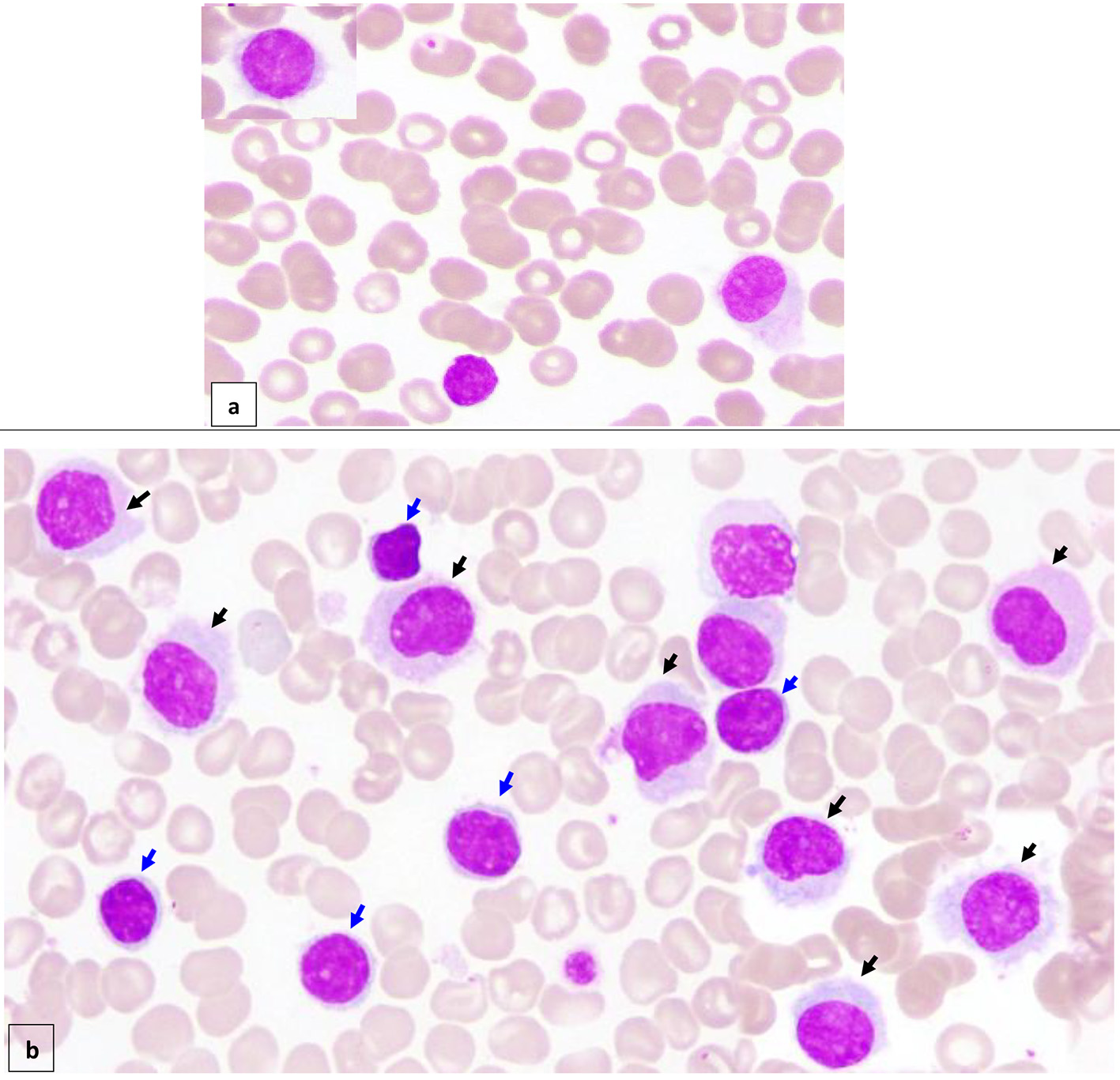
Figure 1. Peripheral blood (× 100) shows about 7% circulating abnormal lymphoid cells (hairy cells) of small/medium to large size with oval/indented/kidney shaped nuclei, dispersed nuclear chromatin, few small nucleoli, abundant pale cytoplasm and hairy like projections in some forms (a) (black arrow). Bone marrow aspirate (× 100) is infiltrated by about 16% abnormal lymphoid cells (hairy cells) (black arrow). In addition, there are small mature looking lymphocytes (about 18%) with condensed nuclear chromatin a small rim of cytoplasm (b) (blue arrow).
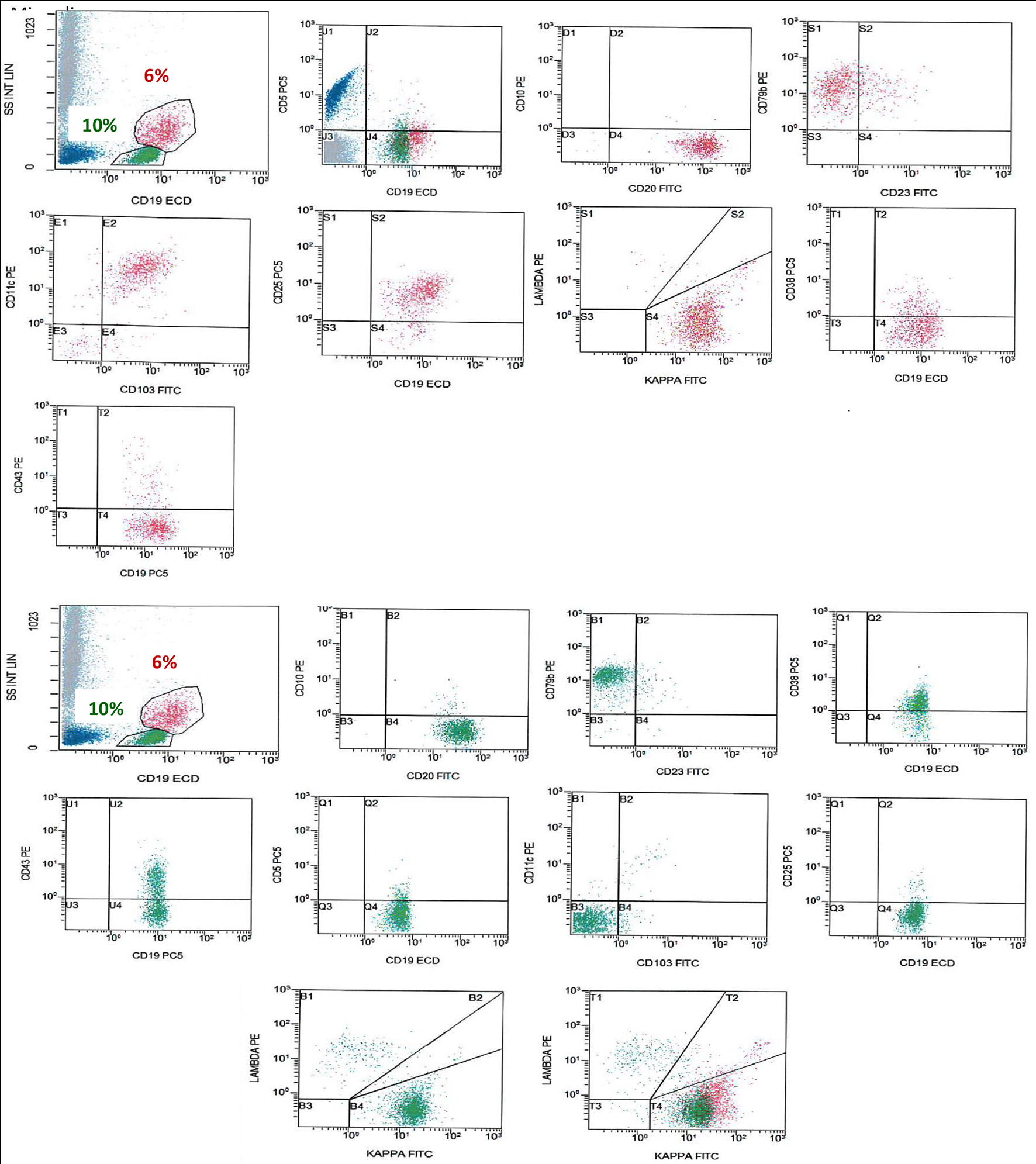
Figure 2. FCM on BM shows a population of monotypic B cells (red), showing high light side scatter and expressing CD19 (bright), CD20 (bright), CD79b, CD103, CD25, CD11c, and shows kappa light chain restriction (bright). This monotypic population is negative for CD5, CD10 and CD43, an immunophenotype consistent with HCL. There is another population of monotypic B cells (green), showing lower light scatter and expressing CD19, CD20, CD79b, and CD38 with partial expression of CD43 and shows kappa light chain restriction. This population is negative for CD5, CD23, CD10, CD25, CD11c and CD103. FCM: flow cytometry; BM: bone marrow; HCL: hairy cell leukemia.
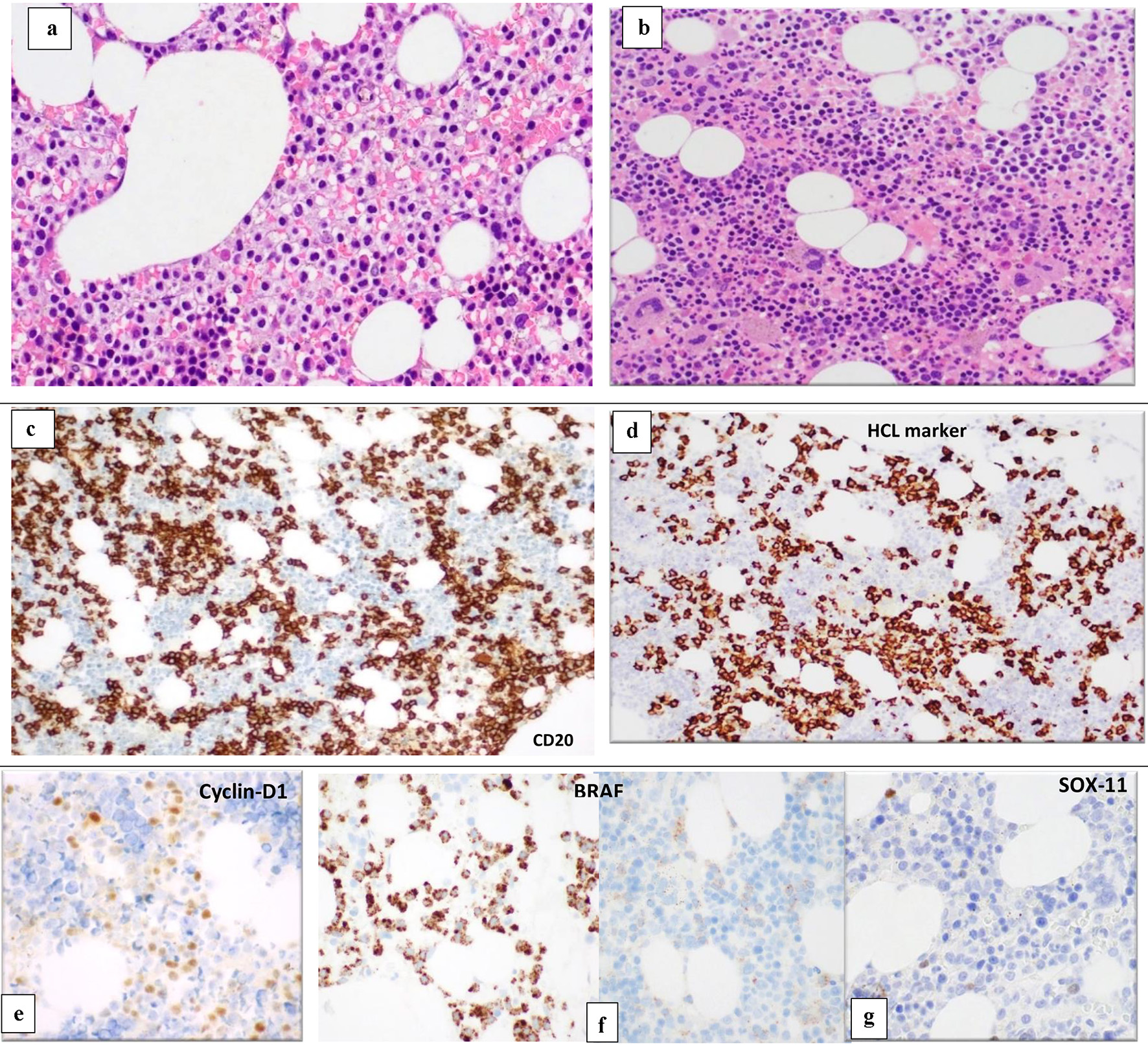
Figure 3. BM biopsy (H&E, × 10) shows variable cellularity with a lymphoid infiltrate showing different patterns of infiltration in different marrow areas. Some areas show widely spaced infiltrate composed of small- to medium-sized lymphoid cells with kidney shaped/indented nuclei and abundant cytoplasm giving the fried-egg appearance (a). Other BM spaces show an interstitial infiltration with small sized lymphoid cells (b). The abnormal lymphoid cells are positive for CD20 (c) and show partial positivity for DBA-44 (HCL marker) (d), cyclin-D1 (e), BRAF (positive in areas with HCL infiltrate) (f) and negative for SOX11 (g). BM: bone marrow; H&E: hematoxylin and eosin; HCL: hairy cell leukemia.
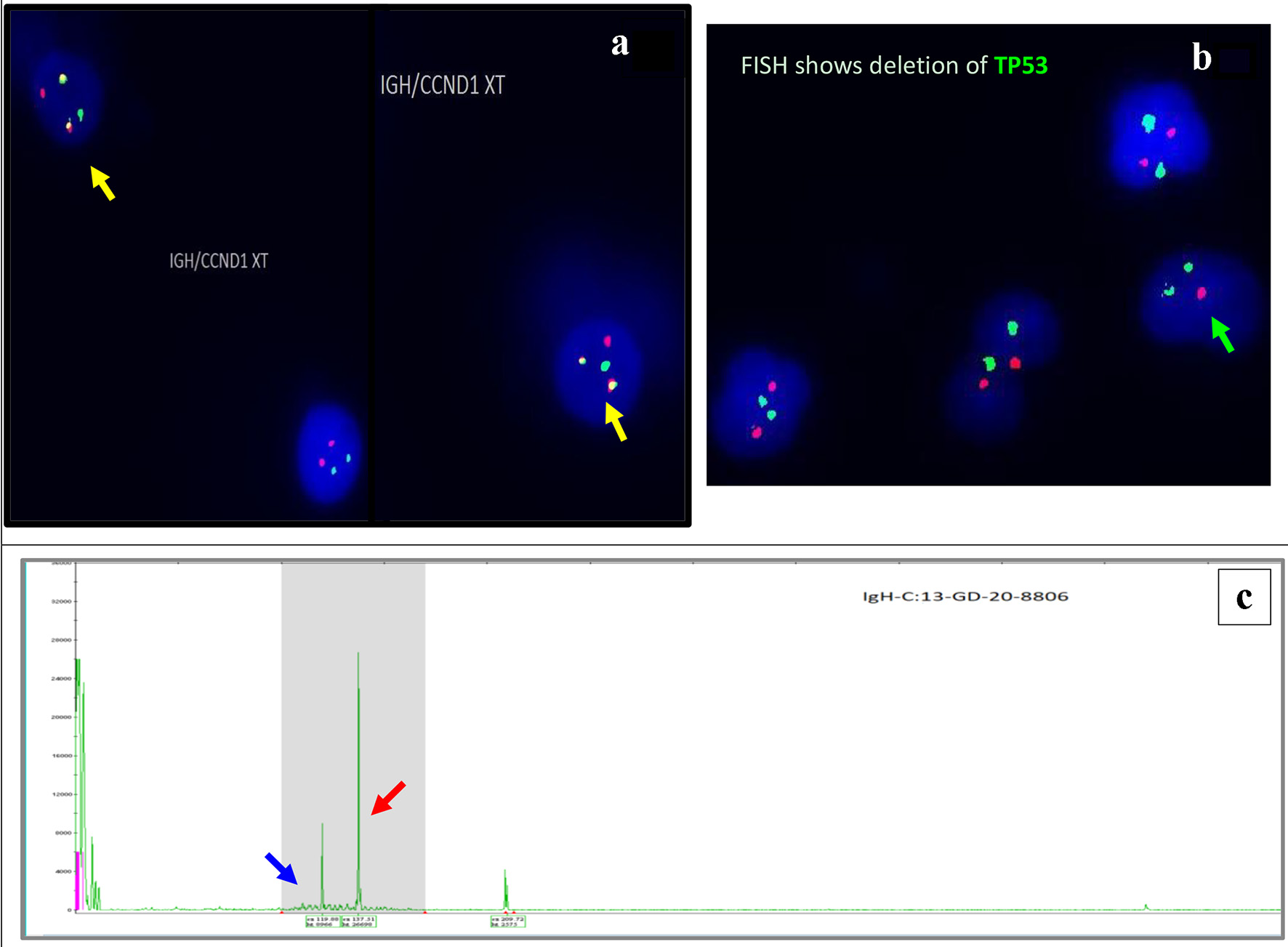
Figure 4. Interphase FISH demonstrating IGH/CCND1 rearrangement (a): IGH (14q32) labelled with spectrum green, CCND1 (11q13) labelled with spectrum orange show dual fusions signals as result of IGH/CCND1 fusion (yellow arrow). Loss of a single copy of CEP17/TP53 indicates deletion of TP53 gene on chromosome 17. CEP17 (17p11.1-q11.1) labelled with spectrum green, and TP53 (17p13.1) labelled with spectrum orange (b) (green arrow). PCR-based clonality testing (Invivoscribe Identiclone) was used to identify clonal B-cell populations with primers that target conserved framework (FR) and joining (J) regions of the IGVH genes. Two clonal B-cell populations were identified: a dominant 335 bp IGH VH (FR1) JH clone (red arrow) and a 317 bp IGH VH (FR1) JH subclone (blue arrow) (c). FISH: fluorescence in situ hybridization; PCR: polymerase chain reaction.



