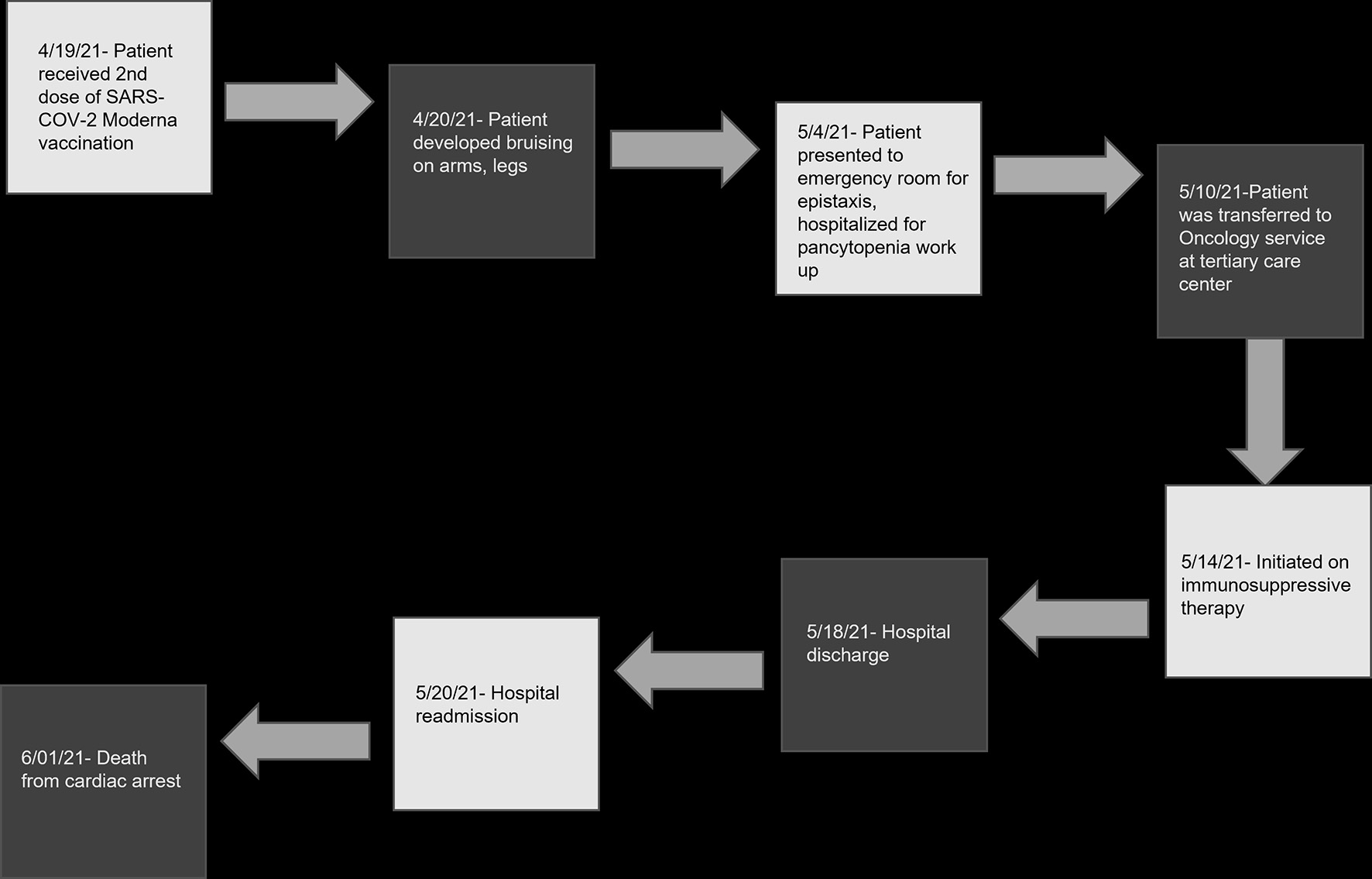
Figure 1. Timeline of clinical events following second SARS-CoV-2 vaccination. SARS-CoV-2: severe acute respiratory syndrome coronavirus 2.
| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Case Report
Volume 11, Number 1, February 2022, pages 34-39
Severe Aplastic Anemia After Receiving SARS-CoV-2 Moderna mRNA Vaccination
Figure

Tables
| Laboratory parameter | At time of initial hospitalization on May 4, 2021 | Reference ranges |
|---|---|---|
| IPF: immature platelet fraction; RETC: reticulocyte count; ANC: absolute neutrophil count; LDH: lactate dehydrogenase. | ||
| White blood cells count, × 103/mm3 | 1.2 | 4 - 11 |
| Hemoglobin, g/dL | 8.0 | 13.5 - 17 |
| Platelet count, × 103/mm3 | 1 | 130 - 450 |
| IPF, % | 0.7 | 1.2 - 8.6 |
| RETC, × 103/µL | 4 | 26 - 168 |
| ANC, × 103/µL | 0 | 1.5 - 7.8 |
| Monocytes, × 103/µL | 0.0 | 0.2 - 1.0 |
| Protime, s | 12.7 | 9.4 - 12.5 |
| Transferrin, mg/dL | 181.9 | 200 - 390 |
| Ferritin, ng/mL | 534 | 20 - 250 |
| Fibrinogen, mg/dL | 478 | 200 - 465 |
| LDH, U/L | 203 | 135 - 225 |
| Haptoglobin, mg/dL | 242 | 43 - 212 |
| Laboratory parameter | At time of first hospitalization on May 4, 2021 | At time of discharge on May 18, 2021 | At time of second hospitalization on May 20, 2021 | Reference range |
|---|---|---|---|---|
| IPF: immature platelet fraction. | ||||
| White blood cell count, × 103/mm3 | 1.2 | 0.1 | 0.1 | 4 - 11 |
| Hemoglobin, g/dL | 8.0 | 7.8 | 8.4 | 13.5 - 17 |
| Platelets, × 103/mm3 | 1 | 26 | 13 | 130 - 450 |
| IPF, % | 0.7 | 0.1 | 0.1 | 1.2 - 8.6 |