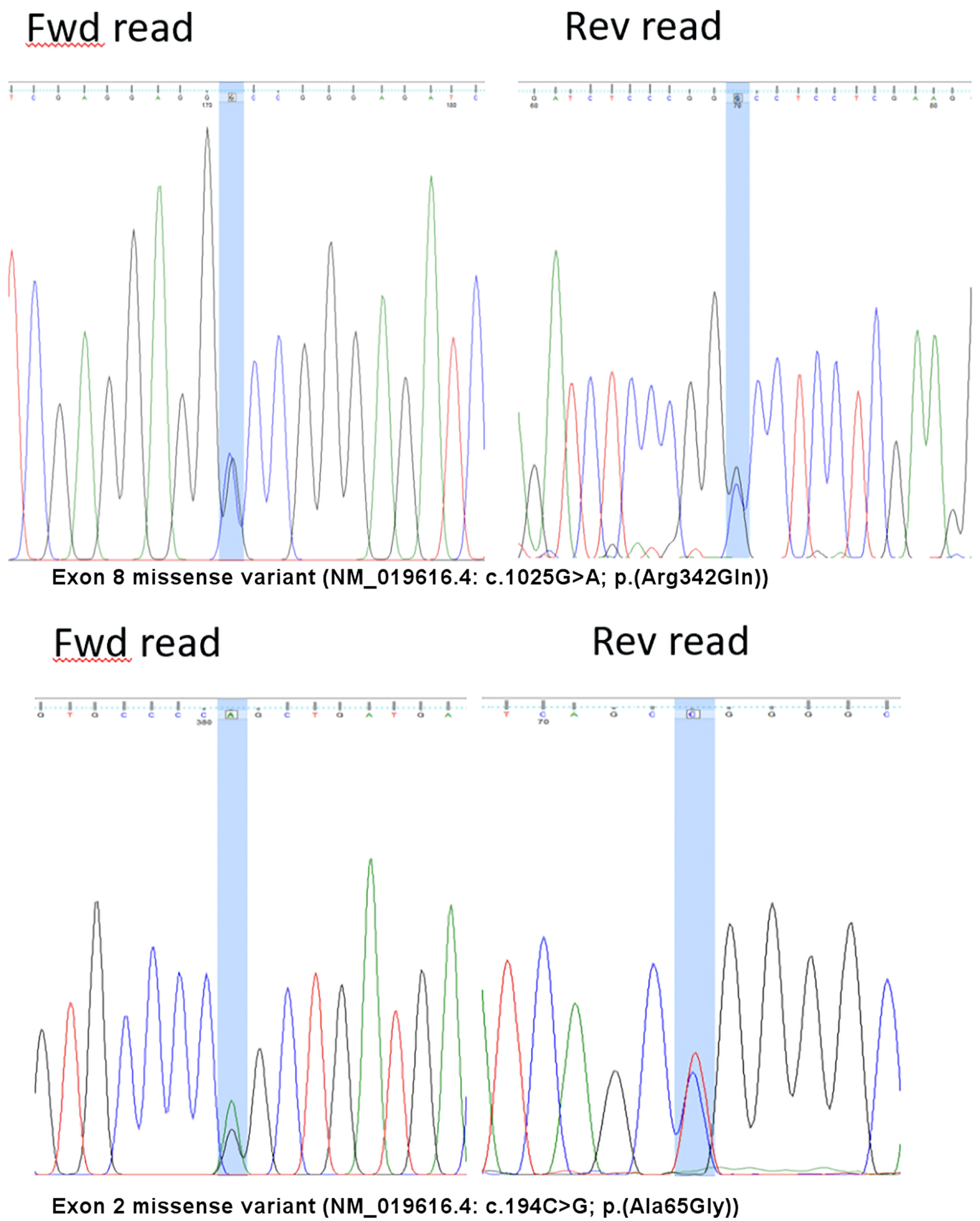
Figure 1. Variants detected on the F7 gene. Fwd: forward; Rev: reverse
| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Case Report
Volume 11, Number 1, February 2022, pages 29-33
Compound Heterozygous Factor VII Deficiency c.1025G>A p.(Arg342Gln) With Novel Missense Variant c.194C>G p.(Ala65Gly)
Figure

Table
| Rabbit brain thromboplastin (Neoplastine CI Plus, Stago Diagnostica) | Recombinant human tissue factor thromboplastin (Dade Innovin, Siemens) | Human placental thromboplastin (Thromborel S, Siemens) | F7 antigen (VisuLize F7 antigen - Affinity Biologicals) | |
|---|---|---|---|---|
| FVII: factor VII; PT: prothrombin time. | ||||
| PT (s) | ||||
| Patient | 27.9 | 14 | 18.4 | |
| Reference range | 11.7 - 14.0 | 9.9 - 11.8 | 10.4 - 12.6 | |
| 50% correction (s) | ||||
| Patient | 14.9 | 9.8 | 13.5 | |
| Control | 13.7 | 9.2 | 12.3 | |
| FVII activity (%) | ||||
| Patient | 4% | 28% | 21% | |
| Reference range (FVII) | 55-170% | 70-120% | 70-120% | |
| FVII antigen | ||||
| Patient | 87% | |||
| Reference range (FVII antigen) | 71-146% | |||