| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website http://www.thejh.org |
Case Report
Volume 3, Number 2, June 2014, pages 46-49
The Clinical Utility of Tranexamic Acid in the Management of Refractory Bleeding in a Patient With Advanced Liver Disease
Lisa Laskiewicza, William Fabriciusb, c, Robert Weinsteina, b, d, e
aDepartment of Pathology, UMass Memorial Medical Center, University of Massachusetts Medical School, Worcester, MA, USA
bDepartment of Medicine, UMass Memorial Medical Center, University of Massachusetts Medical School, Worcester, MA, USA
cDivision of Hematology/Oncology, UMass Memorial Medical Center, University of Massachusetts Medical School, Worcester, MA, USA
dDivision of Transfusion Medicine, UMass Memorial Medical Center, University of Massachusetts Medical School, Worcester, MA, USA
eCorresponding Author: Robert Weinstein, Division of Transfusion Medicine, UMass Memorial Medical Center, University of Massachusetts Medical School, 55 Lake Avenue North, Worcester, MA 01655-0002, USA
Manuscript accepted for publication May 21, 2014
Short title: Management of Refractory Bleeding
doi: https://doi.org/10.14740/jh151w
| Abstract | ▴Top |
Advanced liver disease is associated with coagulopathy and bleeding that develops from several mechanisms. In common clinical practice, the bleeding is treated with blood products and/or pharmacologic agents based on their corrective effect on laboratory tests of hemostasis. We report the case of a 72-year-old man with end-stage liver disease with refractory bleeding in whom multiple therapeutic strategies failed, including massive blood product replacement, but who responded to the infusion of tranexamic acid, an anti-fibrinolytic agent. The hyper-fibrinolytic state is difficult to diagnose with conventional clinical laboratory tests, but it was suspected in this patient with refractory bleeding and hypo-fibrinogenemia. Although largely used in the surgical setting, tranexamic acid may prove useful in medical settings characterized by bleeding and systemic fibrinolysis.
Keywords: Fibrinolysis; Hemorrhage; Cirrhosis; Hemostasis; Tranexamic acid; Antifibrinolytic
| Introduction | ▴Top |
The coagulopathy and bleeding tendency of advanced liver disease develops from several mechanisms including insufficient levels of coagulation factors, thrombocytopenia and platelet function defects, elevated plasma levels of tissue plasminogen activator (t-PA), overproduction of nitric oxide and prostacycline, vitamin K deficiency and dysfibrinogenemia [1, 2]. It is common clinical practice to treat the bleeding with blood products and/or pharmacologic agents. The use of these agents tends to be based on their corrective effect on laboratory tests of hemostatic function (e.g. PT, APTT, fibrinogen level), but their ability to prevent or control bleeding has not been validated by randomized, controlled clinical trials [2, 3]. The fibrinolytic system is altered in 30-50% of patients with end-stage liver disease [4, 5], leading to hyper- or hypo-fibrinolysis and predisposing the patient to excessive bleeding and, less often, thrombosis. We report a patient with end-stage liver disease with refractory bleeding in whom multiple therapeutic strategies failed, including restoration of platelets, coagulation factors and fibrinogen, but who responded to the administration of an anti-fibrinolytic agent.
| Case Report | ▴Top |
A 72-year-old man with advanced cryptogenic cirrhosis presented to the hospital, anuric, jaundiced, feeling ill and requiring total assistance. He had been diagnosed with hepatocellular carcinoma 6 months prior to admission and had undergone radio-frequency ablation of the tumor. His clinical course was complicated by portal hypertension resulting in upper gastrointestinal tract bleeding, recurrent ascites, spontaneous bacterial peritonitis and hepatic encephalopathy. Shortly after admission he developed hepatorenal syndrome requiring transfer to the intensive care unit for hemodynamic support and emergent continuous veno-venous hemofiltration, which was started on the sixth day of hospitalization. On the second week of hospital stay he was noted to have significant oozing of blood from all intravenous (IV) catheter sites including his right subclavian hemodialysis catheter, left internal jugular triple lumen catheter and the site of a previous right internal jugular triple lumen catheter. There were no hematochezia, melena or hemoptysis. At that time, he was on rifaximin, midodrine, pantoprazole, lactulose, insulin and norepinephrine via IV drip. On physical examination, he was afebrile, hypotensive, slightly tachycardic and jaundiced. He had petechiae in his oral mucosa and had blood-soaked dressings over his left internal jugular catheter site, old right internal jugular catheter site and right subclavian hemodialysis catheter site. The abdomen was markedly distended. His extremities had 3+ pitting edema bilaterally to the knees.
The patient’s bleeding was treated with 10 mg of IV vitamin K and multiple blood products (Fig. 1). In the span of 9 days (from day 6 to day 14 of hospitalization), he received 24 units of packed red blood cells (PRBC), 25 units of plasma, 13 apheresis platelets and 15 pools of cryoprecipitate (5 units per pool). Despite this, his bleeding significantly worsened necessitating transfusion of 10 units of PRBC and 12 units of plasma in less than 24 h on the 15th day of hospitalization. The administration of 4 mg of recombinant factor VIIa (50 µg/kg) that same morning did not resolve his bleeding.
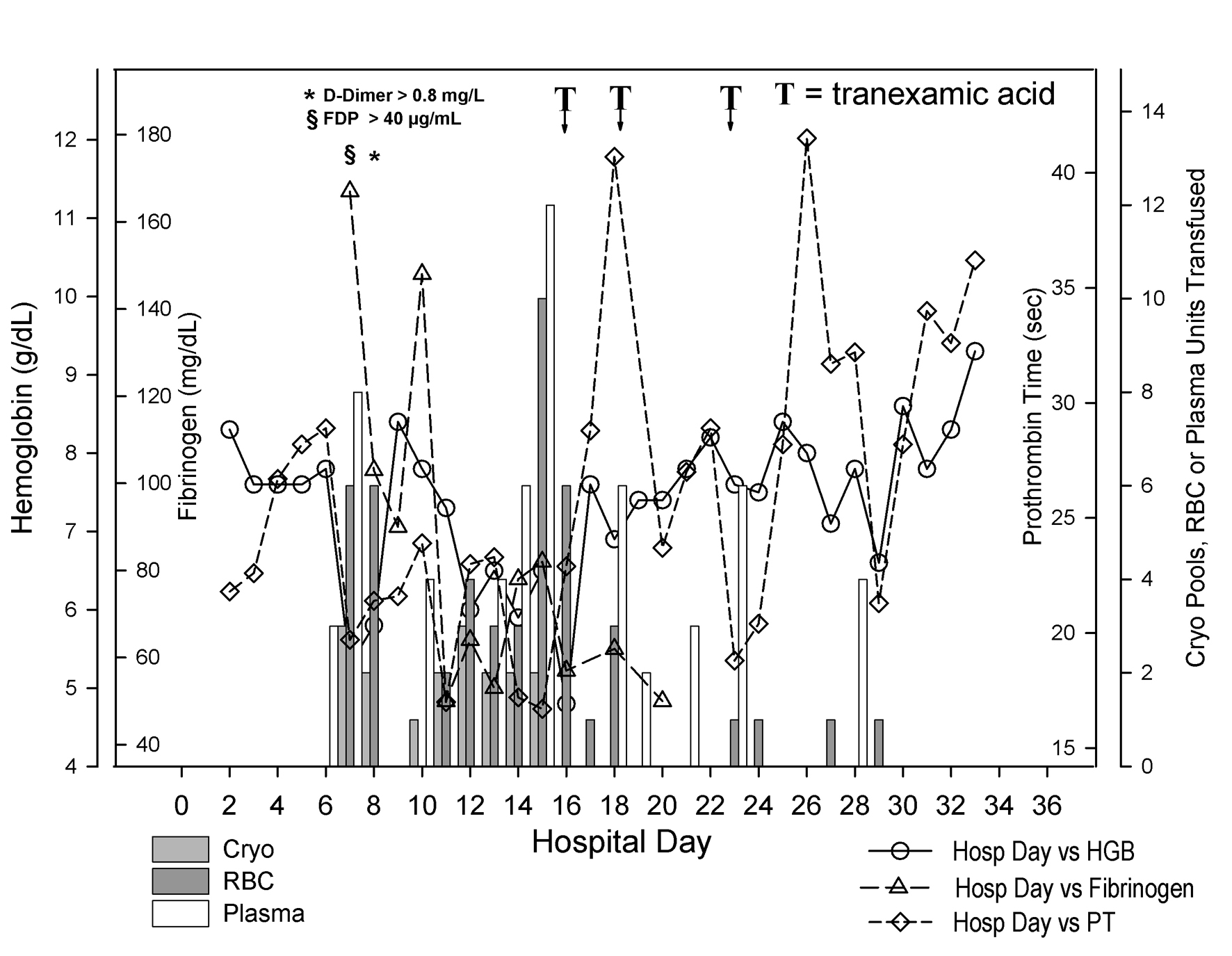 Click for large image | Figure 1. Coagulation status and blood product use during hospitalization. Day of hospitalization is depicted on the X-axis. Separate Y-axes depict hemoglobin (g/dL, open circles), fibrinogen (mg/dL, open triangles), prothrombin time (sec, open diamonds) and pooled cryoprecipitate (Cryo, 5 units per pool, light gray bars), units of red blood cells (RBC, dark gray bars), and units of plasma (white bars) transfused to the patient. The patient’s hemoglobin fell precipitously between hospital days 6 and 7. The fibrinogen began to fall as well. As shown at the top left of the figure, fibrin degradation products and D-dimers were found markedly elevated on hospital day 7 and 8 respectively. Between hospital day 6 and 14, he received 24 units of packed red blood cells, 25 units of plasma and 15 pools of cryoprecipitate. Although not shown on the figure, he also received 13 apheresis platelets during that interval. He received another 16 units of packed red blood cells between hospital day 15 and 16. He then received tranexamic acid on two occasions (the first one in the evening of day 15 through early morning of day 16 and the second one in the afternoon of day 18). After the second dose, his hemoglobin remained stable with the administration of only 4 units of packed red cells beginning with hospital day 19. Despite the persistence of a prolonged prothrombin time, he appeared to have stopped bleeding. |
In light of persistent marked prolongation of the PT and APTT and persistently low plasma fibrinogen, despite massive blood product resuscitation, a transfusion medicine consultant recommended the use of an anti-fibrinolytic agent to control the bleeding. Therefore, in the evening of hospital day 15 and into the early hours of day 16, he received tranexamic acid as an IV bolus of 1,000 mg followed by 1,000 mg as an 8-h continuous infusion. Early on the 16th day of hospitalization, he was still transfused with 6 units of PRBC, but he required only 2 more units of PRBC in the following 48 h. On the 18th day of hospitalization his bleeding from IV catheter sites resumed and 2 more units of PRBC were given. He then received another 1,000 mg IV bolus of tranexamic acid followed by an 8-h continuous infusion, which allowed for deep sutures to be placed at the right internal jugular IV site. His bleeding resolved completely and he had no further active bleeding. A third course of tranexamic acid was given prophylactically on day 23 for a central IV line removal. There was no active bleeding at the time. Unfortunately, he expired on hospital day 33, with pulseless electrical activity and respiratory failure due to overwhelming sepsis and pneumonia.
| Discussion | ▴Top |
This report represents successful use of an anti-fibrinolytic agent in the setting of severe refractory bleeding in a patient with advanced liver failure. Prior to receiving tranexamic acid, our patient received aggressive blood product support, including red cells, plasma, platelets and cryoprecipitate, in an attempt to replete factors involved in multiple aspects of hemostasis. The failure of massive blood product support to control his bleeding, and persistent low serum fibrinogen values and elevated serum levels of D-dimers and fibrin degradation products, were interpreted as an indication that uncontrolled fibrinolysis was the dominant feature of his hemostatic defect. Thus, after weeks of receiving massive amounts of blood products, our patient’s refractory bleeding responded to the use of an anti-fibrinolytic agent within hours. After the effect of tranexamic acid had worn off, and bleeding had resumed, he promptly responded to a second administration of the drug.
Accelerated intravascular coagulation with secondary hyper-fibrinolysis has been reported in 30-46% of patients with end-stage liver disease [1, 4]. The hyper-fibrinolytic state is difficult to diagnose with conventional clinical laboratory tests, but it was suspected in this patient with refractory bleeding and hypo-fibrinogenemia despite massive blood product replacement. There are no randomized clinical trials showing benefit of anti-fibrinolytics in patients with cirrhosis and bleeding diatheses; however, several reports indicate that, when hyper-fibrinolysis is proven or suspected, these agents may be effective and appear safe in cirrhosis [6-9]. The anti-fibrinolytic agent tranexamic acid aids in achieving hemostasis by competitively inhibiting the binding of plasminogen and plasmin to fibrin, thus, interfering with the dissolution of the fibrin matrix [10]. IV tranexamic acid, given with a loading dose of 1,000 mg over 10 min, followed by 1,000 mg over the next 8 h, has been found to decrease the risk of death in patients who have significant bleeding due to trauma when used less than 3 h following the injury event [11].
Our patient was provided massive blood product support in an attempt to replete clotting factors and control his bleeding, an approach based on abnormalities of commonly used clinical laboratory tests of hemostatic function. Despite this, his bleeding persisted and his laboratory test results did not improve, but an appreciation of the role of fibrinolysis in his bleeding diathesis led to the successful use of an anti-fibrinolytic agent. This case illustrates how awareness of the diversity of mechanisms of hemostatic imbalance in patients with liver failure can lead to proper interpretation of common tests of hemostatic function and help in therapeutic decision making. Furthermore, although largely used in the surgical and trauma setting [10, 12], tranexamic acid may prove useful in medical settings characterized by bleeding and systemic fibrinolysis.
Acknowledgments
The authors express their sincere appreciation to Dr. Jan Cerny for critical review of the manuscript.
Funding
None.
Conflicts of Interest
All authors have no financial or other conflicts of interest.
Author Contributions
All authors had access to the data and a role in writing the manuscript. Drs. Laskiewicz and Fabricius are co-first authors of the manuscript.
| References | ▴Top |
- Kujovich JL. Hemostatic defects in end stage liver disease. Crit Care Clin. 2005;21(3):563-587.
doi pubmed - Lisman T, Porte RJ. Rebalanced hemostasis in patients with liver disease: evidence and clinical consequences. Blood. 2010;116(6):878-885.
doi pubmed - Segal JB, Dzik WH, Transfusion Medicine/Hemostasis Clinical Trials N. Paucity of studies to support that abnormal coagulation test results predict bleeding in the setting of invasive procedures: an evidence-based review. Transfusion. 2005;45(9):1413-1425.
doi pubmed - Ferro D, Celestini A, Violi F. Hyperfibrinolysis in liver disease. Clin Liver Dis. 2009;13(1):21-31.
doi pubmed - Nair GB, Lajin M, Muslimani A. A cirrhotic patient with spontaneous intramuscular hematoma due to primary hyperfibrinolysis. Clin Adv Hematol Oncol. 2011;9(3):249-252.
pubmed - Hu KQ, Yu AS, Tiyyagura L, Redeker AG, Reynolds TB. Hyperfibrinolytic activity in hospitalized cirrhotic patients in a referral liver unit. Am J Gastroenterol. 2001;96(5):1581-1586.
doi pubmed - Gunawan B, Runyon B. The efficacy and safety of epsilon-aminocaproic acid treatment in patients with cirrhosis and hyperfibrinolysis. Aliment Pharmacol Ther. 2006;23(1):115-120.
doi pubmed - Molenaar IQ, Warnaar N, Groen H, Tenvergert EM, Slooff MJ, Porte RJ. Efficacy and safety of antifibrinolytic drugs in liver transplantation: a systematic review and meta-analysis. Am J Transplant. 2007;7(1):185-194.
doi pubmed - Massicotte L, Denault AY, Beaulieu D, Thibeault L, Hevesi Z, Roy A. Aprotinin versus tranexamic acid during liver transplantation: impact on blood product requirements and survival. Transplantation. 2011;91(11):1273-1278.
doi pubmed - McCormack PL. Tranexamic acid: a review of its use in the treatment of hyperfibrinolysis. Drugs. 2012;72(5):585-617.
doi pubmed - Roberts I, Shakur H, Coats T, Hunt B, Balogun E, Barnetson L, Cook L, et al. The CRASH-2 trial: a randomised controlled trial and economic evaluation of the effects of tranexamic acid on death, vascular occlusive events and transfusion requirement in bleeding trauma patients. Health Technol Assess. 2013;17(10):1-79.
- Ker K, Edwards P, Perel P, Shakur H, Roberts I. Effect of tranexamic acid on surgical bleeding: systematic review and cumulative meta-analysis. BMJ. 2012;344:e3054.
doi pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.