| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website http://www.thejh.org |
Case Report
Volume 2, Number 1, June 2013, pages 48-50
Hemorrhagic Transformation of Ischemic Stroke in Patient Treated With Rivaroxaban
Robert Feketea, c, Dhruve S Jeevanb, Stephen J Marksa, Virany H Hillardb
aDepartment of Neurology, New York Medical College, Valhalla, NY, USA
bDepartment of Neurosurgery, New York Medical College, Valhalla, NY, USA
cCorresponding author: Robert Fekete, Department of Neurology, New York Medical College, Munger Pavilion, 4th Floor, 40 Sunshine Cottage Road, Valhalla, NY 10595, USA
Manuscript accepted for publication May 27, 2013
Short title: Hemorrhagic Transformation of Ischemic Stroke
doi: https://doi.org/10.4021/jh78w
| Abstract | ▴Top |
Rivaroxaban (BAY 59-7939) is an oral factor Xa inhibitor used for stroke prevention in atrial fibrillation. There are currently no evidence based guidelines for treatment of hemorrhagic side effects of factor Xa inhibitors. We report a case of delayed hemorrhagic conversion of an ischemic infarct in a 60-year-old male treated with rivaroxaban for stroke prevention in a setting of paroxysmal atrial fibrillation. The patient presented with generalized discomfort and malaise as well as worsening of expressive aphasia from a prior infarct. In addition, right superior quadrantanopsia was present. CT head at 140 minutes and 8 hours following symptom onset revealed confluent petechiae and linear gyral hyperintensities of the left temporal lobe without mass effect, classified as type 2 Hemorrhagic Transformation (HI2). The patient received a single unit of fresh frozen plasma (FFP) and three doses of Vitamin K. He remained clinically stable without need for neurosurgical intervention, and was subsequently discharged with a plan to commence aspirin therapy five days after onset and switch to dabigatran two weeks later for management of paroxysmal atrial fibrillation. Hemorrhagic transformation in this case remained clinically stable following conservative therapy, likely due to pharmacological clearance of rivaroxaban. Prothrombin complex concentrates may be considered for emergent reversal of rivaroxaban.
Keywords: Rivaroxaban; Factor Xa; Prothrombin complex concentrates; BAY 59-7939
| Introduction | ▴Top |
Rivaroxaban (BAY 59-7939) is an oral factor Xa inhibitor used for stroke prevention in the setting of atrial fibrillation. There are currently no evidence-based guidelines for the treatment of hemorrhagic complications of factor Xa inhibitors. We report a case of delayed hemorrhagic conversion of an ischemic infarct in a patient treated with Rivaroxaban.
| Case Report | ▴Top |
A 60-year-old right-handed African-American male with history of paroxysmal atrial fibrillation, hypertension, gout, focal segmental glomerulosclerosis, and prior hemorrhagic stroke resulting in chronic abdominal pain, presented with an acute ischemic stroke to an outside institution. At this time the patient’s examination revealed a right facial droop and significant hemiparesis, with an expressive aphasia. His weakness improved within one hour of symptom onset, but expressive aphasia persisted. At this time he was not a candidate for intravenous thrombolysis given the rapidity of symptom improvement. MRI imaging attained at this time confirmed a large area of left-fronto-temporal restricted diffusion consistent with acute infarction, with no evidence of hemorrhage. Noting his failure of therapy on a combination of aspirin 81 mg daily, nebivolol, and dromedarone for paroxysmal atrial fibrillation, the patient was subsequently placed on rivaroxaban 15 mg daily for further stroke prevention.
The patient presented 16 days following this event to the emergency room with a complaint of warm sensation and general malaise at 3 PM, about six hours following his morning dose of rivaroxaban. His residual expressive aphasia worsened. There were no new motor or sensory complaints.
Examination revealed right superior quadrantanopsia as well as expressive aphasia with the ability to name “glasses” and “pen”. To name keys, he used the phrase “You are my keys,” and when shown a twenty-dollar bill, he named it “thirty minted drill.” He was able to follow simple one step commands. Sentences were brief and agrammatical such as “We did right well. I said we it. It we stopped. I thought it low.” Repetition of “no ifs, ands, or buts” was impaired and yielded “no bet and best.” There was no motor drift, and no slowing of fast finger movements. There was no hyperreflexia noted in the previously affected right side. At one-hour post onset, PT, INR, and PTT values were 18.0, 2.5, and 32.5 respectively. Platelet count was 143,000 per mL. Non-contrast CT imaging demonstrated cortical hyperintensity (Fig. 1A) at 140 minutes after onset of symptoms consistent with hemorrhagic conversion of left temporal ischemic stroke.
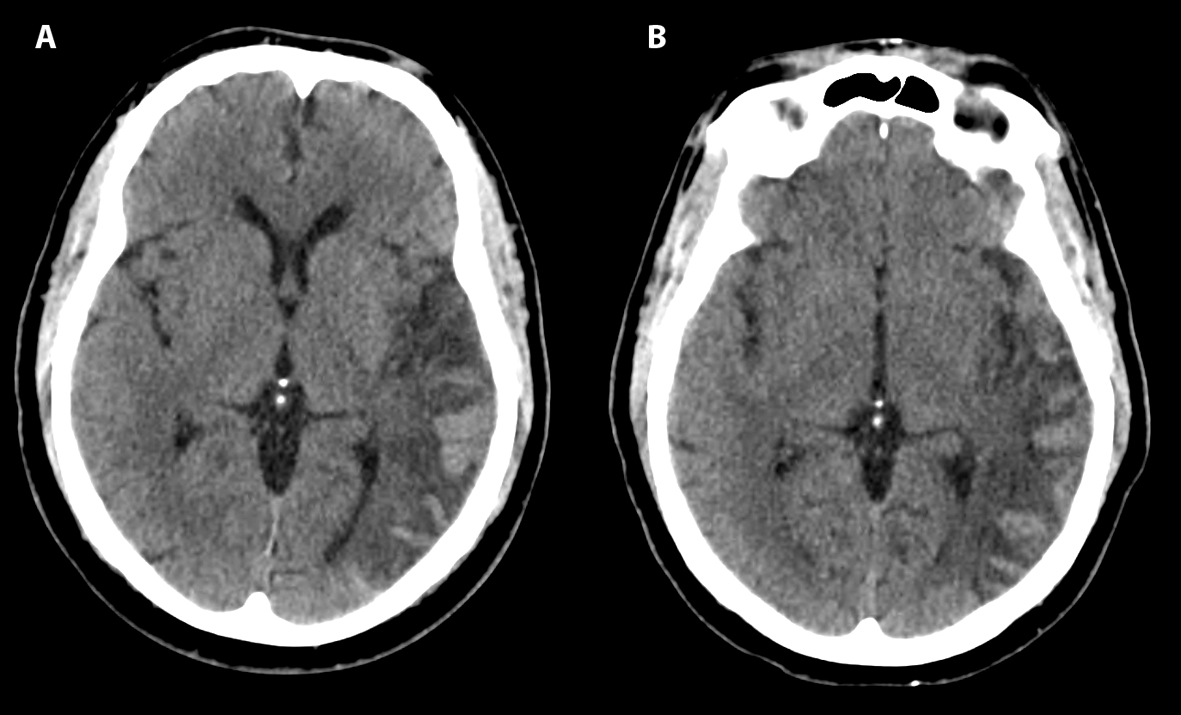 Click for large image | Figure 1. Axial CT head shows hemorrhagic conversion of left temporal ischemic stroke at 140 minutes (A) and 8 hours (B) after onset of symptoms, classified as HI2 according to ECASS criteria. |
The patient received levetiracetam for seizure prophylaxis and one unit of fresh frozen plasma, along with a 10 mg subcutaneous dose of phytonadione (Vitamin K). Subsequent coagulation studies revealed a PT of 14, PTT of 38.9 and an INR of 1.3, approximately 4 hours after symptom onset. Repeat CT scan 8 hours after symptom onset was grossly unchanged (Fig. 1B). According to European Cooperative Acute Stroke Study (ECASS) criteria, the findings were classified as HI2 - confluent petechial hemorrhages and linear hyperintensities without mass effect [1]. Phytonadione 10 mg subcutaneously was also administered on the second and third hospital days, with an INR of 0.99 noted 48 hours following admission. There was mild improvement of aphasia and the patient was subsequently discharged to rehabilitation on the fourth hospital day. Aspirin 81 mg daily was initiated at five days after symptom onset with plan to switch to dabigatran therapy two weeks later for treatment of paroxysmal atrial fibrillation.
| Discussion | ▴Top |
We demonstrate hemorrhagic transformation (HT) of an ischemic infarct 16 days following treatment with rivaroxaban for paroxysmal atrial fibrillation related ischemic infarct. Imaging classification of hemorrhagic conversion was stated at grade HI2 (without mass effect) according to ECASS criteria [1]. As the patient’s main presenting complaint was of generalized malaise and warmth sensation, it remains possible that HT was actually asymptomatic with onset earlier than 16 days post ischemic stroke. In a prospective study of HT by Horning et al, 17%, 23%, and 3% of ischemic stroke patients were found to have onset of HT in weeks 1, 2, and 3 respectively [1, 2]. Rate of HT in the placebo group of ECASS II was 36.8% and cardioembolic source is frequently associated in HT [1]. In the Rivaroxaban versus Warfarin in Nonvalvular Atrial Fibrillation (ROCKET-AF) trial, intracranial hemorrhage rate of 0.5% on rivaroxaban versus 1.2% on warfarin (p = 0.02) was reported. Hemorrhagic stroke rate was 0.41% on rivaroxaban versus 0.71% on warfarin (p = 0.024), but rate of HT of ischemic stroke was not reported [3].
Warfarin is the most frequently used anticoagulant for the prevention of thromboembolic events in the setting of atrial fibrillation. Atrial fibrillation is a causative factor in 15-25% of all ischemic strokes [4]. Direct Factor IIa or Xa inhibition provides an appealing alternative to repetitive monitoring and uncertain dosing of warfarin in light of dietary and drug interactions. Similar stroke prevention rates and reduced overall hemorrhagic complications have been reported for these agents [3]. Rivaroxaban may also have a future role in prevention of deep venous thrombosis in post-operative neurosurgical patients [5]. Guidelines and treatment strategies for hemorrhagic complications of warfarin exist, but there is a paucity of guidance for treatment of acute complications of oral direct factor inhibitors, with lack of antidotes.
In this case, neurosurgical intervention was not necessary. If this patient’s hemorrhage worsened, we would be faced with difficult task of determining the safety of potential surgical intervention as there is no proven method of reversing the effect of Rivaroxaban. This patient was beyond the time frame for use of activated charcoal to reduce gastrointestinal tract absorption.
The half-life of rivaroxaban ranges between 5 - 9 hours to 11 - 13 hours in patients above 75 years old [6]. While PT/INR and PTT may be elevated in the setting of rivaroxaban use, there is no commercially available assay with which the current level of anti-coagulation may be accurately followed. Kaatz et al report that PCC are helpful as an antidote to rivaroxaban, but have thrombotic complications [7]. Rivaroxaban’s effect was completely reversed by PCC as measured by normalization of both PT and endogenous thrombin potential (ETP) in one prospective study [8]. There have been no human studies of recombinant factor VIIa in setting of rivaroxaban use and in animal studies it provides relatively mild reduction of bleeding, independent of rivaroxaban’s interaction with factor Xa [7]. In addition, there is concern about pro-thrombotic side effects of recombinant factor VIIa. Administration of PCC appears to be the only realistic approach to reverse the effect of rivaroxaban given current clinical data.
In our own patient stability of hemorrhage was likely due to clearance of rivaroxaban as opposed to the direct effect of FFP or Vitamin K. Reversal of rivaroxaban’s effect with FFP has not been evaluated in human or animal studies [7]. Murine FFP reduced intracranial hemorrhage volume but did not improve mortality in a mouse model of intracranial hemorrhage in setting of high dose dabigatran (direct Factor IIa inhibitor). Given the need to overcome direct factor inhibition by dabigatran or rivaroxaban, Kaatz et al posit that FFP is unlikely to be helpful in emergent reversal of these agents [7]. Tranexemic acid has been shown to decrease blood loss during orthopedic operations on patients treated with rivaroxaban, but further studies are needed to assess this effect [7].
The case highlights the management decisions faced with the use of these novel anticoagulants and the problems faced from lack of a suitable antidote in a patient that may need surgical decompression. Prothrombin complex concentrates may be considered for emergent reversal of rivaroxaban.
Funding Source
None.
Disclosures
RF served as consultant for Lundbeck, LLC., and Teva Neuroscience, Inc. and received honoraria from Medlink, Inc. DSJ has not disclosures. SJM served as consultant for Boehringer Ingelheim, Inc. VHH has no disclosures.
| References | ▴Top |
- Thanvi BR, Treadwell S, Robinson T. Haemorrhagic transformation in acute ischaemic stroke following thrombolysis therapy: classification, pathogenesis and risk factors. Postgrad Med J. 2008;84(993):361-367.
doi pubmed - Hornig CR, Dorndorf W, Agnoli AL. Hemorrhagic cerebral infarction—a prospective study. Stroke. 1986;17(2):179-185.
doi pubmed - Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883-891.
doi pubmed - Marini C, De Santis F, Sacco S, Russo T, Olivieri L, Totaro R, Carolei A. Contribution of atrial fibrillation to incidence and outcome of ischemic stroke: results from a population-based study. Stroke. 2005;36(6):1115-1119.
doi pubmed - Komotar RJ, Starke RM, Connolly ES, Jr. Orally administered factor xa inhibitor, rivaroxaban: a novel thromboembolic prophylaxis agent. Neurosurgery. 2008;63(4):N10-11.
doi pubmed - Shamoun FE, Martin EN, Money SR. The novel anticoagulants: the surgeons' prospective. Surgery. 2013;153(3):303-307.
doi pubmed - Kaatz S, Kouides PA, Garcia DA, Spyropolous AC, Crowther M, Douketis JD, Chan AK, et al. Guidance on the emergent reversal of oral thrombin and factor Xa inhibitors. Am J Hematol. 2012;87(Suppl 1):S141-145.
doi pubmed - Eerenberg ES, Kamphuisen PW, Sijpkens MK, Meijers JC, Buller HR, Levi M. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo-controlled, crossover study in healthy subjects. Circulation. 2011;124(14):1573-1579.
doi pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


