| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website http://www.thejh.org |
Case Report
Volume 3, Number 1, March 2014, pages 10-12
Mitral Valve Repair Surgery in a Patient With Sickle Cell Disease
Anthony Lemairea, b, Peter Scholza, Antonio Chiricoloa, Andrew Israela, Leonard Y. Leea
aDivision of Cardiothoracic Surgery, Department of Surgery, Rutgers Robert Wood Johnson University Hospital, New Brunswick, New Jersey, USA
bCorresponding author: Anthony Lemaire, Division of Cardiothoracic Surgery, Department of Surgery, UMDNJ-Robert Wood Johnson University Hospital, 125 Paterson Street, New Brunswick, New Jersey 08903, USA
Manuscript accepted for publication October 11, 2013
Short title: Mitral Valve Repair Surgery
doi: https://doi.org/10.14740/jh102w
| Abstract | ▴Top |
Sickle cell disease (SCD) affects between 70,000 and 100,000 Americans, making it one of the most prevalent genetic disorders in the United States. It is an autosomal, recessive disorder characterized by a single amino acid change in the betaglobin chain of hemoglobin (Hob) leading to its pathological polymerization, red cell rigidity and poor microvascular blood flow. These changes also produce the characteristic sickle shape of the red cells, which lose the pliability required to successfully traverse small capillaries. The use of cardiopulmonary bypass (CPB) during adult cardiac surgery initiates many of the conditions that contribute to the sickling of the hemoglobin S cells. Traditionally during CPB the patients are hypothermic and can become acidotic during intermediate periods of surgery. The experience of adult cardiac surgery with CPB on patients with SCD is limited. The purpose of our manuscript is to review the first case of adult cardiac surgery in a patient with SCD at our institution and also to review the literature on the management of patients with SCD who undergo adult cardiac surgery.
Keywords: Sickle cell disease; Mitral valve; Crisis
| Introduction | ▴Top |
Sickle cell disease (SCD) affects between 70,000 and 100,000 Americans, making it one of the most prevalent genetic disorders in the United States [1]. It is an autosomal, recessive disorder characterized by a single amino acid change in the betaglobin chain of hemoglobin (Hob) leading to its pathological polymerization, red cell rigidity and poor microvascular blood flow [2]. These changes also produce the characteristic sickle shape of the red cells, which lose the pliability required to successfully traverse small capillaries [3]. The use of cardiopulmonary bypass (CPB) during adult cardiac surgery initiates many of the conditions that contribute to the sickling of the hemoglobin S cells. Traditionally during CPB the patients are hypothermic and can become acidotic during intermediate periods of surgery. These two factors make performing cardiac surgery on patients with SCD more difficult. The experience of adult cardiac surgery with CPB on patients with SCD is limited. The purpose of our manuscript is to review the first case of adult cardiac surgery in a patient with SCD at our institution and also to review the literature on the management of patients with SCD who undergo adult cardiac surgery.
| Case Report | ▴Top |
The patient is a 40-year-old African-American male with SCD who recently was diagnosed with non-ischemic cardiomyopathy. His presenting symptoms consisted of shortness of breath and worsening fatigue. On transthoracic echocardiogram he was noted to have severe mitral valve regurgitation due primarily to left ventricular dilation with no evidence of torn or prolapsed chordae. Of note, the patient had previously undergone an open cholecystectomy at the age of 18 years old. On admission to the hospital he was jaundice with evidence of hemolysis. His lactate dehydrogenase was elevated although his total bilirubin was normal. In addition, on hemoglobin electrophoresis his hemoglobin S level was 87%.
The patient was worked up extensively for his cardiomyopathy but no true etiology was determined. Prior to taking the patient to the operating room he was prepared for surgery and the use of CPB. A hematology consult was made for guidance with his management. The initial plan was to perform red blood cell (RBC) exchange prior to surgery so that his hemoglobin S level was less than the accepted level of 30% for CPB [4].
After the patients first RBC exchange his hemoglobin S level was 31% and after the second RBC exchange his hemoglobin S level was 8.7%. Several adjustments were made in the operating room in order to prevent any problems with running the CPB pump. First, the patient’s body temperature was cooled to no lower than 32 °C. Second, the blood cardioplegia was cooled down to only 32 °C as opposed to the standard 4 °C. Third, the patient’s blood was hemoconcentrated. Fourth, because of the presence of antibodies in his blood, prior to the day of surgery, in coordination with the blood bank 10 units of packed RBCs were put aside.
Procedure
The intraoperative transesophageal echocardiogram and operative view (Fig. 1) confirmed the presence of severe mitral valve regurgitation due to annular dilation. The patient underwent a standard median sternotomy. The CPB circuit consisted of a hollow fiber oxygenator with an integrated 32 µm arterial filter (FX15; Terumo, Ann Arbor, MI). An X-coated circuit (poly-2-methoxyethyl acrylate; Terumo) was used with a 3/82X 3/322 arterial and venous line, a centrifugal arterial pump (Delphin; Terumo) and a 4:1 blood cardiopledgia system (Terumo). A hemoconcentrator (Capiox HC11; Terumo, Elkton, MD) was placed in the circuit and an in-line venous saturation monitor (CDI 100; Terumo) was used for continuous monitoring of venous oxygen saturation, hemoglobin and hematocrit. A GEM 4000 (Instrumentation Laboratories, Bedford, MA) blood gas machine was in the room and used for blood gas analysis.
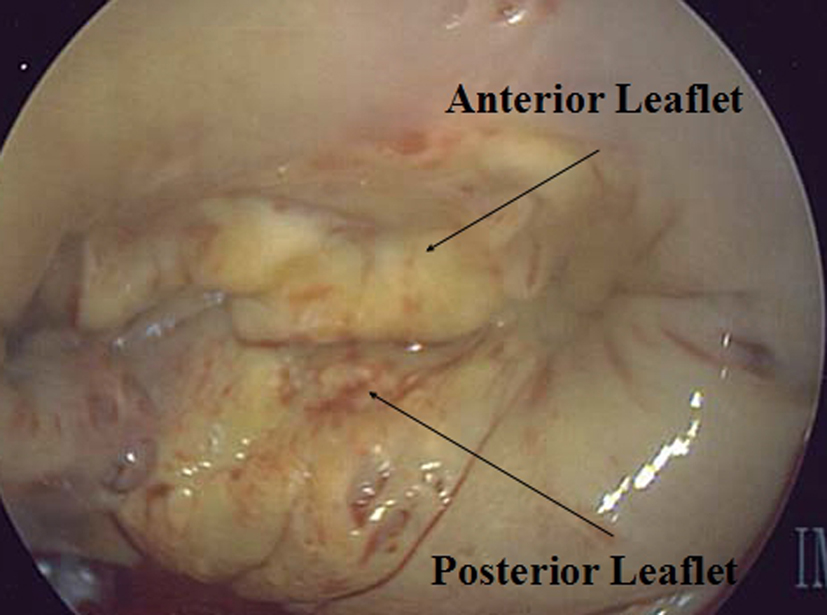 Click for large image | Figure 1. Preoperative image. |
The circuit was initially primed with 1,000 mL of lactated ringers (B Braun, Irvine, CA). After the chest was open and the heart was exposed, the patient was systemically heparinized with 20,000 units of heparin, with a target activated clotting time (ACT) of > 480 s. Both antegrade and retrograde cardioplegia were used for myocardial protection. Retrograde autologous prime and antegrade autologous prime were performed on the patient and 700 mL of prime was removed. At that time 12.5 g of 25% albumin, 25 g of mannitol, 5,000 units of heparin, 50 meq of sodium bicarbonate and 500 mL of autologous RBCs which were washed with the Fresenius continuous autotransfusion system (CATS) (Fresenius AG, Bad Homburg, Germany) in an effort to reduce the potassium were added to the venous reservoir. The CATS was only used for washing the autologous RBCs. Pump suctions were used to return all blood to the CPB circuit after the patient was heparinized and until protamine was given. Any shed blood returned to the CATS post CPB was discarded.
The heart was initially arrested with 1,400 mL of antegrade and retrograde 4:1 blood-crystalloid cardioplegia maintained at 32 °C. Subsequent doses of 32 °C retrograde cardiopledgia were given at 20 min intervals. A standard approach through Sondergaard’s groove was made. On initial inspection the mitral valve was intact with no evidence of prolapse of torn chordae. There was clear evidence that there was no coapting of the anterior and posterior leaflets. The anterior leaflet was sized and a 26 mm Carpentier-Edwards Physio Annuloplasty Ring (Edwards) was implanted. CPB flows were maintained at a minimum cardiac index of 2.4 L/min. Venous saturations were kept above 70% and there was no acidosis during CPB. Arterial pressure was kept between 65 and 87 mmHg. The patient was rewarmed to a bladder temperature of 36 °C. The aortic-cross clamp was removed after 76 min. The patient was ventilated and successfully weaned from CPB. The total CPB time was 114 min.
The post-procedure transesophageal echocardiogram and saline test after placement of the ring showed no residual mitral valve regurgitation (Fig. 2). Postoperatively the patient was transferred to the surgical intensive care unit and extubated shortly upon arrival. He required no additional blood transfusions or blood products. The patient was transferred to a regular room on POD#1 and he was discharged to home on POD#4.
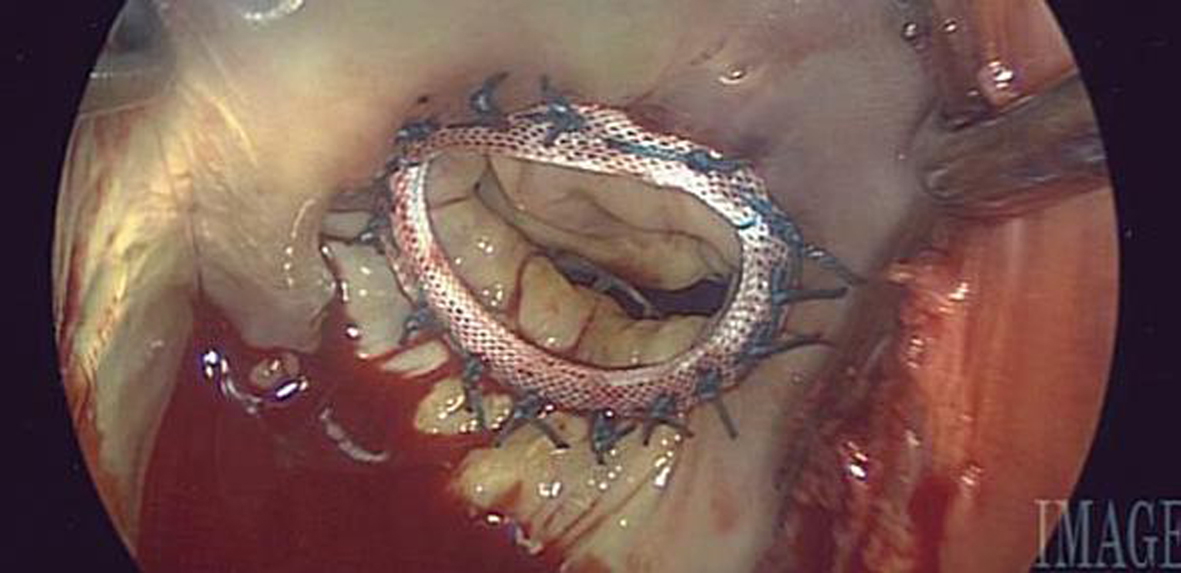 Click for large image | Figure 2. Postoperative image. |
| Discussion | ▴Top |
Between 1989 and 1993, there was an average of 75,000 hospitalizations per year in the United States among individuals with SCD [3]. The current incidence of SCD remains high and the practicing cardiac surgeon needs to be able to manage these patients in the operating room on CPB. Performing open heart surgery with the use of CPB on patients with SCD requires extensive preoperative preparation and coordination by multiple services. As can be seen in this case, the communication between hematology, cardiac surgery, the perfusion team and the blood bank was critical to the patient’s success. The patient underwent successful mitral valve repair with the use of a 26 mm Physio ring. Postoperatively the patient had complete repair of the valve and had no residual mitral valve regurgitation. The patient did well and was later discharged to home. The patient’s success is directly related to the communication and preparation of the different services on the team. Each member of the team played a vital role. Hematology coordinated the RBC exchange so that we could have the patient’s hemoglobin S level below the 30% cutoff that most report that it is safe to have the patient undergo CPB. In addition, the director of the blood bank was able to secure 10 units of pack RBCs which was difficult because of the patient’s known antibodies in his blood. Both the anesthesia and perfusion team coordinated intraoperatively to maintain the patient’s core body temperature above 32 °C and prevent him from becoming acidotic.
The term “SCD” refers to a collection of autosomal recessive genetic disorders characterized by the Hb S variant of the betaglobin gene [3]. The hemoglobin of individuals with SCD polymerizes in RBCs upon deoxygenation. This causes the RBCs to change from the usual biconcave disc shape to an irregular sickled shape [3]. These phenomena cause episodes of microvascular occlusion and premature RBC destruction, which leads to chronic severe hemolytic anemia [5]. Morbidity in SCD arises from vaso-occlusive events or tissue damage resulting from obstructed blood flow. Some of the more common symptoms include pain crises, acute chest syndrome and cerebrovascular accidents. The impact of SCD on the heart is important to note. In order to compensate for the reduced oxygen-carrying capacity, patients often have an increased plasma volume, elevated cardiac output and enlarged heart. In fact, cardiomegaly is an expected consequence of the anemia of SCD [6]. Despite this knowledge, the extent to which SCD impacts myocardial function is not very clear.
This case report describes the difficulties of performing adult cardiac surgery on patients with SCD. The conditions created during CPB often contribute to the sickling of RBCs and thus a balance has to be maintained that allows for successfully completing the procedure safely and preventing the clotting and clumping of cells within the pump. This case shows that successful cooperation by services and technical skill can overcome most operative challenges.
| References | ▴Top |
- Hassell KL. Population estimates of sickle cell disease in the U.S. Am J Prev Med. 2010;38(4 Suppl):S512-521.
doi pubmed - Bunn HF. Pathogenesis and treatment of sickle cell disease. N Engl J Med. 1997;337(11):762-769.
doi pubmed - Ashley-Koch A, Yang Q, Olney RS. Sickle hemoglobin (HbS) allele and sickle cell disease: a HuGE review. Am J Epidemiol. 2000;151(9):839-845.
doi pubmed - Chabot D, Sutton R. Mitral valve replacement in a patient with sickle cell disease using perioperative exchange transfusion. J Extra Corpor Technol. 2008;40(4):275-277.
pubmed - Rees DC, Williams TN, Gladwin MT. Sickle-cell disease. Lancet. 2010;376(9757):2018-2031.
doi - Lindsay J, Jr., Meshel JC, Patterson RH. The cardiovascular manifestations of sickle cell disease. Arch Intern Med. 1974;133(4):643-651.
doi pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.