| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website http://www.thejh.org |
Case Report
Volume 7, Number 4, December 2018, pages 163-166
Non-EBV-Related Aggressive NK-Cell Leukemia: An Oncohematological Great Imitator
Jose L. Lepe-Zunigaa, b, f, Francisco Javier Jeronimo-Lopeza, c, Jorge Gregorio Hernandez-Orantesa, d, Adriana Osiris Mendez-Cigarroaa, e
aHospital de Especialidades Pediatricas, Tuxtla Gutierrez, Chiapas, Mexico
bResearch Department, Hospital de Especialidades Pediatricas, Tuxtla Gutierrez, Chiapas, Mexico
cHematology Lab, Hospital de Especialidades Pediatricas, Tuxtla Gutierrez, Chiapas, Mexico
dCytometry Lab, Hospital de Especialidades Pediatricas, Tuxtla Gutierrez, Chiapas, Mexico
eGeneral Lab, Hospital de Especialidades Pediatricas, Tuxtla Gutierrez, Chiapas, Mexico
fCorresponding Author: Jose L. Lepe-Zuniga, Research Department, Hospital de Especialidades Pediatricas, Chiapas, SS Juan Pablo II S/N, Tuxtla Gutierrez, Chiapas, CP 29070, Mexico
Manuscript submitted September 17, 2018, accepted October 5, 2018
Short title: ANKL in Chiapas, Mexico
doi: https://doi.org/10.14740/jh462
| Abstract | ▴Top |
Aggressive natural killer (NK)-cell leukemia (ANKL) is a very rare oncohematological disease among youngsters in Latin America. Its clinical picture imitates a variety of syndromes and diseases due to its pathophysiology. Its diagnosis is relatively simple due to the prominence of NK malignant cells in peripheral blood and its clinical aggressiveness. In certain circumstances though, the presence of blast NK cells and the natural course of the disease can be so modified by the treatment of one of the imitated diseases, especially when using steroids, that it becomes very difficult to diagnose early in its course. We present a case of a 16-year-old Mexican male who initiated symptoms imitating dengue to severe dengue for which he received steroids, apparently inducing a partial remission; he was then diagnosed as having community acquired pneumonia, then sepsis, septic shock w/disseminated intravascular coagulation, primary hemophagocytic syndrome, severe hepatitis, lupus and finally hyper IgE. It was not until 1 day before dying of hemorrhagic shock, a month after initiating symptoms, when the (re)emergence of blast NK cells in peripheral blood allowed the correct diagnosis to be made. Knowledge of ANKL pathophysiology may raise awareness of this multifaceted malignancy and may open up possibilities for its therapy. Gained knowledge can also be used for guiding NK cell evident aggressiveness against other malignancies.
Keywords: Leukemia; Acute leukemia; NK cell; NK-cell leukemia; Hemophagocytic syndrome
| Introduction | ▴Top |
Ever since the expression “great imitator” was coined to refer to syphilis [1] and later to tuberculosis [2], the term has been applied to several diseases that mimic others, making the real diagnosis more difficult. Pituitary apoplexy [3], cardiac myxoma [4], lyme disease [5] and infectious mononucleosis [6] have been so considered. In more recent times, the preferred great imitator has been lupus, because its clinical picture can vary from predominantly hematological, to renal, hepatic, neurological or dermatological in nature, challenging the clinician before the disease fully reveals itself [7]. We report a novel great imitator: aggressive natural killer (NK)-cell leukemia (ANKL), a very rare disease among Hispanics, which not only imitates other diseases but actually presents itself as well-defined confounding syndromes or diseases. The pathophysiology during its steroid/chemotherapy-induced modified course is analyzed.
| Case Report | ▴Top |
Patient was a previously healthy 16-year-old Mexican adolescent male with no relevant family or personal history. One week before admission, he developed high fever (38 - 39.5 °C), chills, sweating, headache and malaise. Platelet count was 80,000/µL. Dengue was suspected. Three days later, platelets dropped to 60,000/µL, an intermediate action steroid was prescribed and patient was referred to our hospital (HEP) in Chiapas, Mexico, as a probable case of severe dengue.
On admission, patient looked ill, short of breath, diaphoretic and lethargic. Vitals were: blood pressure (BP): 91/57 mm Hg; heart rate (HR): 150/min; respiratory rate (RR): 30/min; SO2: 100% with high flow oxygen; temperature: 36.4 °C. On examination, he was jaundiced; both lung bases were dull with diminished breath sounds. Heart exam revealed no murmurs or other abnormalities. Liver was enlarged 2 cm below the costal margin and spleen was also found enlarged by abdominal ultrasound. A diagnosis of severe community-acquired pneumonia (CAP) and septic shock versus severe dengue was made. He was placed on antibiotics (cephotaxime, clarithromycin and neomycin), IV fluids and dobutamine to sustain BP. Labs showed mild anemia (hemoglobin (Hb): 11.1/12.6 g/dL), neutropenia (680/> 1,800/µL) and thrombocytopenia (43,000/µL); blood smear was negative for plasmodium. Liver function tests: bilirubin: 2.95 mg/dL; direct fraction: 2.32 mg/dL; alanine aminotransferase (ALT): 582 U/L, aspartate transaminase (AST): 1,452 U/L, alkaline phosphatase (ALP): 462 U/L, lactate dehydrogenase (LDH): 10,512 U/L; triglycerides: 214/34 - 140 mg/dL. Acute phase reactants were elevated (C-reactive protein (CRP): 17 mg/dL; procalcitonin (PCT): 33 ng/mL; ferritin: 52,460 ng/mL). All coagulation assays were abnormal (prothrombin time (PT): 31.5”; international normalized ratio (INR): 2.9; partial thromboplastin time (PTT): 102.7”/31.2”; fibrinogen: 40 mg/dL; D-dimer: 1,790/< 600 ng/mL). IgE: > 2,500/< 100 UI/mL. Serology was negative for: dengue IgM, thyphi O, thyphi H, parathyphi A, parathyphi B, proteus OX-19, brucella, leishmania, HIV; Hep C, Hep B eAg, Hep B sAg, Hep B core IgM, herpes simplex virus (HSV) IgM, cytomegalovirus (CMV) IgM, Epstein-Barr virus (EBV) capside IgM and parvovirus B19 IgM. Chest X-ray showed lung condensation in both bases with bilateral pleural effusions. All cultures were negative. A bone marrow aspirate showed increased cellularity, platelet producing megakaryocytes, megaloblastic changes and increased histiocytes, some with phagosomes (hemophagocytes). Bone marrow immunophenotype was reported as non-malignant. Karyotype was not performed. Acid fast bacilli test was negative in bronchial and gastric aspirates. Bone marrow giemsa staining was negative for leishmania. A diagnosis of hemophagocytic syndrome (HPS) secondary to CAP with septic shock and disseminated intravascular coagulation (DIC) was made; dengue was ruled out. Two doses of etoposide were administered 3 days apart. Dexamethasone, fresh frozen plasma, IV immunoglobulin (1g/kg/bolus) and apheresis platelets were added to the treatment.
He continued with high fever, tendency to hypotension and alternating states of alertness with loss of consciousness. Neurological deterioration led to orotracheal intubation and mechanical ventilation. The possibility of brain vasculitis (lupus) was raised. Antinuclear antibody (ANA) (immunofluorescent assay (IFA)) test was positive 1:320 (fine nuclear pattern); 1:160 (cytoplasm), anti-native DNA: 20/< 20 U/mL, anti-SSA(Ro): 4.6/< 20 U/mL; anti-SSB(La): 3.5/< 25 U/mL; anti-RNP/SM: 1.6/< 25 U/mL; anti-SM: 1.6/< 25 U/mL; coombs + IgG, hematuria 18rc/field, cylinders 3-5/field. The diagnosis of systemic lupus erythematosus (SLE) was made. Dexamethasone was replaced by methylprednisolone. Milrinone and norepinephrine were added and continued with antibiotics (cephotaxime, clindamycin and neomycin). From day 16 to day 20, patient improved overall status and was extubated. However, he remained icteric in DIC and with abnormal liver enzymes. He had upper gastrointestinal (GI) bleeding on several occasions. On day 30, lab reported Hb: 7.6 g/dL and 32% blast cells (5,648/µL) in peripheral blood (Fig. 1); blasts immunophenotype was CD45+, CD56+, CD16+, CD2+, CD7+, CD38+, HLA-DR+, CD1a-, CD2-, cCD3-, CD3-, CD4-, CD5-, CD8-, CD10-, CD19-, CD13-, CD34-, CD36-, CD117-, nTdT- and cMPO-. The diagnosis of mature NK-cell leukemia (aggressive) was made. The next day patient had a massive GI hemorrhage and died.
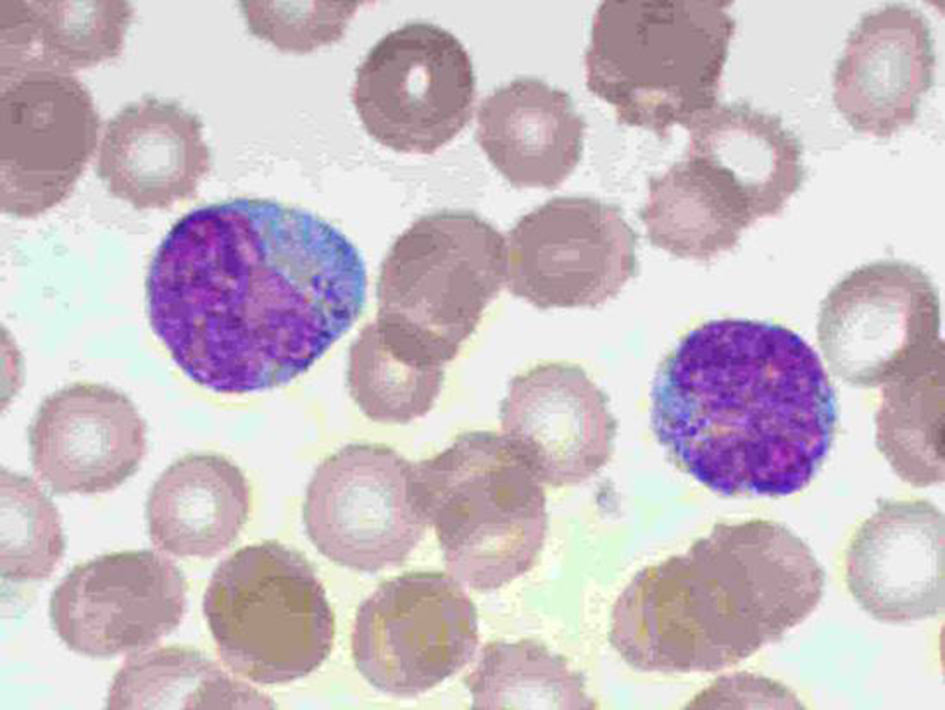 Click for large image | Figure 1. Peripheral blood mature NK blastic cells. |
| Discussion | ▴Top |
The World Health Organization International Classification of Diseases for Oncology (WHO; ICD-O-3) includes three variants of mature NK-cell malignancies: chronic lymphoproliferative disorder of NK-cells (9831/3), extranodal NK-/T-cell lymphoma, nasal type (9719/3) and the ANKL (9948/3), previously known as “large granular lymphocyte leukemia (LGL) or aggressive NK-cell lymphoma”. ANKL is a rare, very aggressive neoplastic proliferation of mature NK cells considered as an entity separated from T-cell LGL leukemia [8-10]. The disease is most prevalent among Asians with occasional reports in Hispanics [10-12]. More than 90% of cases reported have been EBV-related, which makes an EBV-negative case even more unusual [13].
ANKL clinical picture is a direct result of the malignant transformation/proliferation of mature NK cells, which in this case could have been spontaneous or related to an unknown infectious agent (virus?). The NK cells infiltrate a variety of tissues releasing non-specifically cytotoxic granules and perforins onto the surrounding cells (tissue macrophages, lymphocytes, hepatocytes, pneumocytes, endothelial and others) leading to extensive cell necrosis, intravascular coagulation, hyperactivation of the mononuclear phagocytic system causing HPS and the release into the circulation of an assortment of cytokines [14-16]. This outburst of inflammatory mediators induces autoimmune phenomena in some patients [17, 18]. At the end, the patient dies of multiorgan failure or from hemorrhagic shock secondary to DIC, like the present case.
In a tropical area like Chiapas, Mexico, where dengue is endemic, the initial clinical picture was presumed to be dengue because of febrile thrombocytopenia. Soon after, according to WHO guidelines [19], decreasing platelet count, respiratory distress and jaundice, suggested plasma leak syndrome and liver involvement due to severe dengue, prompting referral to our tertiary care hospital. Once admitted and dengue and viral hepatitis had been discarded by serology, the diagnosis was that of CAP with septic shock and DIC [20, 21]. Soon after admission, the possibility of HPS was raised and confirmed according to present criteria [14]. The effort then focused on trying to determine a primary cause for HPS (specific lung infection, EBV, hepatitis, malignancy or autoimmunity). HPS was treated in a limited fashion. A thorough investigation of an infectious primary disease by bacteria, viruses and parasites gave negative results. The possibility of a primary hematological malignancy was also discarded by bone marrow aspiration. The search then turned to a potential autoimmune disease affecting lungs, liver, kidneys, vessels and blood. Even though titers for ANA, anti-native DNA and direct Coombs were not highly elevated, clinical features and lab results formally completed the criteria for SLE [22]. Abnormally high titers of IgE were also found. Based on lab results, the case was considered primary SLE with HPS and DIC and treated accordingly until 1 day before his death when blasts in the peripheral blood were identified as mature NK cells by morphology and immunophenotype. As with most, if not all, cases of ANKL [11], the patient passed away just days beyond a month after initial symptoms.
In summary, this rare non-EBV-associated ANKL clinically imitated dengue, severe dengue, CAP, sepsis, septic shock w/DIC, primary HPS, fulminant hepatitis, hyper IgE and even an imitator: LES… a true “great imitator”.
ANKL is a disease relatively easy to diagnose. It just took a glance over the blood smear by a trained hematologist (the first author) to identify the classical cells the day before his death. Why then, the disease was not diagnosed earlier by a consulting hematologist? Lack of suspicion of a very rare disease could have contributed, but in fairness, around the end of the first week it was not possible to diagnose ANKL without NK malignant cells in peripheral blood and with a normal bone marrow aspirate. It is highly probable that prescription of steroids early on and during the course of the disease and etoposide later on had induced a temporary remission obscuring the diagnosis. Steroids are de facto used to induce an initial remission in leukemia (steroid window) and etoposide is a well-known cell cycle cytotoxic alkaloid used to treat leukemias. This could explain the absence of malignant cells in peripheral blood during most of the time. As for the bone marrow aspirate, the same can hold true; but it is also possible that ANKL, at least in this case, was not a disease originated in the marrow, but in a peripheral lymphoid organ like lymphomas do. This would be in line with its previous designation of “aggressive NK-cell lymphoma”.
Another issue relates to the diagnosis of LES in this case and its relationship with HPS and ANKL. LES has been associated with HPS; yet, most reports refer to HPS in the setting of, or secondary to, an aggressive LES [17] and only occasionally the other way around [18]. Moreover, the diagnosis of LES in this case was marginal at best and thus very unlikely a primary disease complicated with HPS. Hence, it is most likely that both phenomena (HPS and LES) were directly related to ANKL. HPS is an integral part of ANKL pathophysiology and is well documented [14-16]. LES, on the other hand, has only rarely been associated to ANKL [23]. In theory, autoimmune phenomena in ANKL with HPS should be no surprise. The disease itself and HPS entail in many ways autoimmune phenomena: the attack of immune cells on self. NK cells activation is controlled by several immune checkpoint molecules (killer inhibitory receptors (KIRs), programmed cell death protein 1 (PD-1), etc.) whose expression prevents them from killing cells sharing MHC class I identities [24]. It is possible that the original non-EBV-related mutational event(s) leading to the uncontrolled proliferation of NK cells had included the loss of checkpoint molecules allowing non-specific attack on self cells all over the body. This event would then recruit and activate macrophages (HPS) and lymphocytes which amplify and perpetuate the inflammatory state, easily mimicking LES (pseudo LES?). The finding of hyper IgE associated to ANKL is unique. In the absence of a history of susceptibility to infectious diseases, it would be very doubtful a background of a congenital “silent” hyper IgE syndrome in this case. Hyper IgE was found associated to myeloid leukemia (M5c) in one case [25] and IL-4 release by lymphoid cells has been found increased in lupus [26]. Thus, it is quite possible hyper IgE was only a consequence of total loss of regulation of a hyper stimulated immune system upon a cytokine storm associated to ANKL/HPS/LES.
ANKL is a complex, very aggressive disease with a poor prognosis which imitates several syndromes and diseases. Sometimes, like in the present case, treatment of the deceptive syndromes obscures and delays its diagnosis. Knowledge of its pathophysiology may open up therapeutic possibilities for this deadly malignancy and can also contribute to hypothetically guide NK cell aggressiveness against other malignancies.
Financial Support
This study has been supported by internal funds from the Hospital de Especialidades Pediátricas de Tuxtla Gutiérrez, Chiapas, Mexico
| References | ▴Top |
- Peeling RW, Hook EW, 3rd. The pathogenesis of syphilis: the Great Mimicker, revisited. J Pathol. 2006;208(2):224-232.
doi pubmed - Jetley S, Jairajpuri ZS, Pujani M, Khan S, Rana S. Tuberculosis 'The Great Imitator': A usual disease with unusual presentations. Indian J Tuberc. 2017;64(1):54-59.
doi pubmed - Camara-Lemarroy CR, Infante-Valenzuela A, Rodriguez-Velver K, Rodriguez-Gutierrez R, Villareal-Velazquez HJ. Pituitary apoplexy presenting as diabetic ketoacidosis: A great simulator? Neuro Endocrinol Lett. 2016;37(1):9-11.
pubmed - Arrieta F. The great simulator; spectrum of left atrial myxoma: case report. Bol Asoc Med P R. 1989;81(11):438-443.
pubmed - Pachner AR. Neurologic manifestations of Lyme disease, the new "great imitator". Rev Infect Dis. 1989;11(Suppl 6):S1482-1486.
doi pubmed - Bower AG. The diagnosis and treatment of the great simulator, infectious mononucleosis. Ariz Med. 1957;14(10):581-588.
pubmed - Kurien BT, Scofield RH. Autoantibody determination in the diagnosis of systemic lupus erythematosus. Scand J Immunol. 2006;64(3):227-235.
doi pubmed - Suzuki R, Suzumiya J, Nakamura S, Aoki S, Notoya A, Ozaki S, Gondo H, et al. Aggressive natural killer-cell leukemia revisited: large granular lymphocyte leukemia of cytotoxic NK cells. Leukemia. 2004;18(4):763-770.
doi pubmed - Mohamed AN. Aggressive natural killer leukemia (ANKL). Atlas Genet Cytogenet Oncol Haematol. Available at: http://AtlasGeneticsOncology.org/Anomalies/AggressiveNKcellLeukID1690.html Accessed August 28, 2018.
- Lima M. Aggressive mature natural killer cell neoplasms: from epidemiology to diagnosis. Orphanet J Rare Dis. 2013;8:95.
doi pubmed - Tang YT, Wang D, Luo H, Xiao M, Zhou HS, Liu D, Ling SP, et al. Aggressive NK-cell leukemia: clinical subtypes, molecular features, and treatment outcomes. Blood Cancer J. 2017;7(12):660.
doi pubmed - Nazarullah A, Dona M, Linhares Y, Alkan S, Huanga Q. Aggressive NK-cell leukemia: A rare entity with diagnostic and therapeutic challenge. Human Pathology: Case Reports. 2016;4:32-37.
- Gao J, Behdad A, Ji P, Wolniak KL, Frankfurt O, Chen YH. EBV-negative aggressive NK-cell leukemia/lymphoma: a clinical and pathological study from a single institution. Mod Pathol. 2017;30(8):1100-1115.
doi pubmed - Zhang L, Zhou J, Sokol L. Hereditary and acquired hemophagocytic lymphohistiocytosis. Cancer Control. 2014;21(4):301-312.
doi pubmed - Petterson TE, Bosco AA, Cohn RJ. Aggressive natural killer cell leukemia presenting with hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2008;50(3):654-657.
doi pubmed - Okuda T, Sakamoto S, Deguchi T, Misawa S, Kashima K, Yoshihara T, Ikushima S, et al. Hemophagocytic syndrome associated with aggressive natural killer cell leukemia. Am J Hematol. 1991;38(4):321-323.
doi pubmed - Sekigawa I, Suzuki J, Nawata M, Ikeda K, Koike M, Iida N, Hashimoto H, et al. Hemophagocytosis in autoimmune disease. Clin Exp Rheumatol. 2001;19(3):333-338.
pubmed - Egues Dubuc CA, Uriarte Ecenarro M, Meneses Villalba C, Aldasoro Caceres V, Hernando Rubio I, Belzunegui Otano J. Hemophagocytic syndrome as the initial manifestation of systemic lupus erythematosus. Reumatol Clin. 2014;10(5):321-324.
doi - WHO Dengue: Guidelines for Diagnosis, Treatment Prevention and Control. 2009. Available at: http://www.who.int/tdr/publications/documents/dengue-diagnosis.pdf. Accessed August 24, 2018.
- Bradley JS, Byington CL, Shah SS, Alverson B, Carter ER, Harrison C, Kaplan SL, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53(7):e25-76.
doi pubmed - Asakura H, Takahashi H, Uchiyama T, Eguchi Y, Okamoto K, Kawasugi K, Madoiwa S, et al. Proposal for new diagnostic criteria for DIC from the Japanese Society on Thrombosis and Hemostasis. Thromb J. 2016;14:42.
doi pubmed - Tunnicliffe DJ, Singh-Grewal D, Kim S, Craig JC, Tong A. Diagnosis, Monitoring, and Treatment of Systemic Lupus Erythematosus: A Systematic Review of Clinical Practice Guidelines. Arthritis Care Res (Hoboken). 2015;67(10):1440-1452.
doi pubmed - Olde Bekkink M, Ahmed-Ousenkova YM, Netea MG, van der Velden WJ, Berden JH. Coexistence of systemic lupus erythematosus, tuberous sclerosis and aggressive natural killer-cell leukaemia: coincidence or correlated? Lupus. 2016;25(7):766-771.
doi pubmed - Beldi-Ferchiou A, Caillat-Zucman S. Control of NK Cell Activation by Immune Checkpoint Molecules. Int J Mol Sci. 2017;18(10):2129.
doi pubmed - Lima M, Orfao A, Coutinho J, Ferreira G, Freitas I, Silvestre F, Justica B. An unusual acute myeloid leukemia associated with hyper IgE: another case of AML-M5c? Haematologica. 2001;86(2):216-217.
pubmed - Wong CK, Ho CY, Li EK, Lam CW. Elevation of proinflammatory cytokine (IL-18, IL-17, IL-12) and Th2 cytokine (IL-4) concentrations in patients with systemic lupus erythematosus. Lupus. 2000;9(8):589-593.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.