| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website http://www.thejh.org |
Case Report
Volume 6, Number 4, October 2017, pages 105-108
Two Novel Monoallelic Calreticulin Mutations in a Patient With Essential Thrombocythemia
Eniko Kamorya, d, Thomas Schmidtb, Cedric Broquerea, Hartmut Petersc, Berthold Hochera
aIFLb Laboratoriumsmedizin Berlin GmbH, Windscheidstraße 18, 10627 Berlin, Germany
bHematology and Oncology, Stralsund, Germany
cInstitute of Medical Genetics and Humangenetics, Charite, Berlin, Germany
dCorresponding Author: Eniko Kamory, IFLb Laboratoriumsmedizin Berlin GmbH, Fontanestrasse 12, 14193 Berlin, Germany
Manuscript submitted July 4, 2017, accepted August 30, 2017
Short title: Two CALR Mutations in Patient With ET
doi: https://doi.org/10.14740/jh335w
| Abstract | ▴Top |
Recently, mutations have been identified in the calreticulin (CALR) gene in JAK2 or myeloproliferative leukemia negative patients with myeloproliferative neoplasm. A 49-year-old male patient with incidental thrombocytosis was investigated for CALR mutation by direct sequencing method. The patient carried two novel monoallelic somatic mutations, the L367fs*52 and the p.R368W in the CALR gen, which resulted in a novel C-terminal sequence. The absent endoplasmatic reticulum retention signal in the mutant CALR results in an altered subcellular localization of the mutant protein. The new positively charged C-terminal domain has an importance for oncogenicity, effecting different signaling pathways, activating the cytokine-independent growth of the cells and down-regulating the apoptotic signaling. But the new, alternative C-terminal domain offers an opportunity for immunologic therapy as it represents a cancer-specific epitope.
Keywords: Calreticulin; Essential thrombocythemia; Thrombocytosis; Monoallelic somatic mutations
| Introduction | ▴Top |
The World Health Organization (WHO) classified the myeloid malignancies in five major categories [1]. Myeloproliferative neoplasms (MPNs) are a clonal disease of myeloid stem cells that are characterized by myeloid cell proliferation, bone marrow fibrosis, and symptoms associated with the accompanying peripheral blood cell abnormalities. From the heterogenous group of classical Philadelphia-chromosome-negative MPNs, nearly all polycythemia vera (PV) cases carried a JAK2 mutation, most commonly p.V617F. Meanwhile, only approximately 50-60% of cases with essential thrombocythemia (ET) and primary myelofibrosis (PMF) are associated with JAK2 p.V617F mutation, whereas 5-10% are associated with mutations in MPL [2, 3].
Recently, somatic mutations in the calreticulin (CALR) gene, which encodes the calcium-regulating protein CALR, have been identified in most wild-type JAK2 and myeloproliferative leukemia (MPL) patients with ET or PMF [4-6]. Nearly all reported mutations are insertions or deletions in exon 9 of the CALR gene. Most commonly, a 52-base pair (bp) deletion (type I mutation, p. L367fs*46) or a 5-bp insertion (type II mutation, p.K385fs*47) is detected. Although the reported mutations are variable, they collectively produce a 1-bp frameshift that results in a mutant protein with a novel C-terminus, eliminating the KDEL amino acid sequence required for endoplasmatic reticulum retention [4, 5, 7].
| Case Report | ▴Top |
The patient is a 49-year-old male who presented to hematology with incidental thrombocytosis with a history of psoriasis arthritis. The patient was cured for the psoriasis arthritis with prednisolone and then methotrexate therapy. As a result of the therapy, the patient had elevated liver enzymes (GOT, GPT, and GGT) and he developed diabetes mellitus and hyperlipidemia. The patient had no thrombosis before the diagnosis. Beside the thrombocytosis, the patient’s blood showed inflammation (with elevated CRP, ferritin level). The patient showed elevated level of thrombocyte (780 × 109/L), leukocyte (14.8 × 109/L) and reticulocyte (2.55 × 109/L), but normal level of hemoglobin. The lymphocyte level was sank (13%). The patient carried neither JAK2 nor MPL mutation.
For analysis of CALR mutation, the genomic DNA was extracted from blood by using the QIAamp DNA Mini Kit (QIAGEN GmbH, Hilden, Germany) according to the manufacturer’s instruction.
Oligonucleotide primers targeting exon 9 of CALR were used to amplify a 513-bp product. The primers were CALR-forward: 5′-gcctggtcctggtcctga-3′ and CALR-reverse: 5′-ggtgagggctgaaggagaat-3′. About 50 ng genomic DNA was amplified by using FIREPol Mastermix (Solis BioDyne, Tartu, Estonia). The PCR amplification protocol was the following: an initial 3-min denaturation step at 94 °C followed by 30 cycles of 94 °C for 30 s, 58 °C for 40 s and 72 °C for 50 s, and a final 4-min extension at 72 °C.
The PCR product was purified and bi-directionally sequenced using BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, CA, USA) on the ABI 3130 DNA Analyser (Applied Biosystems) using the above described forward primer and a sequencing reverse primer: 5′-aaaagggcggggagggggtg-3′.
Sanger sequencing revealed two separate mutations within exon 9, a 34-bp deletion and a nucleic acid change occurring 3-bp downstream of the deletion (Fig. 1). The 34 bp-deletion occurs between the 1100 and 1133 coding sequence, resulting in L367fs*52 mutation in the amino acid. The nucleic acid change occurs in the original c.1136 position, where the A nucleotide of one allele changed to T nucleotide. Sequencing data indicate that the two alterations occur on the same allele of the CALR, since the third base on the allele with deletion (c.1102, which was before the deletion c.1136) after the deletion start in forward direction is an A/T instead of an A, resulting in an amino acid change at the second position on the novel C-terminal sequence, a tryptophan instead of an arginine (AGG>TGG, on the mutant allele p.R368W). The novel C-terminal sequence has the same length as the original version (Fig. 2). This mutant contains all the mutant amino acid sequence of the type I mutant L367fs*46 and six additional mainly positively charged amino acids before (RWRRQR).
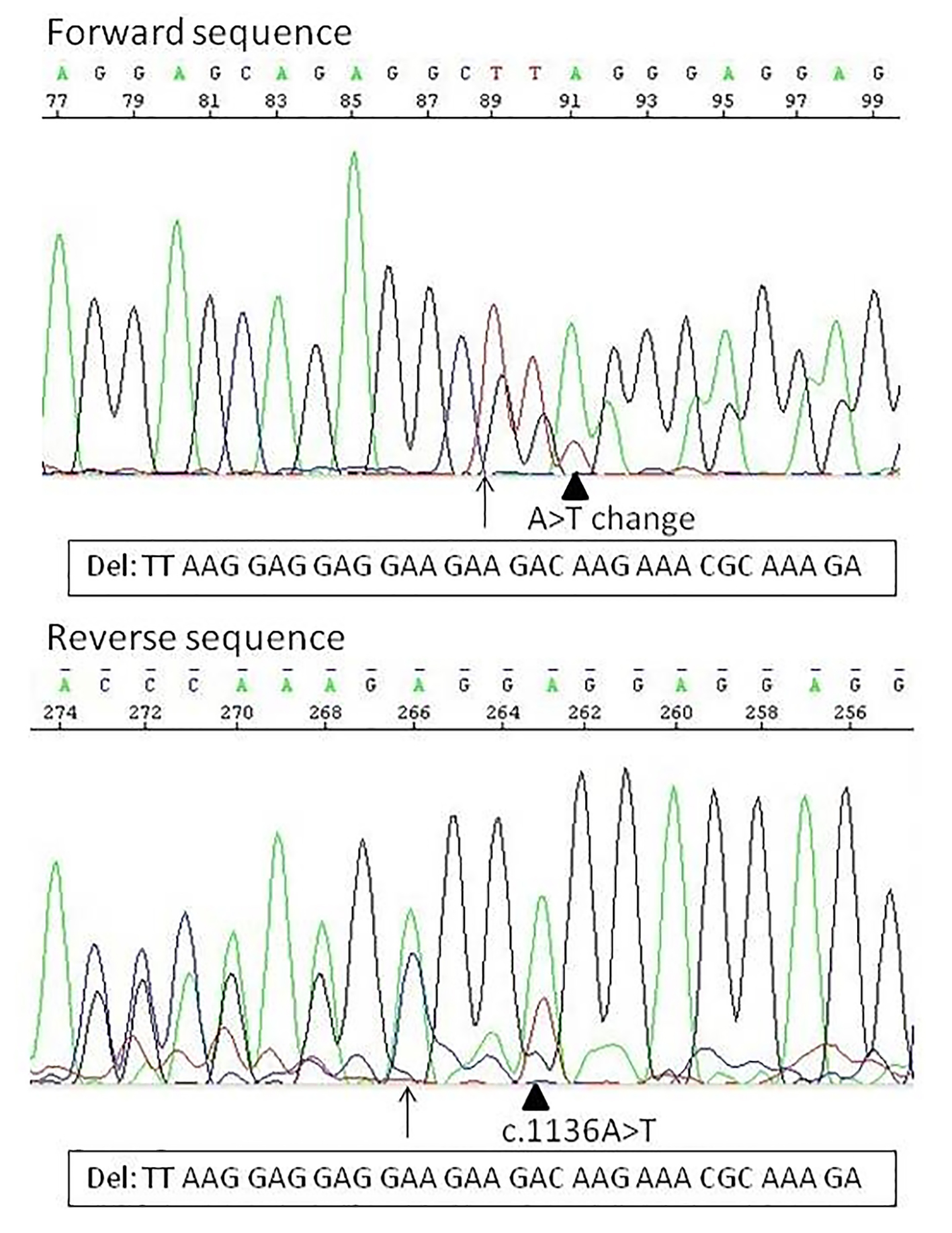 Click for large image | Figure 1. The two types of calreticulin mutation identified in this study. Arrow indicates the position of the deletion. The arrowhead indicates the A>T nucleic acid change, in forward sequence at position c.1102, and in reverse sequence at the original c.1136 position. In forward direction already the nucleic acid change can be seen: by the arrow the normal sequence is TTAAG, the deleted sequence should be GGAGG, what means that at position 91 (at the arrowhead, c.1102) should be only an A, instead of an A/T. The reverse direction confirms that the nucleic acid change is on the deleted allele. |
 Click for large image | Figure 2. The resulting calreticulin protein sequence of a wild type, the most common 52-bp deletion (type I mutation, p. L367fs*46) and the patient sample. |
| Discussion | ▴Top |
Although the novel mutant sequence extends the same length as the original, the change results in a replacement of the C-terminal negatively charged amino acids of CALR by a greater amount of positively charged amino acids rich in arginine and methionine compared to the wild type, and six additional amino acids compared to the most common type I mutation. The novel mutant contains all the mutant amino acid sequence of the type I mutant L367fs*46 and an additional of six altered and mainly positively charged amino acids (four of them are arginine, Fig. 2), therefore this novel sequence has at least the same importance for oncogenicity as the type I CALR mutation. This means that the mutation described in this study increases the activation of JAK-STAT signaling, which is responsible for the cytokine-independent growth of the cells [5]. The involvement of the JAK-STAT signaling pathway in patients with CALR mutation may lead to the effectiveness of JAK2-inhibitor therapy for these patients. Another pathway, called thrombospondin-1-low-density lipoprotein receptor-related protein (TSP1-LRP1) signaling pathway, plays a critical role in increasing cell survival of fibroblasts in anoikis by down-regulating apoptotic signaling and stimulating Akt activity. In mouse embryonic fibroblasts, the TSP1-CALR-LRP1 pathway activates pro-survival signals such as PI3-K and Akt, which precedes the inhibition of apoptosis [8].
The last four amino acids of the CALR gene (KDEL) contain the endoplasmatic reticulum retention signal. This signal is absent in the mutant CALR, resulting in an altered subcellular localization of the mutant protein [5]. As the negatively charged C-terminal domain of CALR is the CA2+ binding domain, the Ca2+ binding function of the mutant protein may be impaired influencing many Ca2+ signaling pathways such as those associated with cardiac development and cellular stress [8]. The presence of the alternative C-terminal of the mutant CALR offers an opportunity for immunologic targeting because it represents a cancer-specific epitope.
Klampfl et al have described a really similar CALR mutation, which they called type 12 [5]. It is a type I-like mutation, a 34-bp deletion (c.1098_1131del) starting only two bases before the mutation that we describe in this study, ending up theoretically at the same frameshift and C-terminal amino acid, L367fs*52. One of the two differences can be seen only in nucleic acid level. But the more important difference is that our mutation contains an amino acid change, which also leads to a nucleic acid chain, and that the second amino acid from the new C-terminal chain is not an arginine but a tryptophan.
CALR-mutated patients have a significantly lower risk of a thrombosis event and better overall survival compared to those MPN patents, who lack CALR mutation, but carry JAK2 or MPL mutation [9-11]. It shows that the mutational status has an impact of the prognostic outcome independently of the chosen therapy, and would be less likely transfusion dependent [12]. Data show that CALR positive MPNs are distinct clinocopathological entities.
CALR mutations in patients with ET have been associated with a lower hemoglobin level, lower leukocyte count and higher platelet count [9, 12, 13], but in our patient, we observed a higher leukocyte count, most likely because the inflammation of the patient influenced the leukocyte count. The earlier treatments of our patient can be the cause that not all blood levels are informative and bounded to the present illness. Other clinical parameters and recent or present treatments of independent illnesses can always influence the blood levels, and in some case, it can be hard to identify which level is relevant for the studied condition. They also found that a patient with CALR mutation has a higher chance of being young and male compared to those with mutated JAK2, which correlates with our findings.
In conclusion, the most known mutations result in the same downstream reading frame as the mutation described in this study, what means that the novel sequence has at least the same importance for oncogenicity as the most common type I CALR mutation, with an effect of different signaling pathways, activating the cytokine-independent growth of the cells and down-regulating the apoptotic signaling. But the presence of an alternative C-terminal domain offers an opportunity for immunologic targeting because it represents a cancer-specific epitope.
Conflict of Interest
The authors declare no conflict of interest.
| References | ▴Top |
- Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon France IARC Press. 2008.
- Pardanani AD, Levine RL, Lasho T, Pikman Y, Mesa RA, Wadleigh M, Steensma DP, et al. MPL515 mutations in myeloproliferative and other myeloid disorders: a study of 1182 patients. Blood. 2006;108(10):3472-3476.
doi pubmed - Pikman Y, Lee BH, Mercher T, McDowell E, Ebert BL, Gozo M, Cuker A, et al. MPLW515L is a novel somatic activating mutation in myelofibrosis with myeloid metaplasia. PLoS Med. 2006;3(7):e270.
doi pubmed - Nangalia J, Massie CE, Baxter EJ, Nice FL, Gundem G, Wedge DC, Avezov E, et al. Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2. N Engl J Med. 2013;369(25):2391-2405.
doi pubmed - Klampfl T, Gisslinger H, Harutyunyan AS, Nivarthi H, Rumi E, Milosevic JD, Them NC, et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. N Engl J Med. 2013;369(25):2379-2390.
doi pubmed - Choi CW, Bang SM, Jang S, Jung CW, Kim HJ, Kim HY, Kim SJ, et al. Guidelines for the management of myeloproliferative neoplasms. Korean J Intern Med. 2015;30(6):771-788.
doi pubmed - Tefferi A, Lasho TL, Finke CM, Knudson RA, Ketterling R, Hanson CH, Maffioli M, et al. CALR vs JAK2 vs MPL-mutated or triple-negative myelofibrosis: clinical, cytogenetic and molecular comparisons. Leukemia. 2014;28(7):1472-1477.
doi pubmed - Sun C, Zhang S, Li J. Calreticulin gene mutations in myeloproliferative neoplasms without Janus kinase 2 mutations. Leuk Lymphoma. 2015;56(6):1593-1598.
doi pubmed - Rotunno G, Mannarelli C, Guglielmelli P, Pacilli A, Pancrazzi A, Pieri L, Fanelli T, et al. Impact of calreticulin mutations on clinical and hematological phenotype and outcome in essential thrombocythemia. Blood. 2014;123(10):1552-1555.
doi pubmed - Tefferi A, Lasho TL, Finke C, Belachew AA, Wassie EA, Ketterling RP, Hanson CA, et al. Type 1 vs type 2 calreticulin mutations in primary myelofibrosis: differences in phenotype and prognostic impact. Leukemia. 2014;28(7):1568-1570.
doi pubmed - Guglielmelli P, Lasho TL, Rotunno G, Score J, Mannarelli C, Pancrazzi A, Biamonte F, et al. The number of prognostically detrimental mutations and prognosis in primary myelofibrosis: an international study of 797 patients. Leukemia. 2014;28(9):1804-1810.
doi pubmed - Kim SY, Im K, Park SN, Kwon J, Kim JA, Lee DS. CALR, JAK2, and MPL mutation profiles in patients with four different subtypes of myeloproliferative neoplasms: primary myelofibrosis, essential thrombocythemia, polycythemia vera, and myeloproliferative neoplasm, unclassifiable. Am J Clin Pathol. 2015;143(5):635-644.
doi pubmed - Tefferi A, Wassie EA, Guglielmelli P, Gangat N, Belachew AA, Lasho TL, Finke C, et al. Type 1 versus Type 2 calreticulin mutations in essential thrombocythemia: a collaborative study of 1027 patients. Am J Hematol. 2014;89(8):E121-124.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.



