| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website http://www.thejh.org |
Case Report
Volume 3, Number 3, September 2014, pages 76-79
Intentional Warfarin Overdose in a Human Patient With Liver Disease and Concurrent Pulmonary Emboli
Nicholas M. Houskaa, c, Jane D’Isa-Smitha, b
aOhio University Centers for Osteopathic Research and Education, Athens, OH, USA
bDepartment of Community Internal Medicine, Cleveland Clinic, Cleveland, OH, USA
cCorresponding Author: Nicholas M. Houska, 1533 Westwood Ave, Lakewood, OH 44107, USA
Manuscript accepted for publication April 1, 2014
Short title: Warfarin Overdose
doi: https://doi.org/10.14740/jh130w
| Abstract | ▴Top |
While there are numerous guidelines in the literature concerning the management of excess anticoagulation with oral vitamin K antagonists, clinicians are often presented with a scenario in which applying these guidelines is difficult. Given the prevalence of oral anticoagulant use and the frequency of toxicity, clinicians are likely to encounter cases of toxicity in patients with uniquely complex histories and presentation. Comorbidities that affect hemostatic physiology, such as hepatic dysfunction, make an algorithmic approach to diagnosis and management less predictable. Given the risk for serious thromboembolism and hemorrhage in this patient population, clinical guidelines must be executed in consideration of physiological knowledge and clinical experience. We present a case of intentional warfarin overdose in a human patient with liver disease and a recent history of thrombosis and hemorrhage. Our management of the patient was based on a combination of published guidelines, case series and clinical experience. Our patient helps to illustrate that the current recommendations for management of excess anticoagulation can be applied to patents with complex hemostatic systems and substantial risk for both thrombosis and hemorrhage, despite mechanism of toxicity and hepatic disease.
Keywords: Warfarin/poisoning; Anticoagulants/poisoning; Hemostasis; Liver diseases/complications; Thrombosis; Hemorrhage; Drug overdose
| Introduction | ▴Top |
Excess anticoagulation with warfarin is a common problem faced by clinicians, therefore clinical guidelines have been developed to provide general principles for management of bleeding and non-bleeding patients presenting with warfarin toxicity [1]. With the number of prescriptions of warfarin dispensed per year over 33 million [2], the patient population receiving anticoagulant therapy is diverse. These patients often have a unique combination of clinical characteristics and comorbidities which can make the use of clinical guidelines less straightforward. Our patient had combination of comorbidities that contributed to a complex hemostatic system, mainly liver disease caused by alcohol use and chronic hepatitis. Liver disease has been shown to disrupt the balance of hemostasis primarily by impairing coagulation factor production [3]. Historically it has been thought that this promotes a state of impaired thrombosis, causing so-called autoanticoagulation, though in more advanced liver disease, thrombotic complications are often seen [4]. This hinders the clinician’s ability to accurately predict the hemostatic state of patients with liver disease.
Our patient presented with a case of intentional warfarin overdose, of which reported cases are few [5]. While theoretical guidelines for the management of intentional overdose have been proposed, a PubMed search found no studies supporting these guidelines [5]. Further complicating management decisions was the patient’s history of a hypercoaguable state of unknown origin and bleeding esophageal varices; moreover, she had concurrent bilateral pulmonary emboli (PE) at the time of presentation. Given the risk for extension or development of thrombi and possibility of post-treatment warfarin resistance, our choices in reversing anticoagulation were perilous.
We report a unique case of intentional overdose of warfarin in a patient with liver disease, hypercoagubility and bleeding, with concurrent PE. Our goal is to help guide the management of future patients presenting with warfarin toxicity and complex hemostatic physiology.
| Case Report | ▴Top |
A 36-year-old African-American female presented to the hospital with 2 days of worsening bilateral lower extremity pain and inspiratory chest pain accompanied by shortness of breath. The patient admitted to consuming an extra 15 doses of 7.5 mg warfarin tablets earlier in the day in an effort to relive her pain. Her international normalized ratio (INR) at the monitoring clinic earlier in the day was 2.9. The patient had been discharged from the hospital 3 weeks prior with a diagnosis of extensive bilateral PE and left lower extremity deep venous thrombosis (DVT). During this previous hospital stay, an inferior vena cava (IVC) filter was placed and the patient was anticoagulated with heparin and bridged to warfarin. She was discharged with a therapeutic INR and had been on a dose of 7.5 mg daily since. The patient’s past medical history is significant for numerous PE and deep venous thrombi, for which she had been tested for hypercoagubility, with no definitive diagnosis. The patient had been on prior warfarin therapy and had a history of non-compliance with her medication and monitoring. She had multiple episodes of bleeding while on anticoagulants, the most recent of which was a Mallory-Weiss tear 1 year prior that required reversal of anticoagulation. The patient also had a history of hepatitis B, hepatitis C, alcoholic fatty liver disease, elevated transaminases, atrial septal shunt, depression and asthma. She admitted to smoking a quarter of a pack of cigarettes a day for 20 years as well as consuming a pint of alcohol daily for the same duration. Other current medications include zolpidem 7.5 mg and citalopram 20 mg daily, and an albuterol inhaler as needed. Allergies included aspirin, morphine, ibuprofen, oxycodone, sulfa drugs and penicillins. Family history is non-contributory. Review of systems was significant for right-sided abdominal pain since placement of the IVC filter. On physical exam the patient was cooperative and anxious with HR 130, BP 121/86 mm Hg, temperature 36.7 °C, and SpO2 of 96% on room air. Cardiac and lung exams were normal. Examination of the extremities showed bilateral calf tenderness, intact distal pulses, normal capillary refill and no evidence of bruising or bleeding. Abdominal exam exhibited lower right quadrant tenderness near the IVC filter incision site. Incision site was intact with no evidence of bleeding, swelling or infection. Her initial INR was 3.3. Liver and renal function was within normal limits. Complete blood count was normal except for a mildly decreased hematocrit and red cell distribution width of 18.6 (reference range 11.5-15%). Serum alcohol was 218 mg/dL and urine was positive for phencyclidine. Spiral computed tomography of the chest with contrast showed a small amount of thrombus in the lobar branches of the right pulmonary artery and left lower lobe, with interval improvement since previous admission. Duplex ultrasound showed chronic occlusive DVT in the left femoral and popliteal veins with no improvement since study on previous admission. Opiates were given for pain control, trypsin balsam castor oil was applied to IVC filter entry site, and psychiatry was consulted. On admittance her warfarin was withheld and her INR was monitored every 6 h. Her INR was 5.0 at 6 h after presentation climbing to 6.7 at 12 h. At this point, on the advice of hematology, three units of fresh frozen plasma (FFP) were transfused and 5 mg of oral vitamin K1 was administered. Post-transfusion, her INR was 2.6 at 12 h, 4.9 at 24 h, rising to 7.5 at 36 h. An additional 5 mg of oral vitamin K1 was then administered. Twelve hours after the second dose of vitamin K, the INR was 6.2, falling to 3.0 at 24 h. Figure 1 illustrates the trend of the patient’s INR. The patient showed no evidence of bleeding or further propagation of thrombi, and was deemed appropriate for discharge by hematology and psychiatry. The patient was to follow up with her primary care physician and hematologist to determine when warfarin therapy would be restarted. She was restarted on the same dosage of warfarin 1 month later on orders from her primary care provider. She had no hemorrhagic or thrombotic complications between discharge and restarting anticoagulation.
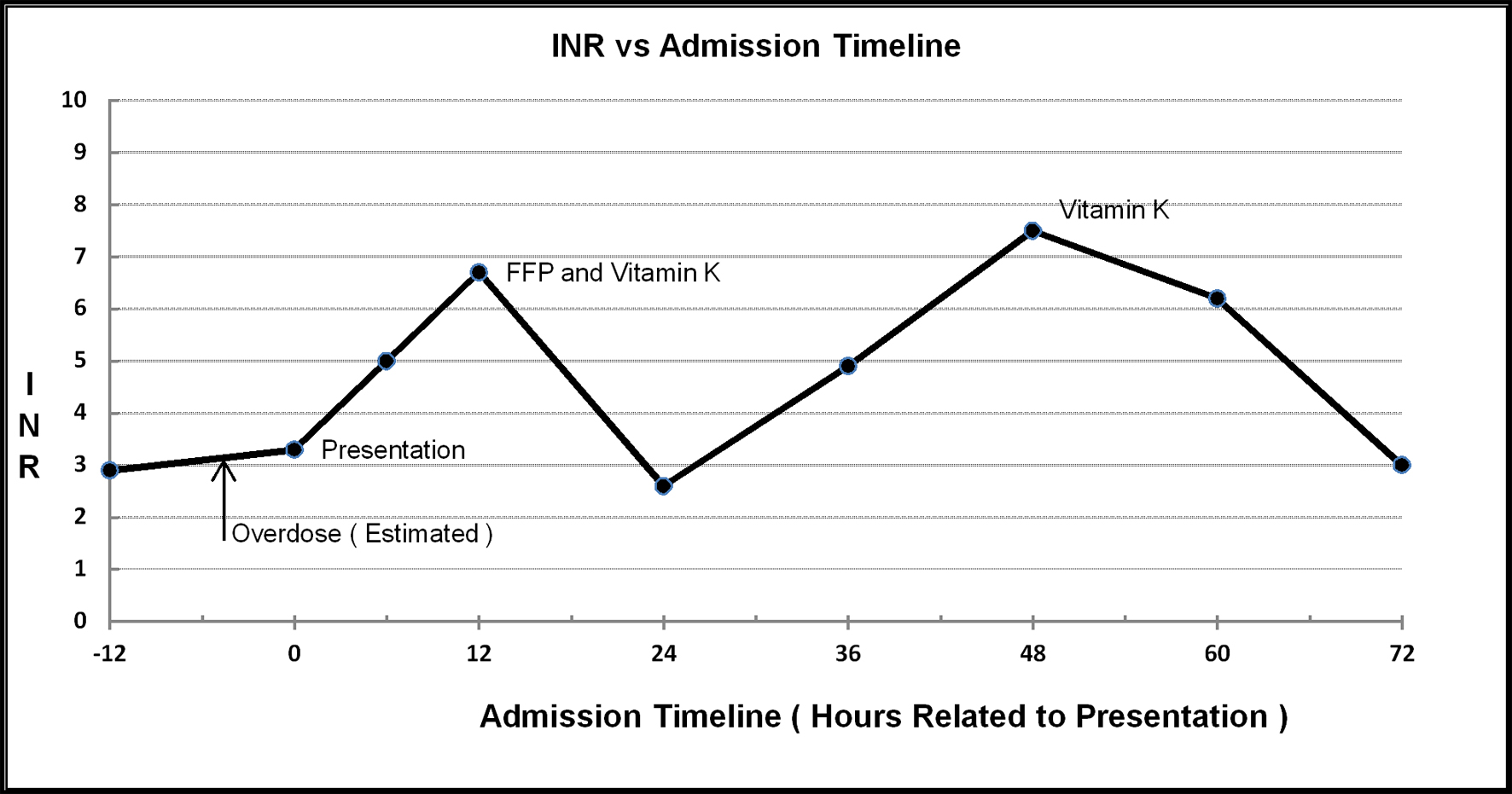 Click for large image | Figure 1. INR versus time. |
| Discussion | ▴Top |
Intentional warfarin overdose is a rare clinical situation, primarily because the typical patient population prescribed warfarin is less likely to intentionally overdose [5]. Our goal is not to focus on the mechanism of overdose, but rather the management of a supratherapeutic dose of warfarin in a patient with a complex hemostatic system. Considering the mechanism of overdose, only ignorance to the exact time and quantity of the overdose was of great importance in our decisions involving management.
Given the risk for major bleeding with a highly elevated INR and of thrombosis with reversal, we had several important considerations in management: monitoring of the INR, when to expect peak effect, when and how to reverse, how to monitor efficacy of reversal, and how to prevent thrombosis and warfarin resistance.
Warfarin is completely orally absorbed and reaches peak concentrations in 2 - 8 h undergoing hepatic metabolism with a half-life of 25 - 60 h [6]. These values can be affected by a number of genetic and clinical factors, including liver disease and alcohol use [7, 8]. Studies have shown that acute alcohol use may decrease warfarin metabolism, while chronic alcohol use may increase metabolism [8]. Our patient was a chronic alcoholic who presented acutely intoxicated, which made the pharmacokinetics of her overdose unpredictable. We initially chose a 6 h interval for INR monitoring, which has been shown in the literature as a suggested frequency [5]. The patients INR peaked at more than 48 h after her overdose, post-interventions.
While current guidelines typically categorize management of supratherapeutic INR based whether or not the patient is actively bleeding or needs urgent invasive intervention, it is pertinent for the clinician to consider risk for bleeding [9]. Our patient had numerous factors that are associated with higher odds of bleeding, including a history of ethanol abuse, liver disease and prior gastrointestinal bleed [1]. Given this risk and the large dose of warfarin taken, we felt it was in the patient’s best interest to intervene more aggressively even though the patient was not actively bleeding. Our choices for reversal included vitamin K, FFP and recombinant factors. Historically, withholding warfarin and administering oral vitamin K would be the mainstay of treatment [1]. Previous studies have shown impaired metabolism of vitamin K in patients with liver disease, and our concern was that given the patient’s history, a suboptimal response would be achieved with this therapy alone [10]. In addition to vitamin K, FFP was administered to provide coagulation factors that the patient might not be effective at producing efficiently. Based on the rapidly climbing INR, the dosage of vitamin K was based on guidelines for INR > 9 in a non-bleeding patient at high risk for bleeding [1, 9]. Per these guidelines, appropriate dosage of oral vitamin K1 ranges from 2.5 to 10 mg [1, 9]. We felt that with the administration of the FFP, a moderate dose of 5.0 mg would be sufficient for reversal, and not so large as to raise concern for warfarin resistance. Recommended monitoring of reversal is every 6 - 12 h; given the stability of our patient, we chose the upper limit of monitoring [1, 9]. This allowed us to observe the second rise in the INR after intervention, administer an additional dose of vitamin K and track the final reversal of the overdose.
Classification and treatment of patients with intentional warfarin overdose based on their requirement for anticoagulation have been proposed based on case series [5]. These guidelines balance the risks and benefits of reversing warfarin against the dangers of thrombosis and hemorrhage. Per these guidelines, our patient would be considered moderate risk for thromboembolic, given her history of DVT and PE [5]. It is suggested that such patients will tolerate complete reversal for short periods of time, and that risk of bleeding outweighs risk of thrombosis [5]. Our patient’s thrombi had remained stable since her prior admission, so we felt comfortable with the recommendations to reverse warfarin. Based on the literature, it was also decided that it would be safe to proceed without bridging anticoagulation [9].
Our patient helps to illustrate that the current recommendations for management of excess anticoagulation can be applied to patents with complex hemostatic systems and substantial risk for both thrombosis and hemorrhage, despite mechanism of toxicity and hepatic disease.
Grant Support
No financial or grant support or other assistance was received for this study.
| References | ▴Top |
- Ageno W, Gallus AS, Wittkowsky A, Crowther M, Hylek EM, Palareti G. Oral anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e44S-88S.
- IMS Institute for Healthcare Informatics. The use of medicine in the United States: review of 2011. Parsippany, New Jersey: IMS Institute; 2011.
- Lisman T, Leebeek FW, de Groot PG. Haemostatic abnormalities in patients with liver disease. J Hepatol. 2002;37(2):280-287.
doi - Senzolo M, Sartori MT, Lisman T. Should we give thromboprophylaxis to patients with liver cirrhosis and coagulopathy? HPB (Oxford). 2009;11(6):459-464.
doi pubmed - Isbister GK, Hackett LP, Whyte IM. Intentional warfarin overdose. Ther Drug Monit. 2003;25(6):715-722.
doi pubmed - Weitz JI. Blood Coagulation and Anticoagulant, Fibrinolytic, and Antiplatelet Drugs. In: Brunton LL, Chabner B, Knollman B, eds. Goodman & Gilman's The Pharmacological Basis of Therapeutics. 12th edition. New York: McGraw-Hill; 2011.
- Demirkan K, Stephens MA, Newman KP, Self TH. Response to warfarin and other oral anticoagulants: effects of disease states. South Med J. 2000;93(5):448-454; quiz 455.
doi pubmed - Weathermon R, Crabb DW. Alcohol and medication interactions. Alcohol Res Health. 1999;23(1):40-54.
pubmed - Baker RI, Coughlin PB, Gallus AS, Harper PL, Salem HH, Wood EM. Warfarin reversal: consensus guidelines, on behalf of the Australasian Society of Thrombosis and Haemostasis. Med J Aust. 2004;181(9):492-497.
pubmed - Mehta AB, McIntyre N. Haematological disorders in liver disease. Forum (Genova). 1998;8(1):8-25.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.


