| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website http://www.thejh.org |
Original Article
Volume 2, Number 1, June 2013, pages 1-7
The Effect of Vitamin D3 on Hepcidin and IL-8 Expression in Monocytes
Sarah Jane Bell
Cardiff School of Health Sciences, Cardiff Metropolitan University, Llandaff Campus, Western Avenue, Cardiff, CF5 2YB, Wales UK
Manuscript accepted for publication April 22, 2013
Short title: Effect of Vitamin D3
doi: https://doi.org/10.4021/jh71e
| Abstract | ▴Top |
Background: It has been shown that optimal levels of vitamin D3 offer many health benefits and confer resistance to many diseases, notably those related to metabolic syndrome. Vitamin D3 status is of particular relevance in elderly populations wherein deficiency of this vitamin is increasingly being reported. Anaemia is also a common finding with approximately 10 percent of persons older than 65 years of age suffering from mild to moderate anaemia. In about 1/3 of these patients, the type of anaemia observed is anaemia of inflammation (AI) and is linked to the presence of high levels of the iron regulatory peptide hormone hepcidin. Hepcidin is antimicrobial peptide (AMP) structurally similar to other members of this family, in particular the defensins. Hepcidin negatively regulates iron absorption in the intestine and inhibits reticuloendothelial macrophages from releasing recycled iron. During periods of infection and inflammation, hepcidin levels increase due to the action of many cytokines including IL-8. Hepcidin has therefore a dual role in the immune response. It has been demonstrated that in monocytes, vitamin D3 is also able to induce the expression of the AMP cathelicidin due to the presence of a vitamin D3 response element (VDRE) in promoter regions of the cathelicidin (Camp) gene. It is however currently unknown whether vitamin D3 also plays a role in the signalling pathway that controls hepcidin expression.
Methods: In the present work, we address the possibility that vitamin D3 is able to modulate hepcidin expression by stimulating monocytic THP-1 cells with varying concentrations of vitamin D3, extracting mRNA and ascertaining hepcidin expression by RT-PCR and gel electrophoresis. We also postulate that vitamin D3 stimulation in these cells is able to regulate the expression of IL-8 and use the supernatant from cell culture to explore this hypothesis.
Results: The results presented here confirm previous reports that these cells constitutively express hepcidin mRNA and extend these observations by demonstrating that vitamin D3 can indeed modulate hepcidin expression. Modulation was dependent on the concentration of vitamin D3 used with maximal effect at 10 nM under the conditions used. Results also demonstrate that non-stimulated THP-1 supernatants release low levels of IL-8 and treatment with vitamin D3 at all concentrations tested reduced the levels of IL-8. A further observation was that IL-8 levels were very similar after 4 and 20 hours of vitamin D3 treatment, suggesting that the production and secretion of IL-8 happened at relatively early time points.
Conclusion: The present work has demonstrated that THP-1 cells when stimulated with higher concentrations of vitamin D3 (10 and 100 nM) decrease their expression of hepcidin mRNA while no change in the housekeeping gene GAPDH was observed. This downregulation of hepcidin mRNA expression may be translated in vivo with increases in serum iron and therefore may be potentially relevant in the treatment of AI/ACD. The present work also confirms previous reports that stimulation with vitamin D3 causes THP-1 cells to decrease the production of the pro-inflammatory cytokine IL-8, suggesting that vitamin D3 insufficiency may play a role in inflammatory disease and may offer new therapeutic options.
Keywords: Hepcidin; Monocytes; Anaemia of Chronic Inflammation; Vitamin D; Antimicrobial Peptide
| Introduction | ▴Top |
It has been shown that optimal levels of vitamin D3 offer many health benefits and confers resistance to many diseases, notably those related to metabolic syndrome- obesity, cardiovascular disease, diabetes mellitus and chronic inflammatory states [1-8].
The active form of vitamin D3 - 1, 25 (OH)2D3 affects the immune system and its components in different ways; in the case of monocytes and macrophages it has been shown that 1, 25 (OH)2D3 is able to decrease the production of pro-inflammatory cytokines (for example, IL-8) upon activation of innate immune receptors such as TLR4 by its specific ligand LPS or TLR2 by its specific ligand Pam3 [1-8], 1, 25 (OH)2D3 has also been shown to stimulate the maturation of monocytes into macrophages and this maturation is accompanied by the up-regulation of the TLR co-receptor molecule - CD14.
A new impetus in 1, 25 (OH)2D3 research in the past few years has revealed new roles for this vitamin. Although its role in regulation of bone and mineral metabolism has been known for many years, 1, 25 (OH)2D3 has been implicated in muscular strengthening, cellular proliferation and differentiation, inhibition of the rennin-angiotensin system and insulin secretion. Also of relevance for the present work it has been implicated in the modulation of the immune response. These findings have opened the way to more research on the clinical consequences of 1, 25 (OH)2D3 deficiency in particular at population level.
The 1, 25 (OH)2D3 status is of particular relevance in elderly populations wherein deficiency of this vitamin is increasingly being reported [9]. Importantly, in this elderly population, anaemia is also a common finding with approximately 10 percent of men and women older than 65 years of age suffering from mild to moderate anaemia. These levels increase even further with advancing age and by 85 years anaemia can affect 20% of this population [10].
Such relatively low/moderate levels of anaemia have, however, a significant impact on quality of life. These patients have a decline in physical performance, increased mortality due to acute myocardial infarction and poorer outcomes linked to heart failure [10]. Furthermore, in about 1/3 of these patients, the type of anaemia observed is anaemia of inflammation (AI) also called anaemia of chronic disease (ACD) and is linked to the presence of high levels of the iron regulatory peptide hormone hepcidin.
Hepcidin is a peptide hormone, discovered in 2000 which negatively regulates iron absorption in the intestine and inhibits reticuloendothelial macrophages from releasing recycled iron [1]. The regulatory mechanism involves the interaction of hepcidin with the only known iron exporter molecule - ferroportin - at the cell surface. Upon interaction, ferroportin is internalized and degraded and iron accumulates in the intracellular environment. Therefore, elevated levels of hepcidin in AI/ACD lead to iron sequestration in the spleen, reduced iron absorption by intestinal enterocytes, reduced iron supply to the bone marrow and the development of anaemia [9].
It is envisaged that during infection and inflammation, hepcidin levels increase in an attempt by the immune system to help in the fight against pathogens. However, the undesired consequence of elevated hepcidin levels in the circulation is the development of anaemia -AI/ACD [11].
AMPs are considered the “natural antibiotics” of the body and are currently attracting much attention due to the emergence of antibiotic resistant bacteria. AMPs are small, gene encoded amphipathic molecules, typically less than 100 amino acids in length, that participate in the immune response. There are now more than 12,000 known antimicrobial peptides [12]. Little resistance to AMP has been reported and for this reason AMPs are currently being researched. Because hepcidin is structurally related to other AMPs (in particular the defensins), it was hypothesized that hepcidin may also have a role in the immune system.
It has been demonstrated that in monocytes, 1, 25 (OH)2D3 is also able to induce the expression of the AMP cathelicidin due to the presence of a 1, 25 (OH)2D3 response element (VDRE) in promoter regions of the cathelicidin (Camp) gene [13]. Furthermore, cathelicidin appears to be induced in monocytes by stimulation of TLR1/2 with CD14 acting as a co-receptor [14]. Therefore, many signalling pathways are activated by 1, 25 (OH)2D3 leading to suppression of several pro-inflammatory cytokines. It is however currently unknown whether 1, 25 (OH)2D3 also plays a role in the signalling pathway that controls hepcidin expression. Furthermore, the observed association between AI/ACD and 1, 25 (OH)2D3 deficiency and their potential consequences have not been studied in detail.
In the present work, we have addressed the possibility that 1, 25 (OH)2D3 is able to modulate hepcidin expression.
| Materials and Methods | ▴Top |
Cell culture
The human monocytic cell line THP-1 was purchased from the European Collection of Animal Cell Culture (ECACC). Cells were grown in complete medium consisting of RPMI (Roswell Park Memorial Institute) 1640 medium (Invitrogen, UK), supplemented with 10% v/v foetal bovine serum (FBS; Biosera, UK). Cultures were maintained in tissue culture flasks (Greiner-BioOne, Germany) and incubated at 37 °C in a 5% CO2 incubator. The cells were passaged weekly to maintain exponential cell growth. Cell viability and cell counting used the Trypan Blue staining method.
Cell stimulation
THP-1 cells (1 × 106cells/ml in medium containing 5% FCS) were stimulated in 96-well plates (100 µL/well) with 1, 25 (OH)2D3 (Sigma-Aldrich) at three different concentrations (1, 10 and 100 nM) for the time points indicated in the corresponding figures. In control experiments 1, 25 (OH)2D3 was replaced with the corresponding volume of ethanol (diluent). All experiments were run in triplicates. After incubation, the cell culture supernatants were harvested and stored at -20 °C until IL-8 content was analysed by ELISA. Cell pellets were lyzed with TRIzol® Reagent (Invitrogen) and used for RNA isolation.
RT-PCR- RNA extraction
RNA was obtained by collecting the THP-1 cells (cells from triplicates were pooled into a single tube) and treating each cell pellet with 0.5 mL of TRIzol. After 5 minutes incubation at room temperature, 200 µL of chloroform were added, mixed well (approx. 15 second) and vials kept at room temperature for 3 minutes. The samples were then centrifuged in a pre-cooled micro-centrifuge at 4 °C for 15 minutes at 12,000 RCF. Following centrifugation, the upper aqueous phase was transferred into new tube and RNA was precipitated by adding 500 µL of isopropanol. After 10 minutes incubation, the centrifugation was repeated under the same conditions. Then, the supernatants were discarded and the pellets (RNA) washed with 1 mL of ice-cold 75% (v/v) ethanol/water and centrifuged at 4 °C for 5 minutes at 7,500 RCF. The washing procedure was repeated twice. Finally, the ethanol was removed and the pellets left to dry for approximately 30 minutes. The RNA pellets were dissolved in 50 µL of water (molecular biology water) and used immediately for preparation of cDNA or stored at -80 °C.
Determination of RNA purity and concentration
The RNA purity and concentration were assessed using spectrophotometry (Nanodrop, Thermoscientific UK). The absorbance of the samples was taken at 260 nm and 280 nm. The ratio 260 nm and 280 nm (A260/A280) provide an estimate of the RNA purity.
First strand cDNA synthesis using M-MLV RT
Thirty ng of total RNA were diluted to reach a final volume of 10 µL with molecular biology water. The complementary DNA (cDNA) was prepared in three steps by using Moloney Murine Leukaemia Virus (M-MLV) Reverse Transcriptase (RT) in PCR tubes. During the first step, 10 µL of diluted RNA (1 µg), 1 µL of random primers and 1 µL deoxyribonucleotide triphosphates mixture (dNTP; 10 mM) were added and the sample incubated for 5 minutes at 65 °C in the thermocycler (Peltier Thermal Cycler, DNA Engine DAYD, UK). In the second step, the sample was transferred from the thermocycler into ice and 4 µL of 5× buffer, 2 µL of 0.1 M Dithiothreitol (DTT) and 1 µL of RNAseOUT were added and the samples incubate for 2 minutes at 37 °C. In the third step, 1 µL of M-MLV RT was added and the samples incubated in the thermocycler for 50 minutes at 37 °C followed by 15 minutes at 70 °C (to inactivate the enzyme and finalise the reaction).
Polymerase chain reaction
One tenth of the RT reaction was used for amplification by adding 0.5 µL of the corresponding forward and reverse primers specific for hepcidin or the housekeeping gene GAPDH and 10 µL of Mastermix (Fermentas, Germany). The sizes of fragments obtained were 99 bp for GAPDH and 94 bp for hepcidin. Forward and reverse sequences are shown in Table 1.
 Click to view | Table 1. Forward and Reverse Sequences of the Primers Used in the RT-PCR |
The samples were transferred to the thermocycler and subject to an initial denaturation step for 5 minutes at 95 °C followed by 34 cycles under the following condition: annealing for 40 seconds at 60 °C, extension for 1 minute at 72 °C, followed by 60 seconds extension. A final 10 minutes step at 72 °C was also performed.
Analysis of RT-PCR product by gel electrophoresis
Gel electrophoresis was used to separate the RT-PCR fragments using a 2% agarose gel (UltraPure Agarose; Invitrogen). Before loading, samples were mixed with loading buffer (6× Gel Loading Dye; Sigma-Aldrich), which contains Bromophenol Blue as tracking dye. Furthermore, a PCR marker (Invitrogen, UK), was also loaded to allow the identification of the different bands according to their size. Electrophoresis was carried out for 1 hour at 60 V using Tris-Borate EDTA (TBE) buffer. After electrophoresis, the gel was stained with 0.5 mg/mL of ethidium bromide for 15 minutes and then washed with water for another 10 minutes. Finally, the gels were analysed and documented under UV light (GelDoc, Bio-Rad, USA). Gel densitometric analysis was performed using Quantity One software (BioRad).
Enzyme-linked immunosorbent assay (ELISA)
The supernatants obtained from the stimulation experiment, were analysed for IL-8 content by ELISA (DuoSet ELISA; R and D) following the manufacturer instructions in 96-well ELISA plates (Nunc-ImmunoMaxisorp, Denmark). Briefly, the wells on the ELISA plate were coated overnight with 100 mL of IL-8 coating antibody (diluted in phosphate buffer saline/PBS), sealed and incubated overnight at room temperature. The plate was then washed out 3 times with ELISA washing buffer (PBS supplemented with 0.05% Tween-20) and blocked with 300 mL/well of blocking buffer (1% bovine serum albumin/BSA in PBS). Plates were sealed and incubated for 1 hour at room temperature. The wells were then washed 3 times with ELISA washing buffer and then 100 mL of either sample or standards diluted in reagent diluent (Tris buffer supplemented with 0.1% w/v BSA) were applied to the appropriate wells. Plates were sealed and incubated at room temperature for 2 hours. The washing step was repeated and then 100 mL of biotinlylated anti-IL-8 detecting antibody was added to each well, the plate was sealed and incubated at room temperature for 2 hours. The washing step was repeated again, and 100 mL of Streptavidin-HRP at a dilution of 1/200 in reagent diluent were added to each well and incubated for 20 minutes at room temperature. The washing step was repeated again, and 100 mL of substrate SureBlueä TMB (Kirkegaard & Perry Laboratories, Inc.) were added to each well and the plate placed in a dark place and incubated for approximately 15 minutes. Once the colour was fully developed in the standards, 50 mL of 1M HCl were added to each well to stop the reaction. The results were then read in an ELISA reader (Tecan, UK) which determined the optical density at 450 nm as a indirect measure of IL-8 concentration in the samples.
| Results | ▴Top |
Hepcidin Expression by THP-1 Cells before and after treatment with 1, 25 (OH)2D3
It was the main objective of the present work to test the capacity of THP-1 monocytic cells to express hepcidin mRNA constitutively or upon treatment with 1, 25 (OH)2D3. To this aim, THP-1 cells were left untreated or were treated with 3 different concentrations of 1, 25 (OH)2D3 (1, 10 and 100 nM). In untreated control cultures 1, 25 (OH)2D3 was replaced with ethanol (the solvent used to dissolve the 1, 25 (OH)2D3 After 4 hours, cells were collected and hepcidin mRNA expression analyzed by RT-PCR.
Results presented in Figure 1 and Figure 2 show that under the culture condition used, THP-1 cells constitutively express hepcidin mRNA as the corresponding 94 bp band was clearly observed. Treatment with 1 nM 1, 25 (OH)2D3 only minimally altered this basal expression. However, a significant but differential down regulation of hepcidin mRNA was observed when cells were treated with 1, 25 (OH)2D3 at 10 and 100 nM.
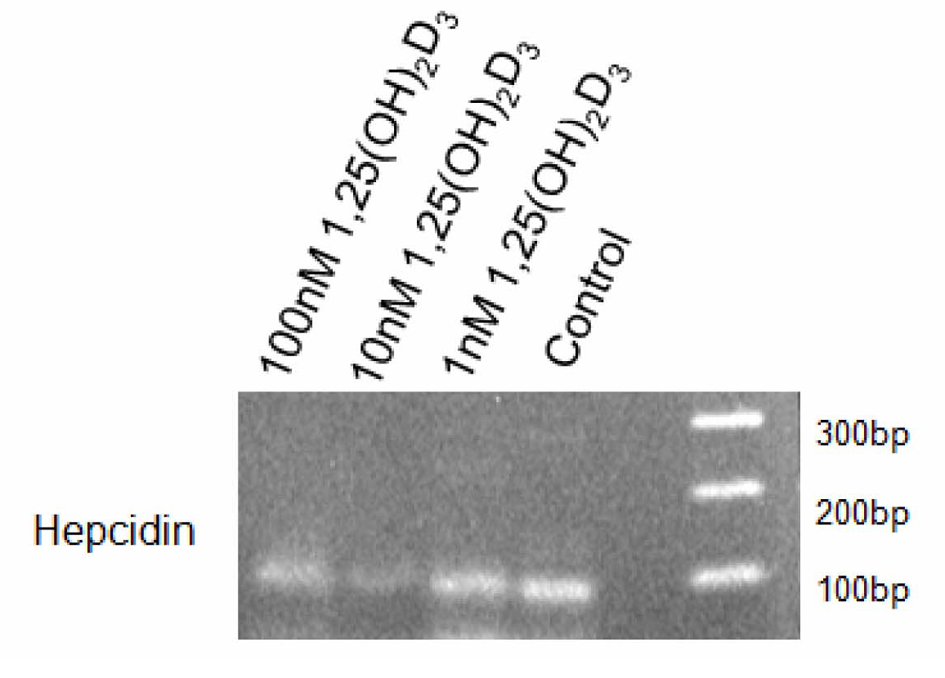 Click for large image | Figure 1. Hepcidin Expression in THP-1 Cells Treated With 1, 25 (OH)2D3. THP-1 cells (100,000 cells per well) were cultured in RPMI medium containing 5% FBS and stimulated with 1, 10 or 100 nM 1, 25 (OH)2D3 or diluent (control). After 4 hours, cells were collected, RNA isolated and hepcidin expression analysed by RT-PCR. Fragments were analysed by electrophoresis using a 2% agarose gel. |
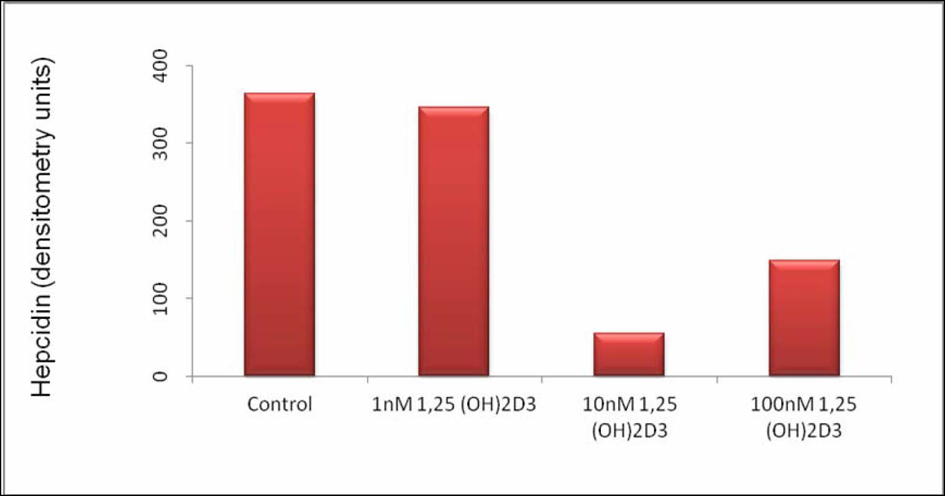 Click for large image | Figure 2. Hepcidin Expression in THP-1 Cells Stimulated With 1, 25 (OH)2D3. Hepcidin expression in arbitrary units after gel densitometry of Figure 1. |
Densitometric analysis shows a reduction on the intensity of the hepcidin band of approximately 86% when 1, 25 (OH)2D3 was used at 10 nM and of approximately 60% when 1, 25 (OH)2D3 was used at 100 nM. These results suggest that at the time point selected, the intermediate concentration of 1, 25 (OH)2D3 used has the strongest effect down-regulating hepcidin mRNA.
Further work will be needed to test whether, under the conditions used analysis at earlier time points (less than 4 hours) and using higher concentrations of 1, 25 (OH)2D3 are more efficient at suppressing hepcidin mRNA. It should be noted that no changes in the housekeeping gene GAPDH were observed after 1, 25 (OH)2D3 stimulation (data not shown).
Production of IL-8 by THP-1 cells treated with 1, 25 (OH)2D3
Hepcidin expression has been linked to cytokine production. In particular in the case of hepatocytes, it has been demonstrated that cytokines are able to upregulate hepcidin expression [13].
To determined if cytokines could be involved in the downregulation of hepcidin mRNA observed in the experiments described above, culture supernatants of 1, 25 (OH)2D3 stimulated cells were analyzed for cytokine content by ELISA. The cytokine studied was IL-8 as this chemokine has an important role in attracting immune cells - in particular neutrophils - to sites of inflammation.
Results shown in Figure 3 demonstrate that non-stimulated THP-1 supernatants release low levels of IL-8 and treatment with 1, 25 (OH)2D3 at all concentrations tested reduced the levels of IL-8. However, it should be noted that IL-8 levels were very low under all conditions tested. A further observation was that IL-8 levels were very similar after 4 and 20 hours of 1, 25 (OH)2D3 treatment, suggesting that the production and secretion of IL-8 happened at relatively early time points.
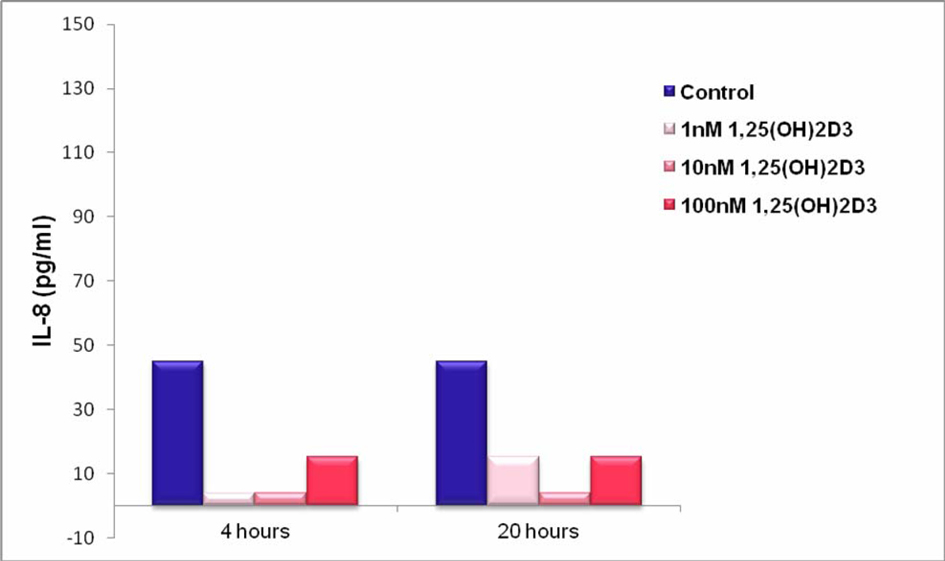 Click for large image | Figure 3. IL-8 Production by THP-1 Cells Stimulated in the Absence and Presence of 1, 25 (OH)2D3. THP-1 cells (100,000 cells per well) were cultured in RPMI medium containing 5% FBS) and stimulated with 1, 25 (OH)2D3 at 1, 10 and100 nM or diluent (control). After 4 and 20 hours, supernatants were collected and analysed for IL-8 content by ELISA. |
| Discussion | ▴Top |
Prior to this study, it was acknowledged that hepcidin levels in the circulation are mainly determined by its production and release by hepatocytes. However, other cells of the immune system such as monocytes, macrophages and lymphocytes [15] as well as other tissues like the pancreas [16] and the stomach [17] can also express hepcidin. The contribution of these alternative sources to the overall levels of systemic hepcidin is at present unknown, but it is believed that the production of hepcidin by these cells is low and therefore only capable of affecting the local microenvironment, influencing localized responses and regulating iron levels at the site of infection.
Several investigators have demonstrated that AMP expression can be modulated by 1, 25 (OH)2D3. This important vitamin has many effects on the immune system including its capacity to increase macrophage differentiation and phagocytosis and to suppress pro-inflammatory responses. However, little is known about the capacity of 1, 25 (OH)2D3 to regulate hepcidin expression.
The present work has addressed this possibility by looking at the effect of 1, 25 (OH)2D3 on hepcidin mRNA expression on monocytic cells. Initial experiments tested whether THP-1 monocytic cells constitutively express hepcidin mRNA. The results presented here show this to be the case, as hepcidin mRNA was clearly detected by semi-quantitative RT-PCR.
Of particular relevance to the present work, it has been demonstrated that 1, 25 (OH)2D3 is a direct inducer of the AMP cathelicidin in human monocytes, keratinocytes, and neutrophils [13], suggesting a potential effect of 1, 25 (OH)2D3 on modulating the expression of other AMPs including hepcidin.
Results presented here show this to be the case. However, contrary to what was observed with cathelicidin, 1, 25 (OH)2D3 downregulated hepcidin mRNA expression suggesting that cathelicidin and hepcidin are regulated differently by 1, 25 (OH)2D3.
In the present work, the maximal effect on hepcidin mRNA expression was observed when 1, 25 (OH)2D3 was used at 10 nM - with a reduction on the intensity of the hepcidin band of approximately 86%. When a lower concentration of 1, 25 (OH)2D3 was used (1 nM) no effect on hepcidin mRNA expression was observed and using a higher concentration (100 nM), the down modulatory effect although still observed was smaller, with a reduction on the intensity of the hepcidin band of approximately 60%. Therefore, the results presented here and those of other investigators point to an optimal concentration of 1, 25 (OH)2D3 during monocyte to macrophage differentiation and hepcidin mRNA modulation. More work will be needed to elucidate the threshold for maximum effect of 1, 25 (OH)2D3 on hepcidin expression.
It has been reported that 1, 25 (OH)2D3 upregulates the expression of the MAPK phosphatases resulting in decrease expression of inflammatory cytokines [3]. Based on these observations, the final part of the present project looked at the expression of the pro-inflammatory cytokine IL-8 upon treatment of THP-1 cells with 1, 25 (OH)2D3.
The results presented show that non-stimulated THP-1 supernatants release low levels of IL-8 and treatment with 1, 25 (OH)2D3 - at all concentrations tested - reduced the levels of IL-8. However, it should be noted that IL-8 levels were low under all conditions tested. A further observation was that IL-8 levels were very similar after 4 and 20 hours after1, 25 (OH)2D3 treatment, suggesting that the production and secretion of IL-8 happened at relative early time points.
Further work will be needed to assess the role of 1, 25 (OH)2D3 on hepcidin mRNA expression during inflammatory conditions that can be mimicked by in vitro stimulation of THP-1 cells with microbial components like the TLR4-specific agonist LPS.
The induction of hepcidin is believed to have a similar mechanism to that of cathelicidin. Macrophage hepcidin expression is controlled by the action of STAT1, NF-κB and C/EBPβ increasing hepcidin promoter activity, which acts synergistically with IFN-γ and M. tuberculosis and also TLR2 and TLR4 [18]. It should be noted that in the present study the expression of TLR2 and TLR4 mRNA on THP-1 cells before and after treatment with 1, 25 (OH)2D3 was tested and that no changes were observed (data notshown).
The relationship between monocytes, 1, 25 (OH)2D3 and IL-8 has been shown to be important in vivo. Repletion of 1, 25 (OH)2D3 in patients with 1, 25 (OH)2D3 deficiency and end stage renal disease (ESRD) resulted in a decrease in IL-8, IL-6 and TNF-α levels in the circulation [19]. These results are important as these markers have been linked to increased morbidity and mortality in this patient population and suggest that nutritional therapy with 1, 25 (OH)2D3 has a biologic effect on circulating monocytes and associated inflammatory markers in patients with ESRD.
The results presented can be particularly relevant when looking at diseases such AI/ACD where a primary pathology causes inflammatory cytokines to be produced resulting in an increase in hepcidin expression.
As 1, 25 (OH)2D3 may suppresses inflammatory pathways, future studies will be needed to expand these in vitro studies to determine if in vivo 1, 25 (OH)2D3 supplementation ameliorates conditions linked to high levels of hepcidin like AI/ACD.
In summary, the present work has demonstrated that THP-1 cells when stimulated with higher concentrations of 1, 25 (OH)2D3 (10 and 100 nM) decrease their expression of hepcidin mRNA while no change in the housekeeping gene GAPDH was observed. This downregulation of hepcidin mRNA expression may be translated in vivo with increases in serum iron and therefore may be potentially relevant in the treatment of AI/ACD.
The present work also confirms previous reports that stimulation with 1, 25 (OH)2D3 causes THP-1 cells to decrease the production of the pro-inflammatory cytokine IL-8, suggesting that 1, 25 (OH)2D3 insufficiency may play a role in inflammatory disease and may offer new therapeutic options.
| References | ▴Top |
- Schoenmakers I, Goldberg GR, Prentice A. Abundant sunshine and vitamin D deficiency. Br J Nutr. 2008;99(6):1171-1173.
doi pubmed - Hintzpeter B, Mensink GB, Thierfelder W, Muller MJ, Scheidt-Nave C. Vitamin D status and health correlates among German adults. Eur J Clin Nutr. 2008;62(9):1079-1089.
doi pubmed - Zhang Y, Leung DY, Richers BN, Liu Y, Remigio LK, Riches DW, Goleva E. Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. J Immunol. 2012;188(5):2127-2135.
doi pubmed - Baarnhielm M, Hedstrom AK, Kockum I, Sundqvist E, Gustafsson SA, Hillert J, Olsson T, et al. Sunlight is associated with decreased multiple sclerosis risk: no interaction with human leukocyte antigen-DRB1*15. Eur J Neurol. 2012;19(7):955-962.
doi pubmed - Litonjua A. Vitamin D deficiency as a risk factor for childhood allergic disease and asthma. Current Opinion in Allergy in Clinical Immunology. 2012;12(2):179-185.
doi pubmed - Weinstein SJ, Stolzenberg-Solomon RZ, Kopp W, Rager H, Virtamo J, Albanes D. Impact of circulating vitamin D binding protein levels on the association between 25-hydroxyvitamin D and pancreatic cancer risk: a nested case-control study. Cancer Res. 2012;72(5):1190-1198.
doi pubmed - Churilla T, Lesko S, Brereton H, Klem M, Donnelly P, Peters C. Serum vitamin D levels among patients in a clinical oncology practice compared to primary care patients in the same community: a case-control study. The British Medical Journal. 2011;1(2):1-7.
- Thill M, Cordes T, Hoellen F, Becker S, Dittmer C, Kummel S, Salehin D, et al. Influence of calcitriol on prostaglandin- and vitamin D-metabolising enzymes in benign and malignant breast cell lines. Anticancer Res. 2012;32(1):359-365.
pubmed - Perlstein TS, Pande R, Berliner N, Vanasse GJ. Prevalence of 25-hydroxyvitamin D deficiency in subgroups of elderly persons with anemia: association with anemia of inflammation. Blood. 2011;117(10):2800-2806.
doi pubmed - Schrier SL. Hematology, ASH, and the anemia of the aged. Blood. 2005;106(10):3341-3342.
doi pubmed - Theurl I, Schroll A, Sonnweber T, Nairz M, Theurl M, Willenbacher W, Eller K, et al. Pharmacologic inhibition of hepcidin expression reverses anemia of chronic inflammation in rats. Blood. 2011;118(18):4977-4984.
doi pubmed - Pasupuleti M, Schmidtchen A, Malmsten M. Antimicrobial peptides: key components of the innate immune system. Critical Reviews in Biotechnology. 2011.[Online] Available: http://informahealthcare.com/doi/abs/10.3109/07388551.2011.594423 [01/03/2012].
- Wang TT, Nestel FP, Bourdeau V, Nagai Y, Wang Q, Liao J, Tavera-Mendoza L, et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol. 2004;173(5):2909-2912.
pubmed - Shin D, Yuk J, Lee S, Son J, Harding C, Kim J, Modlin R, et al. Mycobacterial lipoprotein activates autophagy via TLR2/1/CD14 and a functional vitamin D receptor signalling. Cell Microbiology. 2010;12(11):1-12.
- Pinto JP, Dias V, Zoller H, Porto G, Carmo H, Carvalho F, de Sousa M. Hepcidin messenger RNA expression in human lymphocytes. Immunology. 2010;130(2):217-230.
doi pubmed - Kulaksiz H, Fein E, Redecker P, Stremmel W, Adler G, Cetin Y. Pancreatic beta-cells express hepcidin, an iron-uptake regulatory peptide. J Endocrinol. 2008;197(2):241-249.
doi pubmed - Schwarz P, Kubler JA, Strnad P, Muller K, Barth TF, Gerloff A, Feick P, et al. Hepcidin is localised in gastric parietal cells, regulates acid secretion and is induced by Helicobacter pylori infection. Gut. 2012;61(2):193-201.
doi pubmed - Sow FB, Alvarez GR, Gross RP, Satoskar AR, Schlesinger LS, Zwilling BS, Lafuse WP. Role of STAT1, NF-kappaB, and C/EBPbeta in the macrophage transcriptional regulation of hepcidin by mycobacterial infection and IFN-gamma. J Leukoc Biol. 2009;86(5):1247-1258.
doi pubmed - Stubbs JR, Idiculla A, Slusser J, Menard R, Quarles LD. Cholecalciferol supplementation alters calcitriol-responsive monocyte proteins and decreases inflammatory cytokines in ESRD. J Am Soc Nephrol. 2010;21(2):353-361.
doi pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.