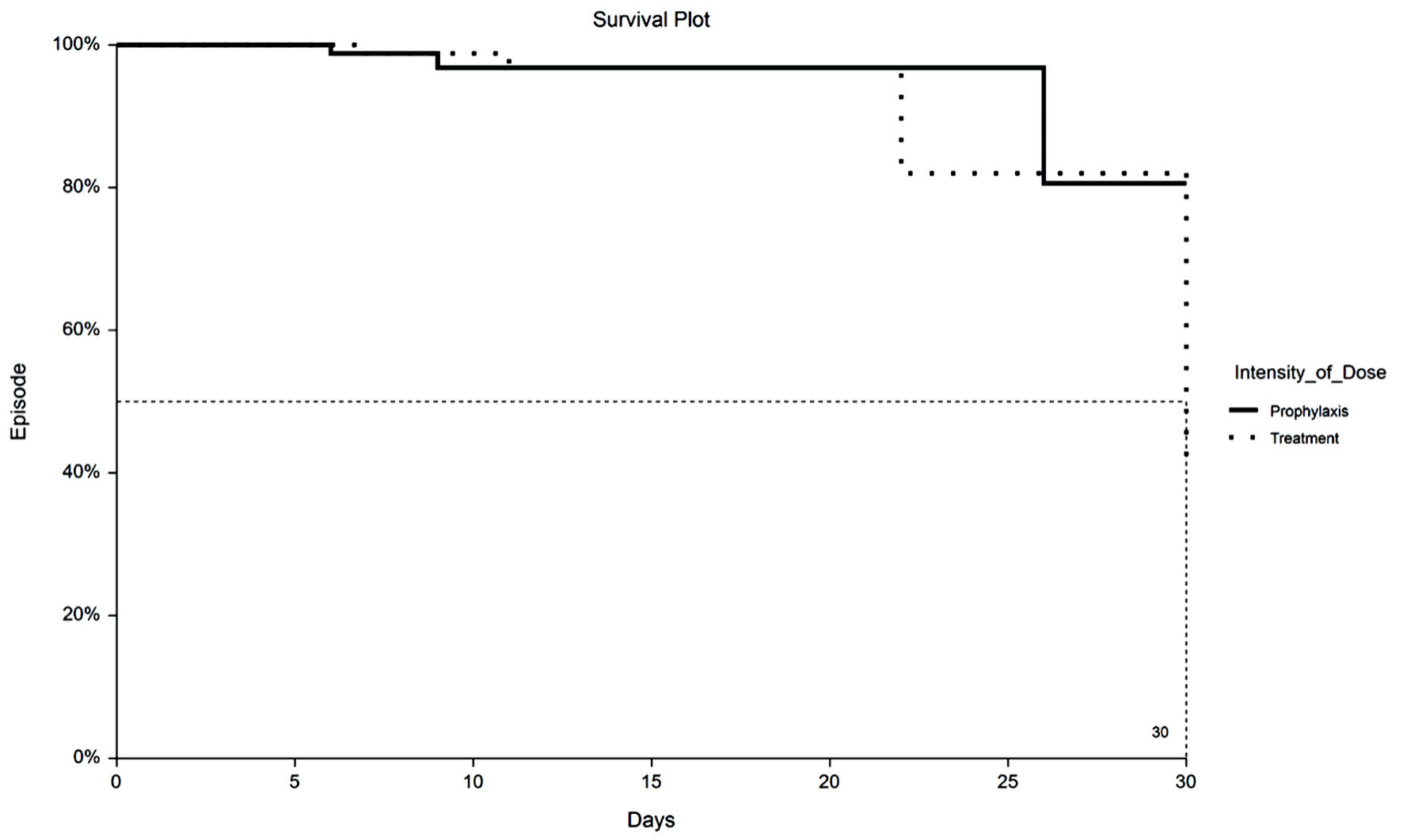 Click for large image | Figure 1. Kaplan-Meier plot of 30-day VTE by TX or PPX group. VTE: venous thromboembolism; PPX: prophylaxis-intensity; TX: therapeutic-intensity. |
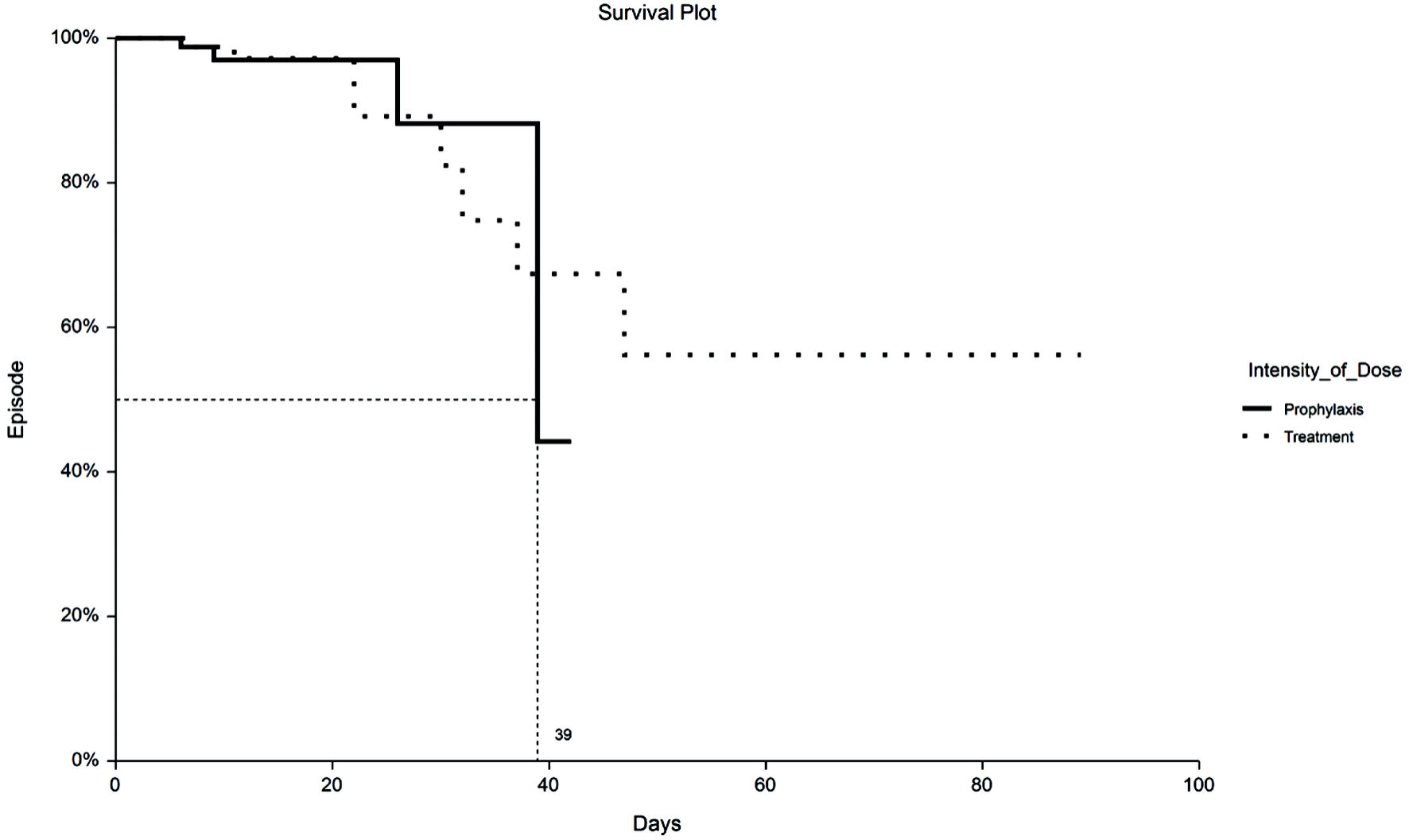 Click for large image | Figure 2. Kaplan-Meier plot of VTE by TX or PPX group with no truncation at 30 days. Fifty percent to VTE time for PPX group is 39 days vs. undefined for the TX group. VTE: venous thromboembolism; PPX: prophylaxis-intensity; TX: therapeutic-intensity. |
 Click to view | Table 3. Restricted Mean Survival Time (RMST) Analysis |
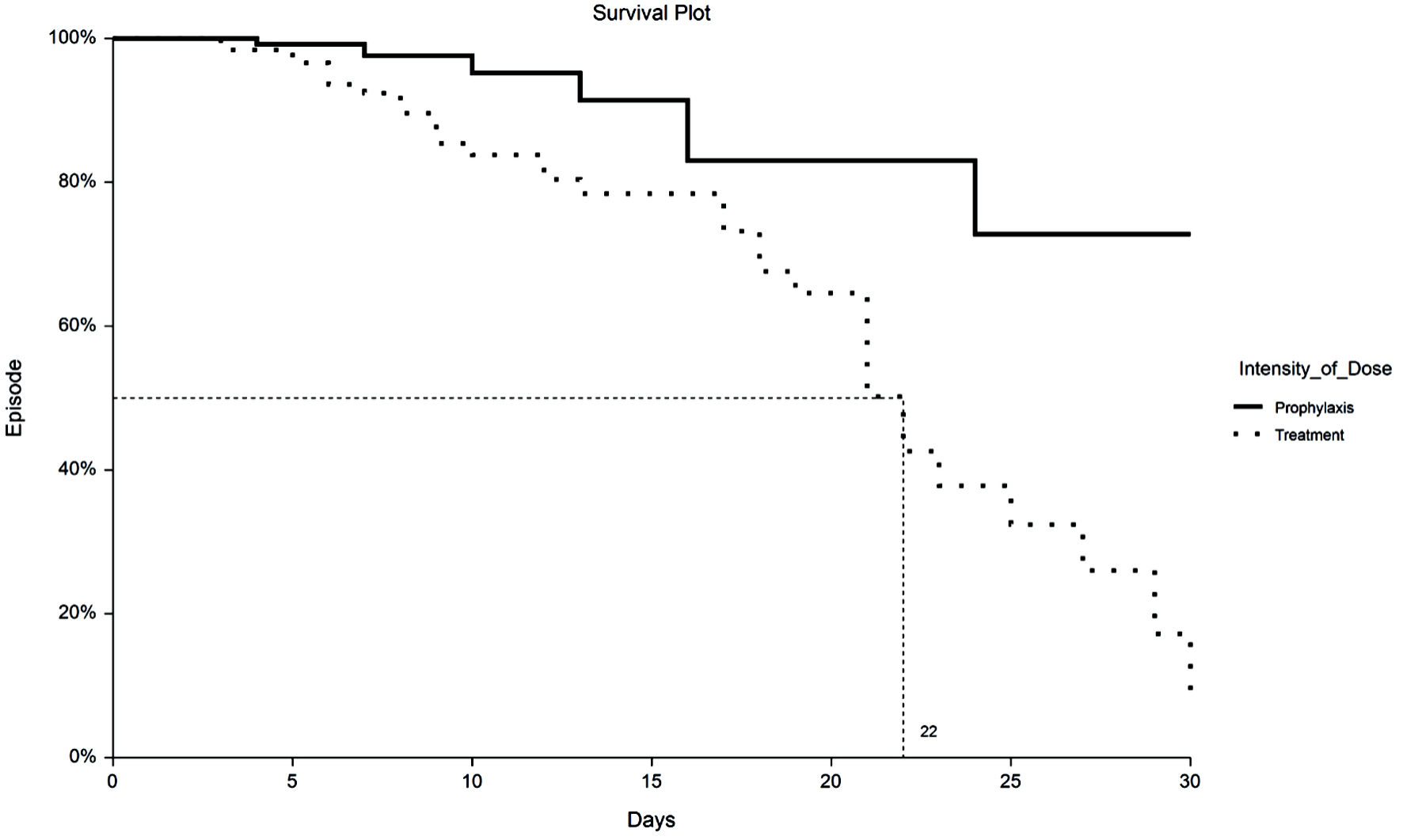 Click for large image | Figure 3. Kaplan-Meier plot of 30-day all-cause mortality by TX or PPX group. Fifty percent survival time for the TX group is 22 days vs. undefined for the PPX group. PPX: prophylaxis-intensity; TX: therapeutic-intensity. |
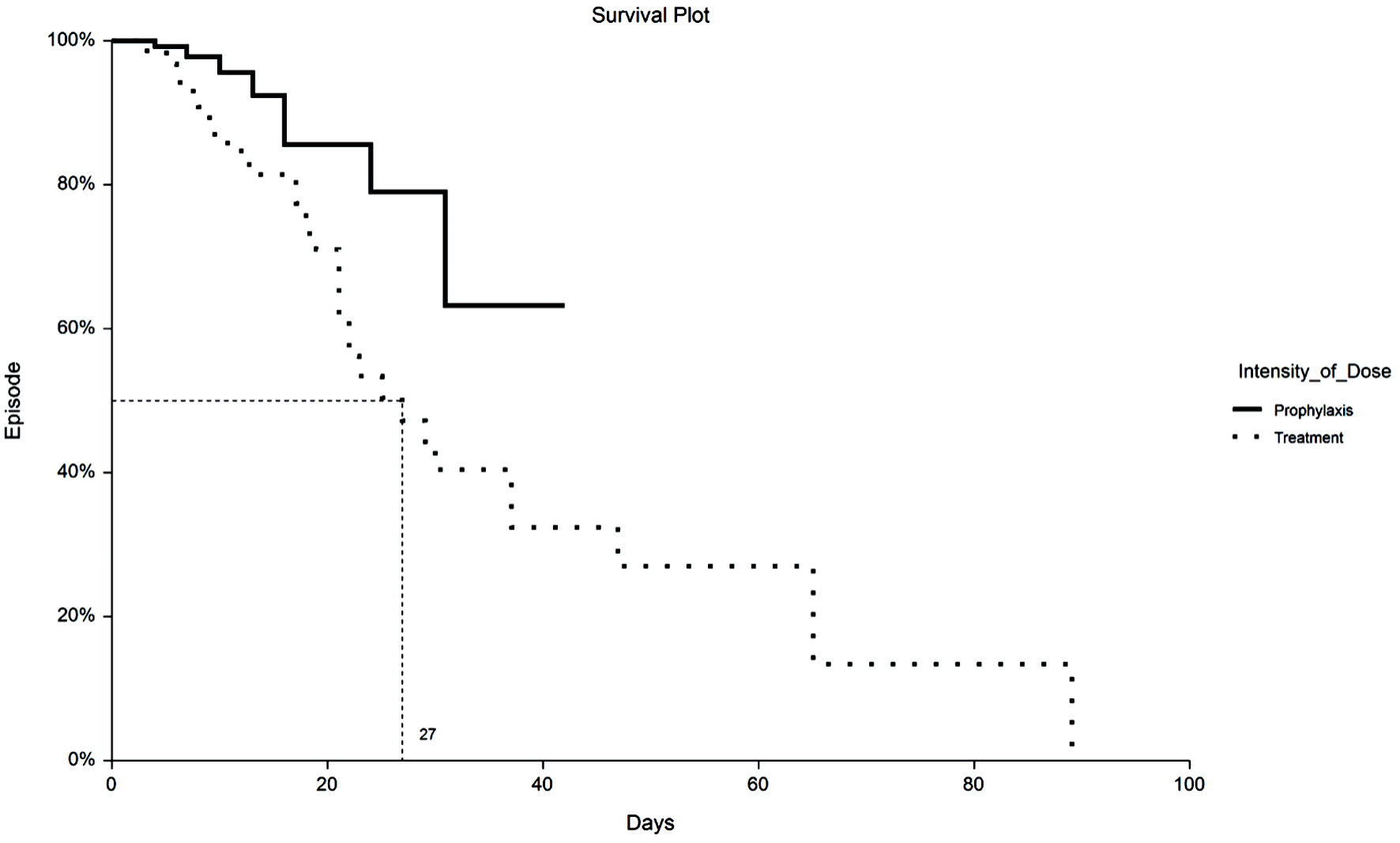 Click for large image | Figure 4. Kaplan-Meier plot of mortality by TX or PPX group with no truncation at 30 days. Median time to death for TX group is 27 days vs. undefined for the PPX group. PPX: prophylaxis-intensity; TX: therapeutic-intensity. |
Cox regression analysis was performed to identify parameters associated with occurrence of VTE. Baseline and in-hospital interventions that were statistically significant (P < 0.05) in univariate Chi-square analysis were entered into a hierarchical forward stepping multivariate Cox regression algorithm. Additional indicators evaluated consisted of antiplatelet drugs, intensity of anticoagulation, and anticoagulant used. COPD, ESRD, and the use of heparin regardless of dose were removed from the analysis as no episodes of VTE occurred in patients with these interventions or disease states. When tested in this multivariate manner, the analysis did not find any significant associations with VTE.
Cox regression analysis was performed to identify parameters associated with occurrence of bleeding (Table 4). When tested in this multivariate manner, the analysis did not find any significant associations with bleeding.
 Click to view | Table 4. Cox Regression of Factors Related to Mortality |
Cox regression analysis was performed to identify parameters associated with mortality. Cox regression can provide some insight into the confounding by physician choice present in this study. We included variables in the Cox model that are not significant by themselves, but when combined with another variable became significant. This is hierarchical forward stepping. The impact of ventilation and intensive care unit (ICU) admission was one such case. Admission to ICU and mechanical ventilation (regardless of patient care area) raised the risk of mortality when considered independently. However, if a patient was in the ICU while ventilated, the risk of death was decreased by 50%. COPD and age ≥ 65 were also significant predictors of mortality but within the treatment group it was not significant in this model.
Secondary
Bleeding episodes occurred in 35/282 (12.4%) patients (Table 2). Secondary outcomes were assessed at 30 days and during unrestricted length of stay. There was a statistically significant difference in hemorrhagic events between the two groups, regardless of length of stay restriction. Fifteen (11.0%) patients in the prophylactic group and 27 (19.1%) patients in the therapeutic group (OR: 0.5 (95% ci: 0.3-1.0); P = 0.049) had a documented case of a bleeding. The breakdown of bleeding cases of the overall population is as followed: 29 gastrointestinal (GI) bleeds, four hematomas, two intracranial hemorrhages, one epistaxis, one pulmonary hemorrhage, one wound bleeding, and one vaginal bleed. One case of intracranial hemorrhage was observed in the prophylaxis dosed group whereas most cases (20) involved GI bleedings. Of the 20 patients with GI bleeding, five were on heparin, 13 were on enoxaparin, one on argatroban, and one on fondaparinux. Six of the 20 patients with GI bleeding received prophylactic dose of anticoagulation (AC) (three UFH, three enoxaparin), and 14 were on therapeutic doses (two UFH, 10 enoxaparin, one argatroban, one fondaparinux).
The median length of stay was 8 days with an IQR of 5 to 15 days. However, as expected with COVID-19 infection, there were outliers leading to a length of stay from 2 days to 87 days. The median length of stay in the PPX group was 6 days and 9 days in the TX group (P < 0.01). Table 3 contains the results of RMST analysis which are consistent with the median analysis of length of stay.
Subgroup
ICU
Subgroup analysis of mortality between the non-ICU group and ICU group were undertaken to further probe the mechanical ventilation and ICU intervention noted above. Regardless of study group, mortality was 5.6% in the non-ICU patients vs. 67.4% in the ICU patients. A similar pattern of excess death in the TX group was observed; 78% of patients in the TX group admitted to the ICU expired, compared to 38% of patients in the PPX group admitted to the ICU.
Enoxaparin vs. UFH
Most patients in this study (282) received either enoxaparin or UFH. Enoxaparin was dosed therapeutically in 128 (55%) of 231 patients on enoxaparin, and heparin was dosed therapeutically in seven (24%) of the 29 patients on heparin. Twenty-eight (22%) of patients on therapeutic dose of enoxaparin expired, while only five (5%) of patients on prophylactic enoxaparin dose expired. Six patients (6/7, 86%) dosed therapeutically in the heparin group expired, while three (3/22, 14%) patients dosed prophylactically in the heparin group expired. Dose-dependent bleeding was significant for patients receiving enoxaparin (7.8% PPX vs. 17.2% therapeutic, P = 0.03). A similar dose-dependent bleed response was also observed for heparin, although the sample size in this group was much smaller than in the enoxaparin group. This finding was not statistically significant (19% vs. 43%, P = 0.18).
| Discussion | ▴Top |
From this retrospective observational study of hospitalized COVID-19 patients receiving pharmacological thromboprophylaxis, therapeutic-intensity anticoagulation did not provide any benefit on reducing the occurrences of VTE and mortality. VTE prevalence may be under detected due to institutional limitations and lowered use of computed tomography angiography (CTA)/ultrasound (US) during the peak of the pandemic. From the study of Mumoli et al [7], VTE prevalence was notably higher among their second COVID wave compared to the first wave (18.1% to 13.9%). While there may be several factors that affect VTE incidences, the higher use of imaging diagnostics during their second wave may attribute to increased incidences. Among the patients given PPX-intensity dosed anticoagulation, lower mortality rates were observed when compared to the TX-intensity anticoagulation group and experienced shorter hospital length of stay. The non-randomized observational design of this study means there is possible prescriber bias in the assignment of anticoagulation intensity. Although an “order set” with prescribed patient characteristics exists (Supplementary Material 1, www.thejh.org), ultimately the physician determined the intensity of anticoagulation and therefore the treatment groups in this study. We matched patients based on ICU admission and age; however, we could not match all previous existing conditions, and discrepancy between groups still exists. The optimization of anticoagulation management among COVID-19 patients remains a pressing issue due to increased rates of both microvascular and macrovascular thrombosis [8]. The anticipated number of higher bleeding events was expected in the therapeutic anticoagulation group due to higher doses. Most cases of bleeding episodes in the study were documented as GI bleeds. When patient factors were examined those treated with therapeutic anticoagulation in this study had a more severe presentation of COVID-19 and higher D-dimer levels at baseline. However, even in the setting of more complex patients, therapeutic doses of anticoagulation should be used with caution.
Cox regression analysis was used to look for predictors of incidence of VTE, mortality, and bleeding events. No predictors of VTE were found in our cohort; and no predictors of bleeding were found in our cohort. Predictors of mortality include ICU admission, COPD, age > 65, ventilator use and dose intensity; however, ICU admission and ventilator use are confounded as the interaction term containing both of them in the Cox regression model has an odds ratio of 0.46.
Parenteral anticoagulants such as heparin and enoxaparin are widely used for COVID-19-associated coagulopathy though differences between the two agents have yet to be thoroughly studied in this population. From our cohort, patients who were treated with enoxaparin displayed a dose dependent bleeding relationship. This association was also noted among those treated with heparin, but a significant difference was not detected due to a smaller sample size. This was an interesting finding when considering the results of the study of Pawlowski et al [9], which directly compared patients who received exclusively one anticoagulant (enoxaparin or UFH). The cohort who was only administered UFH had a higher 28-day mortality with a risk ratio of 4.3 [9].
In a recent meta-analysis that pooled safety and efficacy data from several studies irrespective to critical illness of hospitalized COVID patients, mortality benefits were not found with an escalated anticoagulation dose over standard prophylaxis doses [10]. Overall, with the findings of increased bleeds aligned with our study, the risks may outweigh benefits with therapeutic anticoagulation doses.
The Surviving Sepsis Campaign COVID-19 Guidelines has already conveyed their preference for enoxaparin over UFH due to less bleeding risk in non-COVID-19 patients [11]. The INSPIRATION trial evaluated intermediate enoxaparin dosing in the critically ill COVID-19 infected patients [12]. Main findings support the results as the escalated dosing did not improve the composite outcome of thrombosis, extracorporeal membrane oxygenation (ECMO), or mortality. Among other pertinent findings in the INSPIRATION trial, the rates of VTE in the study were low (3.3% in intermediate and 3.5% in standard); and there was a trend towards increased bleeding with the intermediate dose that was not statistically significant. In the recent ACTIV-4a randomized trial, a benefit was shown with full anticoagulation compared to prophylaxis dosed anticoagulation in non-critically ill COVID-19 patients; but within critically ill patients, full anticoagulation showed no benefits [13]. In this open labeled, randomized controlled clinical trial of 1,098 critically ill COVID-19 patients, patients were randomly assigned to receive therapeutic anticoagulation or pharmacological thromboprophylaxis. No statistical differences were observed among the results, in which percentage of patients who survived to hospital discharge was similar between the two groups (adjusted OR: 0.84, 95% CI: 0.64 - 1.11) [14]. In addition, from the multiplatform trials of REMMAP-CAP, ACTIV-4a, and ATTACC, the randomized control trial with the noncritically ill population (defined by the authors as absence of critical care-level organ support), discovered that an increased in therapeutic-dose anticoagulation increased was clinically beneficial in increasing the increased probability of survival until hospital discharge with reduced use of ICU-level organ support [15].
It should be acknowledged that there were several potential limitations from this study. First, The single-center retrospective study design of this study allows for inherent selection bias for the treatment groups. Also, the institution does not routinely screen patients for VTE which may result in unidentified cases. With any chart review study, the data collection is dependent on accurate documentation by the members of the healthcare team. Critically ill COVID-19 patients may only have documentation of clinically overt bleeding due to difficulties in conducting imaging studies. Another limitation includes changes in therapy throughout the course of the study. The time frame of the study was noteworthy as COVID-19 cases were at a peak in this region. Many institutions encountered national shortages on medications that were used in the management of COVID-19. It may also be possible that nonpharmacological patient care changes were made for patients as practitioners learned more about the disease over that time.
Conclusions
COVID-19 remains an ongoing, international public health crisis with thrombotic complications secondary to the infection being a significant concern on the spectrum of disease management. This single-centered retrospective study observed that the use of therapeutic anticoagulation for prophylaxis may be associated with negative outcomes among COVID-19 patients. Due to increased risk of bleeding with therapeutic intensity doses, the risk of thrombotic events may not justify the use of higher doses. Larger prospective trials are warranted to evaluate the efficacy and safety of therapeutic anticoagulant therapy.
| Supplementary Material | ▴Top |
Suppl 1. Institutional anticoagulation protocol for inpatient COVID-19 patients.
Acknowledgments
The authors thank PharmD candidates Kelly Desir and Ngoc-Han Pham and the hospital staff for their role in frontline management.
Financial Disclosure
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of Interest
All authors from this study declare no conflict of interest.
Informed Consent
Not applicable.
Author Contributions
Jimmy Huang aggregated data, analyzed data, reported findings, and wrote the manuscript. Daniel Diaz and Jenny Martinez constructed the study design, analyzed data, and reviewed and revised the manuscript. William Wolowich constructed the study design, performed the statistical analysis, analyzed the data, and reviewed and revised the manuscript.
Data Availability
Data supporting the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
BMI: body mass index; CI: confidence interval; CrCl: creatinine clearance; COVID-19: coronavirus disease 2019; CKD: chronic kidney disease; ICU: intensive care unit; IQR: interquartile range; LMWH: low-molecular-weight heparin; RT-PCR: reverse transcriptase-polymerase chain reaction; UFH: unfractionated heparin; aPTT: activated partial thromboplastin; VTE: venous thromboembolism
| References | ▴Top |
- Johns Hopkins University COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University. https://coronavirus.jhu.edu/map.html.
- Cui S, Chen S, Li X, Liu S, Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18(6):1421-1424.
doi pubmed - Klok FA, Kruip M, van der Meer NJM, Arbous MS, Gommers D, Kant KM, Kaptein FHJ, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145-147.
doi pubmed - Helms J, Tacquard C, Severac F, Leonard-Lorant I, Ohana M, Delabranche X, Merdji H, et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46(6):1089-1098.
doi pubmed - Thachil J, Juffermans NP, Ranucci M, Connors JM, Warkentin TE, Ortel TL, Levi M, et al. ISTH DIC subcommittee communication on anticoagulation in COVID-19. J Thromb Haemost. 2020;18(9):2138-2144.
doi pubmed - Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844-847.
doi pubmed - Mumoli N, Conte G, Cei M, Vitale J, Capra R, Rotiroti G, Porta C, et al. In-hospital fatality and venous thromboembolism during the first and second COVID-19 waves at a center opting for standard-dose thromboprophylaxis. Thromb Res. 2021;203:82-84.
doi pubmed - Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, Driggin E, Nigoghossian C, et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC State-of-the-Art review. J Am Coll Cardiol. 2020;75(23):2950-2973.
doi pubmed - Pawlowski C, Venkatakrishnan AJ, Kirkup C, Berner G, Puranik A, O'Horo JC, Badley AD, et al. Enoxaparin is associated with lower rates of mortality than unfractionated Heparin in hospitalized COVID-19 patients. EClinicalMedicine. 2021;33:100774.
doi pubmed - Ortega-Paz L, Galli M, Angiolillo DJ. Updated meta-analysis of randomized controlled trials on the safety and efficacy of different prophylactic anticoagulation dosing regimens in non-critically ill hospitalized patients with COVID-19. Eur Heart J Cardiovasc Pharmacother. 2022;8(3):E15-E17.
doi pubmed - Alhazzani W, Evans L, Alshamsi F, Moller MH, Ostermann M, Prescott HC, Arabi YM, et al. Surviving sepsis campaign guidelines on the management of adults with coronavirus disease 2019 (COVID-19) in the ICU: first update. Crit Care Med. 2021;49(3):e219-e234.
doi pubmed - Inspiration Investigators, Sadeghipour P, Talasaz AH, Rashidi F, Sharif-Kashani B, Beigmohammadi MT, Farrokhpour M, et al. Effect of intermediate-dose vs standard-dose prophylactic anticoagulation on thrombotic events, extracorporeal membrane oxygenation treatment, or mortality among patients with COVID-19 admitted to the intensive care unit: the INSPIRATION randomized clinical trial. JAMA. 2021;325(16):1620-1630.
doi pubmed - NIH ACTIV Trial of blood thinners pauses enrollment of critically ill COVID-19 patients. NIH News Releases. https://www.nih.gov/news-events/news-releases/nih-activ-trial-blood-thinners-pauses-enrollment-critically-ill-covid-19-patients.
- Remap-Cap Investigators. A. CTIV-4a Investigators, Attacc Investigators, Goligher EC, Bradbury CA, McVerry BJ, Lawler PR, et al. Therapeutic anticoagulation with heparin in critically ill patients with COVID-19. N Engl J Med. 2021;385(9):777-789.
doi pubmed - ATTACC Investigators; ACTIV-4a Investigators; REMAP-CAP Investigators, Lawler PR, Goligher EC, Berger JS, Neal MD, et al. Therapeutic anticoagulation with heparin in noncritically ill patients with COVID-19. N Engl J Med. 2021;385(9):790-802.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


