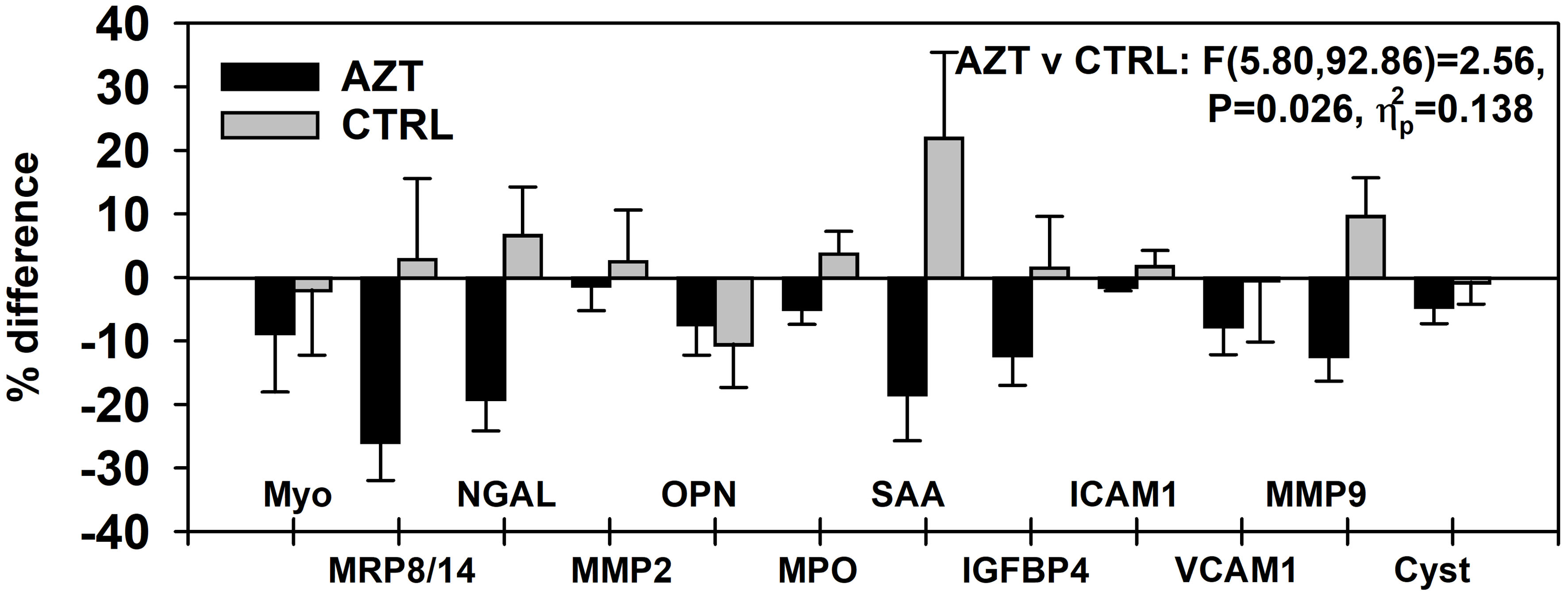
Figure 1. Dynamics of changes in plasma levels of all studied markers of vascular damage in patients treated with azithromycin (AZT) and placebo control (CTRL) groups. Data were analyzed with mixed-model repeated measures analysis of variance (ANOVA) and presented as a percent difference between PRE and POST intervals. MRP: myeloid-related protein; NGAL: lipocalin A; MMP: matrix metalloproteinase; IGFBP: insulin-like growth factor-binding protein; OPN: osteopontin; MPO: myeloperoxidase; SAA: serum amyloid A; ICAM: intercellular adhesion molecule; VCAM: vascular cell adhesion molecule; Cyst: cystatin.
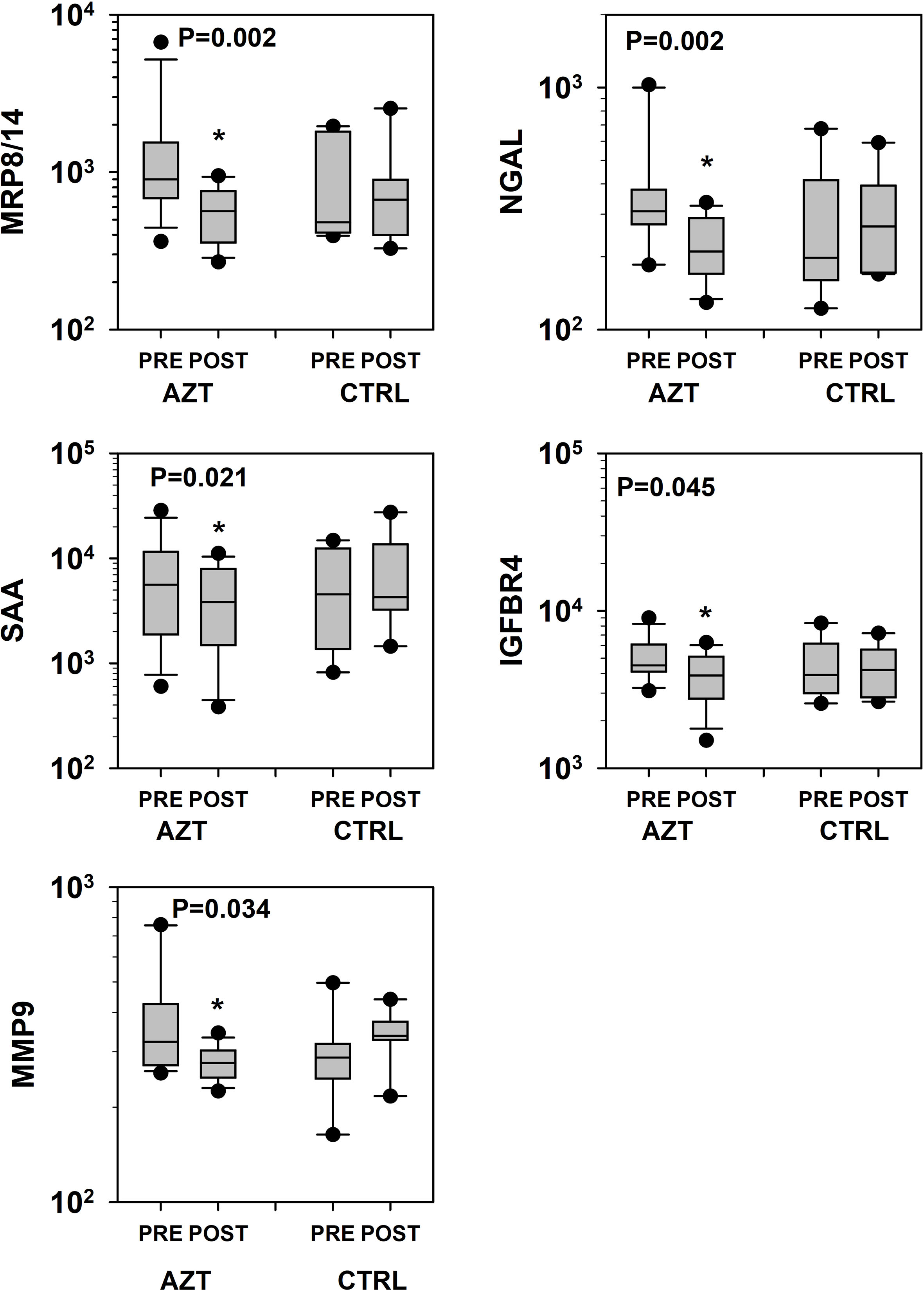
Figure 2. Dynamics of changes in plasma levels of specific markers of vascular damage that revealed statistical significance in patients treated with azithromycin (AZT) and placebo control (CTRL) groups. Data was analyzed with mixed-model repeated measures analysis of variance (ANOVA) followed by the pairwise multiple comparison procedures (Holm-Sidak method). *Statistical significance between PRE and POST intervals. MRP: myeloid-related protein; NGAL: lipocalin A; MMP: matrix metalloproteinase; IGFBP: insulin-like growth factor-binding protein; SAA: serum amyloid A.
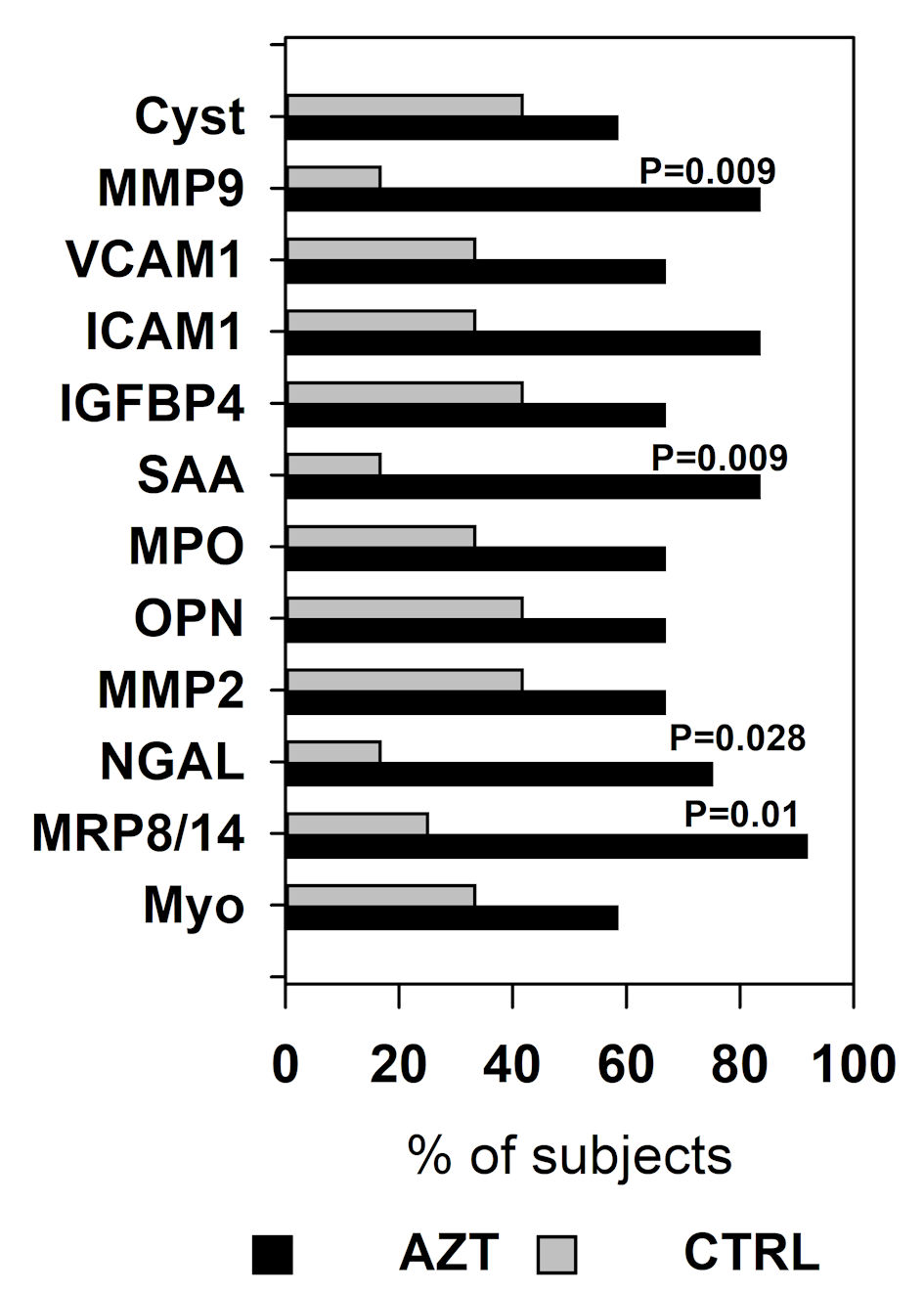
Figure 3. Percentage of the patients in azithromycin (AZT) and placebo control (CTRL) groups whose plasma level decreased at POST interval compared to PRE. Data were analyzed with χ2 test with Yates correction for continuity. MRP: myeloid-related protein; NGAL: lipocalin A; MMP: matrix metalloproteinase; IGFBP: insulin-like growth factor-binding protein; OPN: osteopontin; MPO: myeloperoxidase; SAA: serum amyloid A; ICAM: intercellular adhesion molecule; VCAM: vascular cell adhesion molecule; Cyst: cystatin.


